Abstract
Bioanalytical sample preparation is an essential component in the proper analysis of
biological samples, as it allows for the reliable quantification and characterization of target chemicals. This review discusses the numerous sample preparation techniques used to separate, purify, and concentrate analytes from complex biological matrices such blood, urine, and tissues. The main methods described are liquid-liquid extraction (LLE), solid- phase extraction (SPE), and ultrafiltration (UF). LLE is used to separate analytes based on their solubility in immiscible liquids, whereas SPE increases selectivity and sensitivity by interacting with particular sorbents. UF is used for the size-based separation and concentration of biomolecules. The study focuses on recent advances in these techniques, with an emphasis on increased efficiency, sensitivity, and applicability to complicated samples. Problems like matrix effects and analyte stability are also addressed. This abstract summarizes the principles, applications, and emerging innovations in bioanalytical sample preparation, emphasizing their importance in improving analytical accuracy and dependability in both research and clinical contexts (16) .
Keywords
Self-double emulsifying drug delivery system, Simvastatin, Micro emulsification, DoE, Zeta potential.
Introduction
Bioanalytical procedures are important analytical techniques used to detect and quantify biological molecules in a variety of biological samples, including blood, urine, saliva, tissues, and other body fluids. Pharmacology, toxicology, clinical diagnostics, and biological research all rely on these procedures to some extent. As the demand for precision medicine and individualized healthcare grows, so does the importance of robust bioanalytical procedures (2,6). The fundamental goal of bioanalytical methods is to offer precise and trustworthy information on the concentrations of medicines, metabolites, biomarkers, and other analytes in biological matrices. This data is critical for understanding drug absorption, distribution, metabolism, and excretion (ADME) patterns, as well as evaluating medication safety and efficacy in clinical trials. Furthermore, bioanalytical techniques are crucial in the detection of illness biomarkers, which enable early diagnosis and monitoring of disease progression (16,17). Advances in technology have substantially improved the capabilities of bioanalytical procedures. The combination of high-resolution mass spectrometry with chromatographic techniques has resulted in increased sensitivity and specificity, enabling for the study of complex biological material at lower concentrations than ever before. Furthermore, the introduction of innovative immunoassays and biosensors has enabled quick and point-of-care testing, making bioanalytical techniques more accessible in clinical settings.
SAMPLE PREPARATION TECHNIQUES
Sample preparation is an important stage in the analytical process, connecting sample collection to analysis. It includes processes for isolating and concentrating analytes from complex biological matrices such as blood, urine, and tissues. The success of these approaches has a direct impact on the quality and consistency of analytical results. Proteins, lipids, and salts are common interfering components in biological samples, making it difficult to identify and quantify target analytes. As a result, proper sample preparation techniques are critical for eliminating interferences, extracting analytes, and increasing detectability, eventually assuring accurate and reproducible results (9,16). There are various bioanalytical sample preparation methods for isolating and concentrating analytes from biological matrices. Here are a few common techniques:
- Protein Precipitation (PP)
- Solid Phase Extraction (SPE)
- Liquid-Liquid Extraction (LLE)
- Filtration
- Dialysis
- Ultrafiltration
- Centrifugation
- Liquid Chromatography
- Supercritical Fluid Extraction
- Microextraction Techniques
- Enzymatic Digestion
- Matrix Solid-Phase Dispersion (MSPD)
PROTIEN PRECIPITATION(PP)
In bioanalytical chemistry, protein precipitation is a commonly used sample preparation method, especially for purifying and concentrating analytes by extracting proteins from complex biological matrices such as blood, serum, or urine. Proteins that could obstruct further analytical processes, including mass spectrometry or chromatography, must be removed using this method (3,4).
PRINCIPLE
The basic idea behind protein precipitation is adding a precipitating agent, sometimes called a precipitant, to a biological sample, which causes the proteins to aggregate and then sediment. Through adjustments to the solution's pH, ionic strength, or organic solvent content, the precipitating agent can modify the solubility of proteins. Several different kinds of compounds are frequently utilized as precipitating agents in protein precipitation: In order to cause protein precipitation, organic solvents including acetonitrile, ethanol, and methanol are frequently employed to decrease the solubility of proteins in the aqueous phase. Salts such as sodium chloride and ammonium sulphate can also be used (12,19). By changing the ionic strength of the solution and lowering protein solubility, these salts cause proteins to precipitate. Acids that reduce the pH of a solution, such as perchloric acid and trichloroacetic acid (TCA), cause proteins to denature and precipitate. Proteins can occasionally be precipitated using detergents, especially when proteins need to be extracted from complicated combinations. The nature of the proteins and analytes involved, as well as the particular requirements of the analysis, are taken into consideration when choosing these agents (19).
PROCEDURE
Start with a clear supernatant or a biological sample that has been well-homogenized, like serum, plasma, or tissue extract, to initiate the protein precipitation process. Subsequently, incorporate the selected precipitating agent into the sample; the precise quantity and concentration will differ according to the procedure and the kind of sample (20). Make sure that the precipitant and the proteins are completely mixed in the sample by gently swirling or vertexing it. To encourage successful precipitation, let the combination incubate for a predetermined amount of time, which can be minutes to several hours, at a specified temperature, usually 4°C or room temperature (12). To pellet the precipitated proteins, centrifuge the sample for 10 to 30 minutes at a high speed (typically between 10,000 and 15,000 rpm) following incubation. Since the supernatant contains the important tiny molecules or analytes, remove it carefully and make sure not to disrupt the particle. To get rid of any last traces of impurities or salts, you can choose to wash the protein pellet with a cold solvent like ethanol or acetone (10). Then, centrifuge it once more and throw away the wash solution. Lastly, resuspend the protein pellet in the proper buffer if necessary, so that it can be stored or used for additional analysis.
ADVANTAGES
In the processing of bioanalytical samples, protein precipitation provides a number of important benefits. It is an accessible and effective method that needs little equipment and cheap reagents. It is also reasonably priced. The centrifugation and mixing phases of the procedure are swift, enabling the effective processing of numerous samples. Proteins from biological matrices are efficiently removed, which lowers sample complexity and enhances analyte recovery. This approach is adaptable, appropriate for a range of biological materials, and works with a number of different analytical methods, including mass spectrometry and chromatography. Furthermore, protein precipitation reduces sample loss and can easily scaled up or down based on sample amount (1,6).
LIMITATIONS
There are many restrictions on protein precipitation. It might not eliminate every protein entirely, which could result in impurities that could cause problems for analysis. Target analytes with characteristics similar to the precipitated proteins may also be lost (1). If the procedure is not adequately handled, it may degrade sensitive analytes and be impacted by matrix effects. Accuracy may be impacted by residual impurities from salts or solvents, and the non-specific character of the procedure may make it more difficult to analyse particular proteins. Furthermore, optimizing the conditions for every sample is frequently necessary for protein precipitation, which can be time-consuming and cause problems with repeatability (6).
SOLID PHASE EXTRACTION
Analytes can be separated and concentrated from complicated matrices using Solid Phase Extraction (SPE), a sample preparation method that makes use of a solid adsorbent. In analytical chemistry, this method is frequently applied to clinical diagnostics, pharmaceutical testing, and environmental analysis.
PRINCIPLE
Analytes are partitioned between a liquid sample and a solid adsorbent in solid phase. Extraction: The following steps are involved in the process(5,6) :
- Adsorption:
While interfering chemicals are eliminated, analytes are adsorbed onto the solid phase(adsorbent).
- Cleaning:
To get rid of any leftover contaminants or undesirable materials, the adsorbed analytes are cleaned.
- Elution:
Next, the analytes are extracted from the solid phase and concentrated, making them suitable for further examination.
STEPS IN SOLID PHASE EXTRACTION
Conditioning in Solid Phase Extraction (SPE) is the process of activating the adsorbent material in the SPE column in order to prepare it for sample adsorption (6,7). This is done by running solvents through the column in order to equilibrate the adsorbent, frequently using solvents that will be used in subsequent washing and elution processes. The next step is sample loading, in which the sample is loaded into the SPE column that has been pre-conditioned so that the analytes can adhere to the solid phase (8). The next step involves washing the adsorbent while keeping the target analytes in place by running a wash solvent through the column to get rid of contaminants and non-target materials. The next stage is called elution (5,6), in which the adsorbed analytes are separated from the solid phase and collected in a different vessel using an elution solvent.
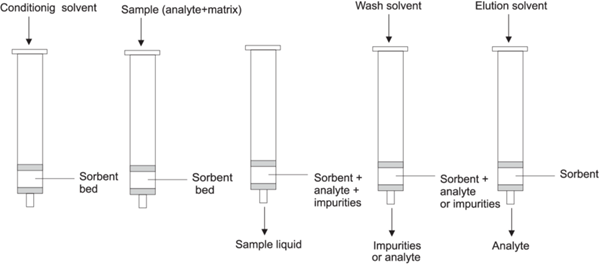
- Optimization Is Necessary:
Optimizing variables such adsorbent type, solvent composition, flow rates, and sample volume is critical to the efficiency of SPE. Ineffective target analyte separation or low recovery can result from improper optimization (1).
- Potential Sample Loss:
Some analytes may be lost or partially recovered during the washing and elution stages, which could have an impact on the precision and comprehensiveness of the results.
- Matrix Effects:
Complex matrices can still provide difficulties even with SPE's reduction of matrix effects. Substances that interfere may contaminate samples (5) or reduce the effectiveness of analyte extraction.
- Cost:
SPE columns, cartridges, and related equipment can be costly, particularly in the case of specialized or high-throughput applications. In certain analytical workflows, this can be a substantial cost component.
- Time-consuming:
The procedure calls for a number of procedures that can take a while, especially when handling a lot of samples (8) : conditioning, loading, washing, and elution.
ADVANTAGES
Depending on how analytes interact with the adsorbent, SPE can isolate particular analytes from complex mixtures.
SPE increases the sensitivity of analytical techniques by concentrating analytes.
Adaptable to a variety of analytes and sample types, including bigger and smaller biomolecules.
- Diminished Matrix Effects:
SPE lessens the influence of matrix elements on the precision of analytical outcomes (1,8).
APPLICATIONS
To separate and gather contaminants from soil, water, or air samples.
To ensure precise assessment of medication levels and contaminants, drug samples are prepared for analysis (5).
Used to separate medications or biomarkers from biological fluids such as blood or urine.
- Food and Beverage Testing:
- To find impurities or additions in samples of food and drink.
- Solid phase extraction is a strong and adaptable method that offers efficient sample preparation, raising the precision and effectiveness of analytical testing in a variety of sectors.
LIQUID PHASE EXTRACTION
Analytes can be separated and purified from a liquid combination using Liquid Phase Extraction (LPE), often referred to as Liquid-Liquid Extraction (LLE), a basic approach that takes use of the solubility differences between two immiscible liquids (10). Chemical analysis, environmental testing, pharmaceutical development, and other fields all make extensive use of this procedure.
PRINCIPLE
Analytes are selectively partitioned between two immiscible liquid phases according to the Liquid Phase Extraction (LPE) or Liquid-Liquid Extraction (LLE) (10). The principle is Partitioning, in which an analyte is divided based on changes in solubility between two immiscible liquid phases (usually an aqueous phase and an organic solvent), is the basic idea behind liquid phase extraction (LPE)(3). The partition coefficient, which measures the analyte's affinity for each phase, is what drives this separation. The ratio of the analyte's concentration in the organic phase to its concentration in the aqueous phase is specifically measured by the partition coefficient (K) (11,12). The partition coefficient plays a vital role in determining the extraction efficiency of an analyte. A higher value indicates a greater tendency of the analyte to go into the organic phase.
K=C aqueous/ C organic (3,12)
- C _organic: Concentration of the analyte in the organic phase.
- C_ aqueous: Concentration of the analyte in the aqueous phase.
STEPS IN LIQUID PHASE EXTRACTION
1.preparation:
Sample Preparation: To enhance the analyte partitioning, the sample is prepared by adding salts or altering its ph. To get rid of particles, it can also be diluted or filtered.
2. Blending:
Phase Contact: In a separation vessel, the sample solution and the organic solvent are combined. This can be carried out mechanically with a mixer or manually in a separatory funnel.
3.Emulsification: The two liquids may need to be split up or given time to settle if they create an emulsion.
4.seperation:
Phase Separation: The two phases are given time to separate following mixing. Centrifugation or gravity (in a separatory funnel) can help with this. There will be a separation between the lighter (usually organic) and heavier (usually aqueous) phases.
5.Collection: Phase Removal: The phases are gathered after being separated. Carefully draining the phase from the bottom of the separatory funnel or using a pipette are common methods for removing the phase containing the target analyte.
6.Drying: Agents for Drying: To eliminate any remaining water, anhydrous sodium sulphate or magnesium sulphate can be used to dry organic extracts.
Focusing (if required):
7. Evaporation: To concentrate the analyte before analysis, the organic solvent may be evaporated using a rotary evaporator at a lower pressure.
8.Evaluation: Preparation for Analysis: Chromatography, mass spectrometry, or spectrophotometry are some of the methods used to get ready the extracted analyte for analysis (3,10,12).
ADVANTAGES
• High Efficiency: Analytes from complicated mixtures can be effectively separated and concentrated using LPE based on variations in solubility.
• Versatility: Suitable for a broad spectrum of analytes and sample kinds, including both organic and inorganic substances.
• Scalability: Capable of being used to large-scale industrial processes as well as little laboratory samples.
• Selectivity: By selecting the right solvents and modifying the conditions, it is possible to selectively extract particular analytes (11).
LIMITATIONS
• Use of Solvents: Necessitates the use of organic solvents, which can be costly, dangerous, and bad for the environment.
• Emulsions: The creation of emulsions can impede phase separation and necessitate further measures for resolution.
• Sample Loss: If analytes are not partitioned selectively, they may be lost in the extraction process.
• Complexity: In order to obtain the best separation, the process might be complicated and requires careful control of several factors (3,10,11,12).
APPLICATIONS
Liquid-Liquid Extraction (LLE) is a widely applied, adaptable technology with several uses in different fields. The following are some important applications:
Liquid-liquid extraction (LLE) is widely utilized across various sectors due to its effectiveness in separating and isolating substances. In environmental studies, LLE is used to detect pollutants in water, soil, and air samples, including pesticides, heavy metals, and organic contaminants. It also aids in monitoring contamination levels and assessing environmental compliance. In the healthcare sector, LLE plays a critical role in drug purification (3) during pharmaceutical development and production, and in metabolite analysis for pharmacokinetic research, where it isolates and concentrates metabolites from biological fluids (10).
In medical diagnostics, LLE is employed to extract biomarkers from biological fluids such as blood and urine for diagnostic testing and disease monitoring. It also assists in drug testing by isolating and measuring pharmaceuticals and their metabolites in clinical samples. The food and drink industry benefits from LLE in detecting contaminants like pesticides and additives, as well as extracting flavour (10) and aroma components for product development and quality assurance. Additionally, in chemical production, LLE is used to recover valuable chemicals and solvents, ensuring effective resource utilization and minimizing waste. It also facilitates separation and purification processes in various industrial applications.
FILTRATION
A basic bioanalytical technique for removing impurities or particles from liquids or gases is filtration. It is crucial for guaranteeing the precision and Caliber of analytical results while preparing samples for analysis (1).
PRINCIPLE
The basic idea behind filtering is to use a filter medium to separate solid particles from a liquid or gas. While the solid particles are trapped, the liquid or gas can pass through the filter media. The size of the filter's pores, the kind of particles, and the filtration method all affect how effective the filter is. Among the filtration techniques are mechanical filtration and matrix filtration. Mechanical filtration uses physical sieving to retain particles larger than the pores on the filter's surface or within its matrix, based on the size of the particle relative to the filter's pore size. By employing a porous media with a fibrous or granular structure to create a multitude of barriers and paths for particle retention, depth filtration collects particles at any depth in the medium (12,8). Membrane filtration separates particles and solutes from liquids by using semi-permeable membranes with precisely calibrated pore sizes. Particles larger than the pores are trapped, but the liquid phase flows through. Reverse osmosis, ultrafiltration, nanofiltration, and microfiltration are just a few of the scales at which membrane filtration can be customized (8)(10). Which particles can pass through a filter is determined by its pore size; finer particles are filtered out by smaller pores, which may also cause faster blockage and greater resistance. Pore size, fluid viscosity, and applied pressure all affect the filtration rate; larger pore sizes enable faster filtering but may also result in less effective particle removal. Filtration efficiency is also affected by pressure and flow rate; while increased pressure or flow rates can expedite the process, they also raise the risk of fouling and shorten filter life(18).
TYPES OF FILTERS
Frequently utilized in laboratory environments, filter sheets are utilized for basic separations by gravity filtering. They are perfect for efficiently separating solid particles from liquid solutions since they are available in a range of pore diameters.
Membrane filters can be made of cellulose, nylon, or PVDF and are made of synthetic or natural membranes with predetermined pore sizes. In both laboratory and industrial procedures, they are frequently employed for precise separations, particle removal, and sterile filtration.
Designed to trap particles throughout the whole filter medium, depth filters are constructed from materials like cellulose or fiberglass. Usually, they are employed in coarse or pre-filtration to get rid of bigger particles before more specialized filtration procedures(1,12).
APPLICATIONS
Filtration is an adaptable method with several uses in many domains:
- Sample preparation:
To guarantee clarity and guard against harm to analytical equipment, particles from liquid samples are removed from the sample using filtration in laboratories.
- Sterilization:
To ensure that solutions are free from microbial contamination, filters with pore diameters of 0.2 µm or less are used to remove germs from liquids and gases.
- Purification:
In the chemical, pharmaceutical, and biotechnology industries, filtration is crucial for separating and purifying chemicals by eliminating undesired solid materials or impurities.
- Environmental Monitoring:
By removing pollutants from the sample matrix, it is utilized to identify and examine pollutants in samples of air, water, and soil.
- Food and Beverage Industry:
To ensure that food is free of pollutants and impurities, filtration is essential (10,12).
ADVANTAGES
- Easy to Implement and Effective: This approach is simple to use and effective in a variety of contexts.
- Adaptable: suitable for a range of particle sizes, gases, and liquids.
- Scalable: Fits both large-scale industrial operations and little lab setups.
LIMITATIONS
- Clogging: Particulate buildup in filters can cause them to lose performance and necessitate routine maintenance or replacement.
- Analyte Loss (18): If some analytes adhere to the filter material or stay in the filter matrix, they may be lost.
- Pressure Requirements: In some cases, high-pressure filtering systems may be required, which can raise the cost and complexity of operations (10).
DIALYSIS
In bioanalytical sample preparation, dialysis is a process used to separate molecules according to size. It is very helpful in separating smaller molecules in a sample from bigger ones (1).
PRINCIPLE
Dialysis utilizes a semi-permeable membrane to selectively diffuse molecules and separate them based on size. This membrane, made from materials like cellulose, polysulfone, or other polymers, has specific pore sizes that allow certain molecules to pass through while blocking others (12). A key feature of these membranes is their Molecular Weight Cut-Off (MWCO), which indicates the maximum molecular weight of solutes that can cross the membrane. For example, a membrane with an MWCO of 10,000 Dalton will allow smaller molecules to pass while retaining larger ones. The dialysis process involves several crucial steps. Initially, a dialysis bag or tube is filled with a mixture of large and small molecules and submerged in a dialysis buffer. This setup creates a concentration gradient between the inside of the bag and the external buffer. Molecules then diffuse selectively across the semi-permeable membrane from areas of higher concentration to areas of lower concentration. Larger molecules, such as proteins, remain inside the bag, while smaller molecules, like salts and chemical compounds, move out into the buffer. To maintain an effective concentration gradient, the external buffer is often changed several times during dialysis. This prevents the buildup of small molecules in the buffer, encouraging further diffusion. The duration of the dialysis process depends on various factors including the size of the molecules, the MWCO of the membrane, the volume of the buffer, and the composition of the sample (10). Dialysis can take from a few hours to several days to achieve the desired level of purification. The rate of diffusion is influenced by the concentration gradient, membrane permeability, and the size of the solutes, with higher gradients leading to faster diffusion.
APPLICATIONS
Liquid-liquid extraction (LLE) is a versatile technique applied across various sectors. In environmental monitoring, LLE is used to detect pollutants in water, soil, and air, including pesticides, heavy metals, and organic contaminants. It supports the assessment of contamination levels and ensures environmental compliance. In the healthcare sector, LLE is essential for drug purification during pharmaceutical production and for isolating and concentrating metabolites in pharmacokinetic studies (18,8). For medical diagnostics, LLE extracts biomarkers from biological fluids such as blood and urine, aiding in diagnostic testing and disease monitoring. It is also crucial for drug testing, helping to isolate and measure pharmaceuticals and their metabolites in clinical samples. In the food and drink industry, LLE detects contaminants like pesticides and additives and extracts flavour and aroma components for product development and quality control. Additionally, in chemical production, LLE recovers valuable chemicals and solvents, optimizing resource use and reducing waste (8,10,18), while also facilitating separation and purification processes in various industrial applications. Dialysis offers several advantages as a purification technique. It provides selective separation based on size, effectively distinguishing between smaller and larger macromolecules. The process is mild, making it a gentle method for purifying samples while preserving the integrity of delicate biomolecules. Dialysis is also user-friendly and cost-effective, requiring minimal equipment and being less expensive compared to other purification methods. Its versatility allows it to handle a wide range of biomolecules and sample types, including nucleic acids, proteins, and small contaminants (10). Additionally, the technique minimizes sample loss, which is crucial for preserving valuable or delicate samples. There is no chemical interference during dialysis, helping to maintain the sample's natural state. Finally, the process is scalable, making it adaptable for both small-scale laboratory work and larger industrial applications.
LIMITATIONS
Dialysis has some limitations as a purification technique. It can be time-consuming, often requiring several hours to several days to achieve adequate separation or purification. The method also has limited resolution, making it less effective for separating molecules of similar sizes or when precise separation is required. Additionally, dialysis may not be ideal for situations where a high concentration of the sample is needed, as the process tends to dilute the sample. Effective dialysis requires a significant volume of buffer, which may be impractical for concentrated or small-scale samples. Moreover, the membrane's Molecular Weight Cut-Off (MWCO) may not always be suitable for all applications, potentially leading to insufficient separation (8,18,10).
ULTRAFILTRATION
A membrane is used in the separation process known as ultrafiltration to filter out particles according to size. It involves a semi-permeable barrier that holds onto larger particles, such proteins, cells, and larger macromolecules, while allowing smaller molecules and solvents to flow(21).
PRINCIPLE
A semi-permeable membrane is used in ultrafiltration, a separation process, to filter molecules according to size. Larger particles like proteins and colloids are retained while smaller molecules and solvents can pass through the membrane under pressure. This is how the process works. Pressure is applied to a feed solution. Which molecules can be separated is determined by the molecular weight cut-off (MWCO) of the holes in the membrane. Centrifugal force can also be employed, however hydraulic pressure is usually the driving force behind this filtration. Two separate streams emerge as a result: the retentate, which is a concentrated solution of the bigger molecules that were held, and the permeate, which contains the smaller molecules that cross the membrane (21,22). The efficiency of ultrafiltration is dependent on a number of factors, including feed concentration, temperature, pressure, and membrane pore size. In order to control fouling and preserve performance, routine maintenance is necessary (20). This includes cleaning and sometimes replacing the membrane. Ultrafiltration is a vital technique for sample preparation and purification in both research and industrial contexts. It is widely utilized in many applications, such as protein concentration, water purification, and desalting.
ADVANTAGES
Ultrafiltration (UF) has a number of noteworthy benefits, especially when it comes to its superior filtration qualities. It is extremely useful in the food and beverage processing, biotechnology, pharmaceutical, and water purification industries due to its capacity to efficiently eliminate bacteria, macromolecules, and tiny particles. The technique offers molecular size-based selective separation, enabling exact control over the filtration process. Comparing UF to other filtration techniques, it is also a more environmentally responsible choice because it requires fewer chemical treatments. Its efficiency in cross-flow configurations and mild operating pressures reduce fouling and maintain a greater flux rate, which eventually improves performance and lowers operating costs(8,10,18).
APPLICATIONS
Ultrafiltration (UF) is widely used across various sectors for its ability to enhance safety, quality, and efficiency. In drinking water treatment, UF removes bacteria, viruses, and particulate matter from both well and municipal water, improving its safety and quality. It also plays a crucial role in wastewater treatment by clarifying effluents and removing impurities before the water is released or reused in municipal and industrial settings. In the food and beverage industry, UF is employed to separate lactose, concentrate proteins in milk and whey, and clarify dairy products like yogurt and cheese. It also extends the shelf life and clarity of juices and other beverages by removing microorganisms and suspended particles (23). In pharmaceuticals, UF is used to purify and concentrate proteins, enzymes, and other macromolecules, ensuring high purity and concentration. It is also integral to the manufacturing of vaccines and other biologics, where it helps to eliminate impurities and concentrate active substances. In biotechnology, UF assists in processing cell cultures by removing cellular debris and clarifying the culture medium. It also separates and purifies various biomolecules such as peptides, nucleic acids, and polysaccharides (20,23). In industrial applications, UF enhances chemical processing efficiency by aiding in the separation and concentration of compounds and intermediates. In the oil and gas industry, it helps recover valuable components from process streams. Additionally, in medicine and laboratories, UF technology is crucial for dialysis, aiding in the removal of waste materials and excess fluid from the blood of patients with renal failure. It also improves the accuracy of analytical results by purifying and concentrating laboratory samples.
LIMITATIONS
Although ultrafiltration (UF) has numerous advantages, it is not without its drawbacks. Membrane fouling, which occurs when particles, microbes, or other contaminants build up on the membrane surface and cause repeated cleaning or replacement requirements, is one of the main problems. Delays in operations and higher operating costs may arise from this fouling. Furthermore, the installation and upkeep of UF systems can be costly, especially in high-capacity or specialized applications. Another issue is membrane longevity; with time, exposure to chemicals, high pressures, or mechanical stress can cause membranes to deteriorate and require frequent replacement. Additionally, UF cannot separate very tiny molecules or dissolved salts, which limits its usefulness in several applications where these kinds of separations are necessary. Even with these difficulties, UF is still a useful technology when its drawbacks are carefully managed and addressed.
CENTRIFUGATION
A key method in the processing of bioanalytical samples is centrifugation, which divides a mixture's constituent parts according to their size, density, and form.
PRINCIPLE
The principle of sedimentation underlies centrifugation, in which particles in a liquid feel force proportionate to their mass and size due to centrifugal force produced by spinning the sample at high speeds. Less dense (25) particles stay closer to the top of the centrifuge tube while denser particles are pushed outward and towards the bottom by the centrifugal force generated by the spinning centrifuge. Due to variations in the rates at which particles settle when subjected to centrifugal force, this separation occurs.
The centrifugal force is calculated as;
F=m× ?2 × r.
Where m is the mass of the particle
? is the angular velocity (speed of rotation)
r is the radial distance from the centre of rotation.
To obtain the best separation, the sample is really treated to different times and speeds. Smaller or less dense particles stay in the supernatant, whereas larger, heavier particles form a pellet at the tube's bottom (23,24). Centrifugation is an effective method for bioanalytical applications because of its gradient of separation, which enables exact isolation and analysis of various components.
METHOD
Centrifugation is a precise process that uses centrifugal force to separate sample components according to their density. Sample preparation is the first step in the process, during which the sample is gathered, combined, and sometimes pre-treated to guarantee consistency. The required speed, sample volume, and kind of separation are taken into consideration while choosing the right centrifuge and rotor. As tubes are placed into the centrifuge, equal-volume tubes are positioned across from one another to ensure balance. Next, the centrifuge's desired settings are entered, including time, temperature, and speed (RPM or RCF) (25).When the centrifuge is operating, less dense components stay in the supernatant while more dense particles sediment at varying speeds and form a pellet at the bottom of the tubes due to centrifugal force. Before opening the cover, the centrifuge must be allowed to stop entirely after centrifugation. Supernatant and pellet are meticulously separated in order to facilitate processing or additional analysis. Equipment needs to be cleaned and maintained on a regular basis after centrifugation to guarantee peak performance. The efficient separation and purification of sample components is ensured by this approach, which is essential for precise bioanalytical studies
APPLICATIONS
Because centrifugation is an effective method for separating and purifying chemical and biological samples, it is used extensively in many different fields. It is essential for blood separation in clinical laboratories so that cellular components, plasma, and serum can be isolated for research and diagnostic testing. Centrifugation is used in the food and beverage industry to separate the components of milk in dairy processing and to clarify liquids and concentrate proteins (12,24). It is used in the biotechnology and pharmaceutical industries to purify macromolecules such as proteins and nucleic acids that are necessary for molecular research and drug development. Centrifugation also helps with environmental testing by removing impurities from soil and water samples. For routine and sophisticated sample preparation in a wide range of scientific and commercial applications, the method's accuracy and adaptability make it invaluable.
ADVANTAGES
Numerous noteworthy benefits are provided by centrifugation in a range of scientific and industrial contexts. Its main advantage is that it can separate components based on density quickly and effectively, which makes it possible to purify and analyse complex mixtures at high resolution. The method is adaptable and works well with a variety of sample sizes in bench-top or industrial centrifuges as well as small volumes in microcentrifuges. Furthermore, centrifugation is typically a simple procedure requiring little sample handling, which lowers the possibility of contamination and maintains sample integrity. Given appropriate conditions, it is extremely reproducible and yields consistent results (24,25). Centrifugation is also useful for sample preparation, concentration, and clarifying in a variety of sectors, including environmental testing, biotechnology, and clinical diagnostics. It can be used for both preparative and analytical applications.
LIMITATIONS
Centrifugation offers benefits, but it also has drawbacks. The risk of sample damage is a major concern; if high centrifugal forces are not adequately managed, they may disrupt cells or denaturize delicate proteins. In order to prevent operating problems, the approach also calls for precise tube balancing. Any imbalance could cause vibrations or even damage to the equipment (21). Furthermore, centrifugation can only be used for very fine separations since it cannot effectively separate particles that are extremely similar in size or density. The process can take a while, especially for high-speed or high-capacity centrifugation, and in order to get the best results, certain factors like speed and duration must be carefully calibrated. Finally, the cost of purchasing and maintaining the equipment can be exorbitant; high-speed or ultracentrifuges, in particular, may require substantial operational costs. These limitations necessitate careful consideration and proper technique to ensure effective and reliable outcomes.
SUPERCRITICAL FLUID EXTRACTION
INTRODUCTION
Supercritical Fluid Extraction (SFE) is a potent and adaptable method used in the production of bioanalytical samples. It takes advantage of supercritical fluids, most commonly carbon dioxide (CO?), to remove desired chemicals from intricate matrices. The fluid's supercritical state, in which it combines the characteristics of a liquid and a gas (25), allows for selective and effective extraction with no need for solvent. Because of this method's accurate.
PRINCIPLE
Supercritical fluids are materials that exist at pressures and temperatures higher than their critical points. At these conditions, they have special qualities that combine those of a liquid and a gas. The fluid possesses strong solvent power like a liquid and high diffusivity like a gas at the supercritical state (12,24). The supercritical fluid is passed through the sample matrix during the extraction process, where it dissolves and removes the target chemicals. The extracted compounds are left behind as the supercritical fluid cools and decompresses, returning to a gaseous form. Temperature and pressure of the fluid are critical factors that may be accurately regulated to maximize yield and selectivity during the extraction process (14,24).
PROCEDURE
The process of supercritical fluid extraction (SFE) involves several detailed steps to efficiently obtain target compounds from a sample. It begins with the assembly of samples, where appropriate samples are collected to ensure they accurately represent the subject under study. Common sample types include plant materials, soils, biological tissues, and food products. Once collected, the samples are homogenized or ground to enhance extraction efficiency by increasing the surface area, which is crucial for achieving uniform extraction, especially in solid samples (22,23). Next, the system is configured for extraction. Carbon dioxide is the most frequently used supercritical fluid due to its relatively low critical temperature (31.1°C) and high pressure (73.8 bar), though other fluids such as ethane or methane may be used depending on the specific application. The extraction chamber of the SFE system is then filled with the prepared sample, where it will interact with the supercritical fluid. The extraction parameters, including pressure and temperature, are set based on the characteristics of the matrix and the target chemicals. Supercritical CO2 is typically used at pressures ranging from 100 to 300 bar and temperatures between 40 to 80 degrees Celsius (25). During the extraction procedure, the extraction chamber is pressurized with the supercritical fluid, which infiltrates the sample matrix. The fluid circulates through the sample for a predetermined period, during which the target compounds dissolve into the supercritical fluid. After extraction, the fluid is passed through a separator where temperature and pressure are reduced. This process causes the dissolved chemicals to concentrate as they return to their gaseous state, while the supercritical fluid is recycled for further use. Finally, in the post-extraction work phase, the extracted chemicals are collected from the container. The recycling of the supercritical fluid not only makes the process more economical but also environmentally friendly (23,25). The extracted compounds are then analysed using techniques such as mass spectrometry (MS), liquid chromatography (LC), or gas chromatography (GC), depending on the nature of the chemicals and the specific analytical requirements. This detailed approach ensures efficient and effective extraction of target compounds from the sample (25).
APPLICATIONS
Supercritical Fluid Extraction finds widespread applicability in several bioanalytical fields.
- Pharmaceuticals: To ensure high purity and concentration while removing active pharmaceutical ingredients (APIs) from plant sources or complicated matrices.
- Food and Beverage: To extract flavours, bioactive components, and essential oils without sacrificing the finished product's integrity or quality.
- Environmental Analysis: To separate impurities or pollutants from soil and water samples and produce purer extracts for additional examination.
- Biotechnology: Improving the effectiveness and selectivity of the extraction process through the extraction of proteins, lipids, and other macromolecules from biological tissues (25).
ADVANTAGES
Supercritical fluid extraction has a number of important advantages, such as:
- excellent Efficiency: Low solvent consumption, quick extraction, and excellent recovery.
- Selectivity: The capacity to extract substances only those that are soluble in the supercritical fluid.
- Ecologically Friendly: Less waste is produced and less organic solvents are used, which results in more environmentally friendly extraction procedures.
- Versatility: Adaptable to a variety of target chemicals and sample matrices (23,24).
LIMITATIONS
Despite its advantages, supercritical fluid extraction (SFE) has several drawbacks. One major challenge is its cost, as maintaining supercritical fluid systems involves significant upfront investment and ongoing operational expenses (25). Additionally, the complexity of the system setup can be a hurdle, requiring precise regulation of temperature and pressure to ensure effective extraction. Moreover, sample preparation can be demanding, as solid samples often require pre-treatments such as grinding or homogenization to optimize extraction efficiency.
CONCLUSION:
Bioanalytical sample preparation is an important part of analytical chemistry that ensures the precision and reliability of results from complicated biological matrices. Recent advances have resulted in more efficient procedures, such as automated liquid handling and microfluidic devices, which increase throughput and precision. Despite these advances, difficulties such as sample matrix complexity and the requirement for contamination-free methods remain. Emerging trends include the use of modern technology, such as mass spectrometry, with novel sample preparation processes that improve sensitivity and specificity. Among these approaches, liquid-liquid extraction (LLE) and solid-phase extraction (SPE) are currently regarded as the most accurate, with SPE frequently preferred due to its excellent selectivity and recovery. Future advances will focus on refining these methodologies, integrating sustainable practices, and harnessing new materials and data science to address existing limitations and enhance bioanalytical capabilities.
REFERENCES
- Kole PL, Venkatesh G, Kotecha J, Sheshala R. Recent advances in sample preparation techniques for effective bioanalytical methods. Biomedical Chromatography. 2011 Jan;25(1?2):199-217.
- Kumar R, Patel N, O'Connor J. Recent advancements in bioanalytical methods for pharmacological research. Pharmaceutical Research. 2023;40(3):489-505.
- Medvedovici A, Bacalum E, David V. Sample preparation for large?scale bioanalytical studies based on liquid chromatographic techniques. Biomedical Chromatography. 2018 Jan;32(1): e4137.
- James CA. Sample preparation. In Principles and practice of bioanalysis 2008 Feb 25 pp. 19-39). Boca Raton, USA: CRC Press.
- Jork H, Funk W, Fischer W, Wimmer H, MuKllner S, Hofmann R, Saar K, Karbe-ThoKnges B. Bioanalytical applications: solid-phase extraction. Journal of Planar Chromatography. 2000; 3: 2142
- Li W, Jian W, Fu Y. Basic sample preparation techniques in LC?MS bioanalysis: protein precipitation, liquid–liquid extraction, and solid?phase extraction. Sample preparation in LC?MS bioanalysis. 2019 Feb 25:1-30.
- Simpson H, Berthemy A, Buhrman D, Burton R, Newton J, Kealy M, Wells D, Wu D. High throughput liquid chromatography/mass spectrometry bioanalysis using 96?well disk solid phase extraction plate for the sample preparation. Rapid Communications in Mass Spectrometry. 1998 Jan 31;12(2):75-82.
- Rogeberg M, Malerod H, Roberg-Larsen H, Aass C, Wilson SR. On-line solid phase extraction–liquid chromatography, with emphasis on modern bioanalysis and miniaturized systems. Journal of pharmaceutical and biomedical analysis. 2014 Jan 18; 87:120-9.
- Bennett DA, Lee J, Patel S. Application of bioanalytical methods in toxicology: A comprehensive review. Journal of Toxicology and Environmental Health. 2022;85(4):267-290.
- Lucena R, Cruz-Vera M, Cárdenas S, Valcárcel M. Liquid-phase microextraction in bioanalytical sample preparation. Bioanalysis. 2009 Apr 1;1(1):135-49.
- Drouin N, Rudaz S, Schappler J. Sample preparation for polar metabolites in bioanalysis. Analyst. 2018;143(1):16-20.
- Li W, Jian W, Fu Y, editors. Sample preparation in LC-MS bioanalysis. John Wiley & Sons; 2019 Mar 12.
- Locatelli M, Tartaglia A, Piccolantonio S, Di Iorio LA, Sperandio E, Ulusoy HI, Furton KG, Kabir A. Innovative configurations of sample preparation techniques applied in bioanalytical chemistry: a review. Current Analytical Chemistry. 2019 Dec 1;15(7):731-44.
- Kieltyka K, McAuliffe B, Cianci C, Drexler DM, Shou W, Zhang J. Application of cassette ultracentrifugation using non-labeled compounds and liquid chromatography-tandem mass spectrometry analysis for high-throughput protein binding determination. Journal of Pharmaceutical Sciences. 2016 Mar 1;105(3):1036-42.
- Berna M, Murphy AT, Wilken B, Ackermann B. Collection, storage, and filtration of in vivo study samples using 96-well filter plates to facilitate automated sample preparation and LC/MS/MS analysis. Analytical chemistry. 2002 Mar 1;74(5):1197-201.
- Adams L, Clark J, Green M. Advances in bioanalytical techniques for improved analytical accuracy and dependability in clinical research. Clinical Biochemistry. 2024; 87:112-122.
- Miller A, Thompson L, Green H. Innovative bioanalytical approaches for assessing drug metabolism and pharmacokinetics. Pharmaceutical Research. 2024;41(3):482-493.
- James CA. Sample preparation. In Principles and practice of bioanalysis 2008 Feb 25 (pp. 19-39). Boca Raton, USA: CRC Press. 19.
- Kirthi A, Shanmugam R, Prathyusha MS, Basha DJ. A review on bioanalytical method development and validation by RP-HPLC. Journal of global trends in pharmaceutical sciences. 2014;5(4):2265-71.
- Lucena R, Cruz-Vera M, Cárdenas S, Valcárcel M. Liquid-phase microextraction in bioanalytical sample preparation. Bioanalysis. 2009 Apr 1;1(1):135-49.
- Chiap P, Hubert P, Crommen J. Strategy for the development of automated methods involving dialysis and trace enrichment as on-line sample preparation for the determination of basic drugs in plasma by liquid chromatography. Journal of Chromatography A. 2002 Mar 1;948(1-2):151-61.
- Garcia-Martínez S, Rico E, Casal E, Grisaleña A, Alcaraz E, King N, Leal N, Navarro I, Campanero MÁ. Bioanalytical validation study for the determination of unbound ambrisentan in human plasma using rapid equilibrium dialysis followed by ultra performance liquid chromatography coupled to mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2018 Feb 20;150 :427-35.
- Dispas A, Jambo H, André S, Tyteca E, Hubert P. Supercritical fluid chromatography: a promising alternative to current bioanalytical techniques. Bioanalysis. 2018 Jan 1;10(2):107-24.
- Ríos A, Zougagh M, de Andrés F. Bioanalytical applications using supercritical fluid techniques. Bioanalysis. 2010 Jan 1;2(1):9-25.
- Liang Y, Zhou T. Recent advances of online coupling of sample preparation techniques with ultra-high performance liquid chromatography and supercritical fluid chromatography. Journal of separation science. 2019 Jan;42(1):226-42.


 JISHA U*
JISHA U*
 PRASANTH S.S
PRASANTH S.S
 SIBINA M.K
SIBINA M.K
 K.T AKSHARA
K.T AKSHARA
 SANOOJA P.K
SANOOJA P.K
 RIYA RAJAN
RIYA RAJAN
 MOHAMMED FAROOQ P
MOHAMMED FAROOQ P
 AJAY A
AJAY A

 10.5281/zenodo.13376811
10.5281/zenodo.13376811