Abstract
Gestational hypertension includes conditions such as preeclampsia, chronic hypertension, gestational hypertension, and eclampsia. Preeclampsia is the leading cause of maternal and fetal death worldwide. One of the main factors contributing to the development of this syndrome is endothelial dysfunction in placental defects. Preeclampsia is considered a disease spectrum, with different disease mechanisms and clinical manifestations in disease groups. A new onset of hypertension after 20 weeks of gestation in pregnant women with normal blood pressure, previously known as "gestational hypertension" or "pregnancy-related hypertension,". Several factors increase the risk of preeclampsia, including obesity, smoking, obesity, family factors, sperm exposure, external factors, socioeconomic burden, infertility, and other maternal conditions. Maternal nutrition is an important risk factor for preeclampsia. However, it is not clear whether the Diet to Avoid Hypertension (DASH) can reduce the development of preeclampsia. Dietary factors associated with reducing the risk of preeclampsia include low blood pressure (DASH), fiber, high fat, sugar and salt, vitamins, folic acid, minerals probiotics/prebiotics, and selenium, etc. Maternal nutrition is an important risk factor for preeclampsia. This review discusses high blood pressure, preeclampsia, pregnancy, risk factors, and types of memory.
Keywords
Hypertension, Pre-eclampsia, Pregancy, Risk factors, Diatary patterns.
Introduction
Hypertensive disorders of pregnancy affect about 10% of pregnant women in the world. This group of diseases and conditions includes preeclampsia and eclampsia, gestational hypertension, and chronic hypertension.[1,2] Gestational hypertension is an important cause of severe acute complications, long-term disability, and death in mothers and newborns. In Asia and Africa, about one in every maternal death is related to gestational hypertension, while in Latin America, a quarter of maternal deaths are related to this complication.[3] Preeclampsia is the most common complication of pregnancy worldwide, although its prevalence varies. Although less common in the Nordic countries, it is an important disease faced by all obstetricians and gynecologists in clinical practice and an important cause of maternal and neonatal death. Despite extensive and clinical research in this area, the pathogenesis of preeclampsia remains unclear. However, our understanding of its pathophysiology is evolving, and its prognosis, diagnosis, and management continue to evolve and improve. This will significantly reduce the incidence of serious complications such as eclampsia, cerebral hemorrhage, kidney failure, coagulopathy, pulmonary edema and maternal mortality. The recognition of magnesium sulfate as the treatment of choice for the treatment and prevention of eclamptic seizures is an important development in this field.[4][5] Preeclampsia is caused by placental hyperperfusion and subsequent cytotrophoblast stress, which secretes soluble factors into the circulation and causes an initial imbalance between anti-angiogenic factors. Pathways that influence the development of preeclampsia include genetic, epigenetic, lifestyle, and environmental factors.[6] However, little information has been published on diet and preeclampsia. This descriptive review will focus on food components that interact with lifestyle and environment. We detail the influencing factors of metabolic function, weight, weight gain, hypertension, adverse lipid profiles, inflammation, dietary patterns, and factors mediating endothelial protection.[7-11]
Types of pre-eclampsia
High blood pressure during pregnancy is a major threat to maternal and fetal health.[12] Preeclampsia is one of the most well-known medical conditions associated with this disease spectrum and is one of the most reported complications of pregnancy with a prevalence of around 2 - 15%.[13][14] These include hypertensive disorders diagnosed after 20 weeks of gestation and various bleeding disorders such as proteinuria or generalized edema and thrombocytopenia, or end-stage blood disorders such as kidney damage, liver failure, and pulmonary edema. with symptoms. and brain and vision disorders.[15] If preeclampsia is severe or untreated, it can lead to serious and long-lasting complications. In such cases, some visceral organ involvement and uterine enlargement can cause pregnancy complications and poor fetal outcomes, including intestinal growth restriction and preterm birth. Changes in morbidity can be dangerous in terms of increased mortality and maternal and fetal complications.[16] A simple way to classify preeclampsia is to divide it into two groups, early and late, based on gestational age (GA). The cutoff point is usually set at GA 34 weeks or 37 weeks GA and classify preeclampsia as early-onset (GA < 34>
Table 1: Classification of preeclampsia according to gestational age
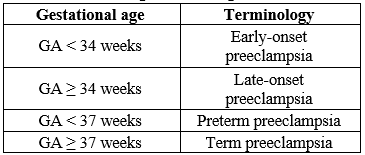
Preeclampsia should be considered a spectrum of diseases that may differ in disease mechanisms and clinical subtypes. A new onset of hypertension after 20 weeks of gestation in pregnant women with normal blood pressure, previously known as "gestational hypertension" or "pregnancy-related hypertension,". In addition to gestational hypertension, a patient meets the diagnostic criteria for preeclampsia if he has proteinuria, thrombocytopenia, renal or hepatic insufficiency, cerebral symptoms, visual symptoms, or pulmonary edema. In terms of severity, preeclampsia is divided into "mild" and "severe", the second group is characterized by 160/100 mm Hg, headache, visual disturbances, epigastric pain, oliguria and serum. . Elevated creatinine levels are associated with thrombocytopenia (<100>
Epidemiology
Up to 10% of women experience high blood pressure during pregnancy.[19]3-8% of these women develop preeclampsia in developed countries[20][21], with an incidence of up to 0.56 per 1000 live births.[22] Because of the lack of epidemiological data in many low- and middle-income countries. The exact prevalence, morbidity and mortality of preeclampsia in low- and middle-income countries is unknown due to a lack of capacity and power to collect data and report important statistics. However, the World Health Organization estimates that approximately 16% of maternal deaths in LMICs are caused by pregnancy-related hypertensive disorders, with eclampsia being the leading cause.[23] Save the Mother 2008-2010: The Fifth Report on Maternal Mortality in South Africa shows that 14% of maternal deaths in South Africa are caused by hypertensive pregnancy (sustained hypertension, preeclampsia, HELLP and liver rupture) informed. The leading cause of death was hypertension with 51.1% of deaths. Preeclampsia accounted for 29.3% of deaths, chronic hypertension 5%, HELLP syndrome 12.7%, liver failure 1.3%, and pulmonary edema 27%. Brain death was the final cause in 51.1% of maternal deaths.[24]
Pathophysiology
The pathophysiology of HDP is not fully understood, but possible causes include placental dysfunction and immune changes leading to uterine maturation (Figure 1). Importantly, the mechanisms underlying vascular dysfunction in preeclampsia are similar to cardiovascular disease and atherosclerosis in nonpregnant individuals. This similarity may help explain why preeclampsia is associated with an increased risk of cardiovascular disease in old age. In normal pregnancy, cytotrophoblast cells proliferate in the myometrium and spiral arteries and form a rich vascular anastomotic system, thereby enriching the placenta and fetus.[25] In preclinical patients, cytotrophoblast cells do not express the invasive phenotype required to form this tight anastomosis, leading to dilation and narrowing of the spiral artery.[26-28] These abnormal blood vessels are of narrow caliber, leading to placental ischemia and ineffective oxygenation.[29] This step is illustrated in Figure 1. In addition, preeclamptic patients have increased levels of various anti-inflammatory molecules, including natural killer cells and other inflammatory markers[30]. "Immune tolerance" in normal pregnancy is mainly due to changes in maternal immunity around T cells.[30] In pregnancy without preeclampsia, Th1 and Th2 cells work together to prevent excessive inflammation and fetal rejection. In the preeclampsia model, this balance is disturbed and many T cells switch to the Th1 phenotype, similar to T cells in chronic autoimmune diseases. Th1 cells promote inflammation through inflammatory cytokines, autoantibodies, and oxidative stress, which exacerbate the damage and ischemia seen in preeclampsia.[30] The complex pathogenesis of preeclampsia may be facilitated by a combination of abnormal placentation and ischemia, which induces the release of proinflammatory and antiangiogenic proteins into the maternal circulation, leading to endothelial dysfunction leading to clinical symptoms. This may include: Preeclampsia. Two of the most studied biomarkers with effects related to the development of preeclampsia are soluble FMS-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF). sFlt-1 is an anti-angiogenic factor that inhibits angiogenesis 31. High levels of sFlt-1 have been reported in the placenta of preeclampsia and preeclampsia patients.[31][32] PlGF levels are low and PlGF sFlt-1 ratio is increased in preglampsia patients.[32][33]
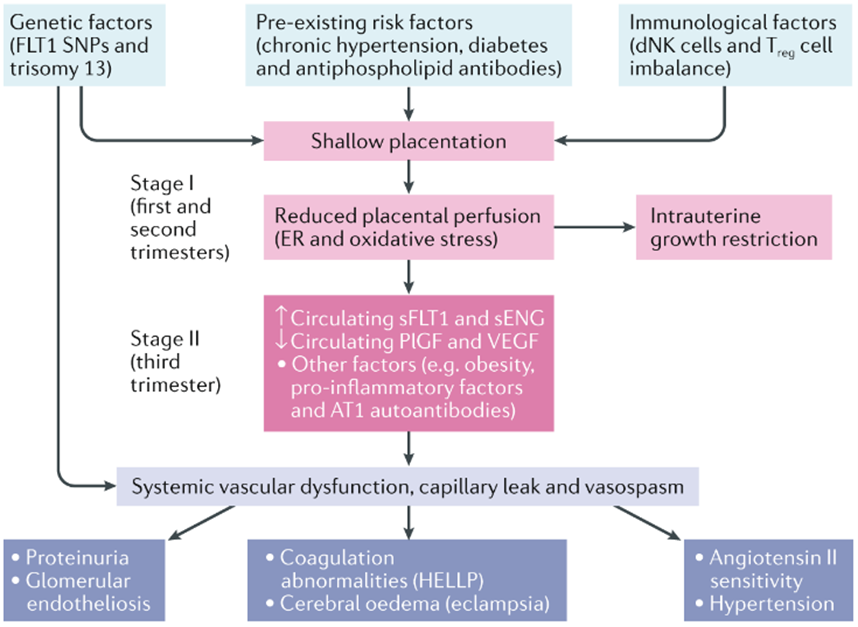
Figure 1: Pathogenesis of Pre-eclampsia[34]
In general, the pathogenesis of preeclampsia can be very complex and multifaceted. The basic theory proposed in development suggests that defective communication leads to spiral artery remodeling and endothelial damage, and tissue hypoxia leads to hypertension pathology. On the other hand, altered maternal immunity in preeclamptic patients causes low-grade inflammation and subsequent endothelial damage. This combination can lead to an imbalance of angiogenic and antiangiogenic factors. The complex interplay between placental pathology, inflammation, and angiogenic changes ultimately lead to the clinical syndrome of preeclampsia, which is detrimental to the health of both pregnancy and postpartum patients.[35][36]
RISK FACTORS
Obesity
Obesity is a clear risk factor for preeclampsia, and the risk increases with increasing BMI. Obesity is strongly associated with insulin resistance, a risk factor for preeclampsia. The exact mechanisms by which obesity and insulin resistance are associated with preeclampsia are poorly understood.[37] One possible explanation is vascular shear stress associated with high dynamic blood flow; dyslipidemia or cytokine-induced oxidative stress. It increases sympathetic nerve activity and increases tubular sodium absorption, and direct interference with insulin resistance causes hyperinsulinemic conditions in the placenta.[37]
Smoking
Several studies have shown that smoking reduces the incidence of preeclampsia by about 50% in a dose-dependent manner. However, this observation was not confirmed by yeast. The risk is not reduced in women who smoke regularly and who quit during pregnancy, but women who start smoking during pregnancy and women who smoke during pregnancy are protected, but smoking increases fetal risk. It is not associated with negative results in the case of pregnancy complications and preeclampsia. These data suggest that although tobacco products are generally harmful in terms of pregnancy outcomes, they may have a protective effect during pregnancy.[38][39]
Co-morbid Conditions
Women with gestational diabetes, insulin-dependent diabetes or non-insulin-dependent diabetes and chronic hypertension are at increased risk of preeclampsia.[40] Studies have shown that women with anti-phospholipid syndrome, thrombocytosis, autoimmune disease, kidney disease, and infertility are at increased risk of developing preeclampsia.[41][42]
Familial factors
Family history of preeclampsia significantly increases the risk of developing preeclampsia, and women with a mother's history of preeclampsia are more likely to develop preeclampsia. Among men with preeclampsia who have difficulty conceiving, other women have pregnancies complicated by preeclampsia.[43][44]
Previous Preeclampsia
Preeclampsia in the previous pregnancy is a strong predictor of preeclampsia in the next pregnancy. The risk of recurrence is about 14%. Odegaard et al reported a 20-fold increased risk of preeclampsia in some women.[45] In a large review of risk factors for preeclampsia, Duckitt and Harrington found that women with a history of preeclampsia had a seven-fold increased risk compared to women without a history of preeclampsia. According to Mostello et al, recurrence during the first pregnancy is inversely related to gestational age.[40][46]
Sperm exposure
Although preeclampsia is more common in young women and women of childbearing age, older women are at risk of developing chronic hypertension with preeclampsia. The risk of preeclampsia in primiparternity women is three times higher than in multiparous women.[47] An early hypothesis suggests that women with limited exposure to their partner's sperm are at increased risk of preeclampsia.[48] Evidence supporting this hypothesis includes a lower risk of preeclampsia in most women, women with a previous pregnancy, women with a previous pregnancy, and women who change partners. A longer birth interval has been proposed as an alternative explanation for the latter phenomenon, but the evidence for this observation is controversial.[49][50]
Heterogeneous Factors
Older age, multiple pregnancies, and periodontal disease have been shown to increase the risk of preeclampsia. Chronic oral infection is considered a causative factor of various systemic diseases, including atherosclerotic cardiovascular disease and cerebrovascular ischemia. Persistent Gram-negative infections are associated with periodontal disease, atherosclerosis, thromboembolism, and hypercholesterolemia.[51-54] Oral pathogens have been identified in atherosclerotic plaques and may play a role in the development and progression of atherosclerosis leading to coronary artery disease. Chronic disease causes an increase in endotoxin and inflammatory cytokines, which can contribute to atherogenesis and thrombus formation and amplification. Pregnant women who develop preeclampsia may develop placental pressure.[55] In a study of 41 women with preeclampsia and 41 healthy normal pregnant women, Kenaki et al found an association between periodontal disease and the risk of preeclampsia.[56]
Socioeconomic Burden
Since preeclampsia is one of the leading causes of serious maternal and fetal outcomes in the world, the treatment of patients with preeclampsia often requires a short-term special medical plan. This creates not only health problems but also socio-economic problems, as the consumption of resources and human resources and additional costs for the health system are unavoidable. However, some studies have aimed to estimate the socioeconomic burden associated with preeclampsia, relevant studies have so far yielded limited results. Jin Hao et al conducted a retrospective study to investigate the economic burden of preeclampsia using US data. The researchers identified three groups: women who are not pregnant for their gestational age, women with high blood pressure but no preeclampsia, and women with a diagnosis of preeclampsia. Maternal and child costs are calculated from 20 weeks to 6 weeks and under 12 months from GA. The average cost of treatment for the preeclampsia group was US$41,790, which was significantly higher than complications (US$13,187) and hypertension, but higher than the preeclampsia group (US$24,182). The difference in price mainly depends on the price of the child.[57] Another retrospective study focusing on the same topic was conducted in the United States by Warren et al. Parent allowance is calculated from 6 to 12 months and child allowance from 12 months. Finally, the birth model suggested an increase in costs per birth of US $ 6,583. On the other hand, the increase in the cost of the baby model has a significant effect on the gestational age at birth. Neonatal costs account for 26% of total medical costs for childbirth and rise to 91% for babies born at less than 28 weeks. The high costs of preeclampsia and preterm delivery are primarily associated with adverse fetal outcomes, including gastrointestinal or fetal bleeding, bronchopulmonary dysplasia, periventricular leukomalacia, and neonatal death.[58]
Nulliparity
One of the risk factors for preeclampsia is impotence, so women often do not have normal means. Consequently, routine screening for hypertension and proteinuria is recommended until predictive tests are performed to stratify women into high-risk and low-risk groups. Whether acute preeclampsia is better managed on an inpatient or outpatient basis is unclear and often depends on hospital policy.[59]

Figure 2: Various risk factors for pre-eclampsia
Other Maternal Conditions
One of the most important risk factors for preeclampsia is a history of preeclampsia in a previous pregnancy. Women with preeclampsia in their first pregnancy are at increased risk of recurrence in their second pregnancy. Several studies have shown this phenomenon. Risk factors for preeclampsia in antenatal appointments: a systematic review of controlled studies. In addition to the woman's medical history, the family history of women with preeclampsia is of special concern. Because a positive family history predicts preeclampsia at all stages of pregnancy.[59][60] Multifetal pregnancy increases the risk of preeclampsia 3-4 times. This may be related to pregnancy, as some pregnancies are more stressful on the cardiovascular system. People with multiple pregnancies are often considered a special population and therefore excluded from general surveys. For women diagnosed with preeclampsia, it can be confusing whether certain adverse outcomes, such as preterm birth, are due to preeclampsia or multiple pregnancies. However, because multiparous women are more likely to experience eclampsia and serious complications, more attention should be paid to the possibility of screening, prevention, or early intervention.[61] Patients with preeclampsia who have persistent hypertension before pregnancy are classified as "multiple preeclampsia". Chronic hypertension accounts for approximately 4% of pregnancies and is often associated with adverse pregnancy outcomes such as preeclampsia, premature birth, intestinal growth restriction, and placental abruption. About 20% of these patients develop preeclampsia, which occurs earlier than normal blood pressure. Unintended pregnancy outcomes were also observed at the same time.[62] Both type I and type II diabetes cause preeclampsia. Statistically, 10-20% of women with diabetes develop preeclampsia during pregnancy, which is significantly higher than women without diabetes. On the other hand, some researchers believe that gestational diabetes mellitus (GDM) is an independent risk factor for preeclampsia, but whether GDM and preeclampsia share a common pathogenic pathway remains to be determined.[63]
DIETARY PATTERNS
Importance of dietary patterns to the risk of pre-eclampsia
Diet before and during pregnancy can play an important role in the development of gestational hypertension, including preeclampsia. Although limited, there is evidence that a diet rich in fruits and vegetables, nuts, whole grains, legumes, fish, and vegetable oils can protect against hypertension.[64][65]
Dietary Approaches to Stop Hypertension (DASH) dietary pattern
The Diet to Prevent Hypertension (DASH) is high in whole grains, fruits, vegetables, low-fat dairy products, and plant-based protein from nuts and beans, but almost no red meat. Processed meat, sweets or sugary drinks.[65] Although the DASH diet was originally developed and evaluated to lower blood pressure, recent studies have shown that it reduces the risk of glioma, non-alcoholic fatty liver disease, cardiovascular disease, type 2 diabetes, gestational diabetes (GDM), and more. Could it be proven effective against other metabolic diseases? However, no study to date has identified a relationship between the DASH diet and the risk of preeclampsia.[66] The OR was 0.53 (95% CI 0.36 to 0.78) for participants in the lowest quartile of the DASH score compared with participants in the fourth quartile of the DASH score. The DASH diet is high in fruits, vegetables, whole grains, low-fat dairy products, and plant-based proteins, but low in red meat, processed meats, sweets, and sugary drinks. In contrast, the Danish National Birth Family Study found that greater adherence to the DASH diet did not significantly reduce the risk of preeclampsia. However, looking specifically at sodium intake, there is a 20% increased risk of preeclampsia in women with a mean of 3.7 g of sodium and a mean of 2.6 g of sodium.[69]
Fibre
A high amount of dietary fiber helps maintain body weight and is associated with a reduction in cardiovascular disease.[70][71] Compared with women without preeclampsia, women with preeclampsia had higher serum triglycerides and low-density lipoprotein (LDL) cholesterol, which are known to increase the risk of hypertension and cardiovascular disease. In a study from Washington State, maternal plasma lipid concentration was measured at 13 weeks of gestation in 57 women with preeclampsia and 510 normal controls. Women who later developed preeclampsia had 10.4%, 13.6%, and LDL-cholesterol, triglyceride, and high-density lipoprotein (HDL) ratios, respectively, compared to controls (p < 0>
High-fat, sugar and salt-rich diets
MoBa found that a diet high in processed meat, salty foods and sugary drinks was associated with an increased risk of preeclampsia (OR 1.21, 95% CI 1.03 to 1.42).[76] The same study found that consumption of sugar-sweetened beverages was associated with a significantly increased risk of preeclampsia. Intake of 125 ml or more per day was associated with an OR of 1.27 (95% CI 1.05 to 1.54). In addition, the OR for drinking 1000 ml or more of sugary soft drinks per day was 2.04 (95% CI 1.21 to 3.45).[77]
Vitamin D
There is controversy about the amount of vitamin D that should be sufficient to prevent dementia and the concentration of vitamin D25 (OH) D. Sufficient intake (recommended / sufficient intake) of vitamin D for women / pregnant women is 15 ?g (600 IU) per day at a concentration below nmol / L (> 20 ng / ml). The rate of 25(OH)D deficiency.[78][79]
Folic acid, vitamin B12 and multivitamins/minerals
About 85% of studies have found that women with preeclampsia have higher serum homocysteine levels than women without preeclampsia. High homocysteine levels may be associated with folate or vitamin B12 deficiency. Fetal cells divide rapidly during pregnancy, and the need for folic acid increases when urine is produced. Adequate intake of folate (as folic acid) during pregnancy is important for fetal growth and development and prevention of neural tube defects. Folic acid may play a protective role in preeclampsia because it is involved in mechanisms that lower blood pressure, reduce oxidative stress, and restore endothelial function.[80] A recent meta-analysis of 19 studies examined serum vitamin B12 concentrations in women with preeclampsia and found them to be significantly lower than serum vitamin B12 concentrations in healthy pregnant women (mean value -15.24 pg/ml, 95% confidence interval (CI): -27.52- from -2.954 (p < 0 xss=removed xss=removed>
Probiotics and prebiotics
Probiotics are live microorganisms, bacteria, and yeast that perform a variety of functions, including restoring the gut microbiome and improving cholesterol levels and blood pressure. Prebiotics are foods that promote the growth of bifidobacteria by bacteria.[80] Probiotic foods have been shown to reduce the risk of preeclampsia in pregnant women by reducing the swelling of placental trophoblast cells, lowering blood pressure, and reducing systemic inflammation. During pregnancy, prebiotics significantly increase the benefits of bifidobacteria in the maternal gut.[84]
Selenium
The biological effects of selenium are mediated mainly by seleno-proteins encoded by a gene containing the amino acid selenocysteine. Selenium has several uses around the world. Consumption is high in North America but low in Europe.[80][85][86]
CONCLUSION
Women at risk for preeclampsia need special evaluation of their nutritional and nutritional status. Several factors increase the risk of preeclampsia, including obesity, smoking, obesity, family factors, sperm exposure, external factors, socioeconomic burden, infertility, and other maternal conditions. Dietary factors associated with reducing the risk of preeclampsia include low blood pressure (DASH), fiber, high fat, sugar and salt, vitamins, folic acid, minerals, probiotics/Prebiotics, and selenium, etc. Maternal nutrition is an important risk factor for preeclampsia. However, it is not clear whether the Diet to Avoid Hypertension (DASH) diet can reduce the development of preeclampsia. RDNs can provide patients with specific dietary recommendations that support nutritional status, reduce disease risk, and meet cultural nutritional needs.
REFERENCE
- Duley L. The global impact of pre-eclampsia and eclampsia. Seminars in Perinatology. 2009;33(3):130–137.
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–44.
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074.
- Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345 (8963):1455–63.
- Acharya G, Bhide A, Rasanen J. Clinical trials in preeclampsia: implications for practice. Acta Obstetricia et Gynecologica Scandinavica. 2017;96:1157–1158.
- Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2022 Feb;226(2S):S907-S927.
- Ahmed A, Rezai H, Broadway-Stringer S. Evidence-Based revised view of the pathophysiology of preeclampsia. Adv Exp Med Biol. 2017;956:355–74.
- Enquobahrie DA, Williams MA, Butler CL, et al. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17:574–81.
- Gratacós E, Casals E, Sanllehy C, et al. Variation in lipid levels during pregnancy in women with different types of hypertension. Acta Obstet Gynecol Scand. 1996;75:896–901.
- Santos S, Voerman E, Amiano P, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG. 2019;126:984–95.
- He X-J, Dai R-X, Hu C-L. Maternal prepregnancy overweight and obesity and the risk of preeclampsia: a meta-analysis of cohort studies. Obes Res Clin Pract. 2020;14:27–33.
- Say L, Chou D, Gemmill A, Tunçalp O, Moller A.-B, Daniels J, Gülmezoglu A.M, Temmerman M, Alkema L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health. 2014;2:E323–E333.
- Jeyabalan, A. Epidemiology of preeclampsia: Impact of obesity. Nutr. Rev. 2013;71:S18–S25.
- Mou AD, Barman Z, Hasan M, Miah R, Hafsa JM, Das Trisha A, Ali N. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci. Rep. 2021;11:21339.
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP., Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
- Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am. J. Obstet. Gynecol. 2022;226:S1071–S1097.e2.
- Von Dadelszen P, Magee L.A., Roberts J.M. Subclassification of preeclampsia. Hypertens. Pregnancy. 2003;22:143–148.
- Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffman BL, Casey BM, Spong CY. Williams Obstetrics, 25th ed.; McGraw Hill/Medical: New York, NY, USA. 2022;88:1086–1089.
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009; 33:130-137.
- Carty DM, Delles C, Dominiczak AF. Preeclampsia and future maternal health. J Hypertens. 2010;1349-1355.
- Khalil AA, Cooper DJ, Harrington KF. Pulse wave analysis: a preliminary study of a novel technique for prediction of pre-eclampsia. BJOG. 2009;116(2):268-276.
- Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979-1986. Am J Obstet Gynecol. 1990 Aug;163(2):460-465.
- Khan KS, Wojdyla D, Say L et al. WHO analysis of cause of maternal death: a systemic review. Lancet. 2006;367:1066-1074.
- Moodley J. Saving Mothers Report 2008-2010: Fifth report on the Confidential Enquiries into Maternal Deaths in South Africa. Available on https://www.kznhealth.gov.za/mcwh/Maternal/Saving-Mothers-2011-2013-short-report.pdf
- Redman CWG, Staff AC. Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2022;226:S907–27.
- Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–79.
- Robertson WB, Brosens I, Dixon HG. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J Pathol Bacteriol. 1967;93:581–92.
- Ridder A, Giorgione V, Khalil A, Thilaganathan B. Preeclampsia: the relationship between uterine artery blood flow and trophoblast function. Int J Mol Sci. 2019;20:1–14.
- De Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol. 1975;123:164–74.
- Amaral LM, Wallace K, Owens M, LaMarca B. Pathophysiology and current clinical Management of Preeclampsia. Curr Hypertens Rep. 2017;19(8):61.
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–9.
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al.. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58.
- Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. 2022;226:S1019–34.
- Phipps EA, Thadhani R, Benzing T. et al. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. 2019;15:275–289.
- Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–93.
- Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–22.
- O ‘Brien TE, Raj JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
- England L and Zhang J. Smoking and risk of pre-eclampsia: a systematic review. Front Biosci. 2007;12:2471-2483.
- Wikstrom AK, Stephansson O and Cnuttingius S. Tobacco use during pregnancy and pre-eclampsia risk: effects of cigarette smoking and snuff. Hypertension. 2010;55:1254-1259.
- Duckitt K and Harrington D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ. 2005;330:565.
- Yasuda M, Takakuwa K, Tokunaga A, et al. Prospective studies of the association between anticardiolipin antibody and outcome of pregnancy. Obstet Gynecol. 1995; 86:555-559.
- Sibai B, Dekker G and Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785-799.
- Mogren l, Hogberg U, Winkvist A, Stenlund H. Familial occurrence of preeclampsia. Epidemiology. 1999;10(5):518-522.
- Lie RT, Rasmussen S, Brumborg H, et al. Fetal and maternal contribution to risk of pre-eclampsia: population based study. BMJ. 1998;316:1343-1457.
- Odegard R, Vatten LJ, Nilsen ST, et al. Risk factors and clinical manifestation of preeclampsia. BJOGN 2000;107:1410-1416.
- Mostello D, Kallogjeri D, Tungsiripat R, Leet T. Recurrence of pre-eclampsia: effects of gestational age at delivery of the first pregnancy, body mass index, paternity and interval between births. Am J Obstet Gynecol. 2008;199:55.e1- 55.e7.
- Hartikainen AL, Aliharmi RH and Rantakillo PT. A cohort study of epidemiologic associations and outcomes of pregnancies with hypertensive disorders. Hypertens Pregnancy. 1998;17:31-41.
- Robillard PY, Hulsey TZ, Alexander GR, et al. Paternity patterns and the risk of pre-eclampsia last pregnancy in multipara. J Reprod Immunol. 2003;59:245-251.
- Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and risks of preeclampsia. N Engl J Med. 2002;346:33-38.
- Dekker G, Robillard PY. The birth interval hypothesis-does it really indicate the end of the primiparternity hypothesis? J Reprod Immunol. 2003;59:245-251.
- Boggess KA, Lieff S, Murtha AP, et al. Maternal periodontal disease is associated with an increased risk for pre-eclampsia. Obstet Gynecol. 2003;101:227-231.
- Beck JD, Pankow J, Tyroler HA, Offenbacher S. Dental infections and atherosclerosis. Am Heart J. 1999;138:528-533.
- Beck JD, Offenbacher S, Williams R, Gibbs P, Garcia R. Periodontitis: A risk factor for coronary heart disease? Ann Periodontol. 1998;3:127-141.
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123-1137.
- Haraszthy VI, Zambon JJ, Trevisa M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554-1560.
- Canakci V, Canakci CF, Canakci H, Canakci E, et al. Periodontal disease as a risk factor for preeclampsia: A case control study. Aust NZ J Obstet Gynaecol. 2004;44:568-573.
- Hao J, Hassen D, Hao Q, Graham J, Paglia M.J, Brown J, Cooper M, Schlieder V, Snyder S.R. Maternal and infant health care costs related to preeclampsia. Obstet. Gynecol. 2019;134:1227–1233.
- Stevens W, Shih T, Incerti D, Ton TG, Lee HC, Peneva D, Macones GA, Sibai BM, Jena AB. Short-term costs of preeclampsia to the United States health care system. Am. J. Obstet. Gynecol. 2017;217:237–248.e16.
- English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control. 2015 Mar 3;8:7-12.
- Boyd HA, Tahir H, Wohlfahrt J, Melbye M. Associations of personal and family preeclampsia history with the risk of early-, intermediate- and late-onset preeclampsia. Am. J. Epidemiol. 2013;178:1611–1619.
- Bergman L, Nordlof-Callbo P, Wikstrom AK, Snowden JM, Hesselman S, Edstedt Bonamy AK, Sandstrom A. Multi-fetal pregnancy, preeclampsia, and long-term cardiovascular disease. Hypertension. 2020;76:167–175.
- Battarbee AN, Sinkey RG, Harper LM, Oparil S, Tita AT. Chronic hypertension in pregnancy. Am. J. Obstet. Gynecol. 2020;222:532–541.
- Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr. Diab. Rep. 2015;15:9.
- Raghavan R, Dreibelbis C, Kingshipp BL, et al. Dietary patterns before and during pregnancy and maternal outcomes: a systematic review. Am J Clin Nutr. 2019;109:705S–28.
- Vogt TM, et al. Dietary Approaches to Stop Hypertension: rationale, design, and methods. DASH Collaborative Research Group. J. Am. Diet. Assoc. 1999;99:S12–18.
- Cao Y, Liu Y, Zhao X, Duan D, Dou W, Fu W, Chen H, Bo Y, Qiu Y, Chen G, Lyu Q. Adherence to a Dietary Approaches to Stop Hypertension (DASH)-style Diet in Relation to Preeclampsia: A Case-Control Study. Sci Rep. 2020;10(1):9078.
- Cao Y, Liu Y, Zhao X, et al. Adherence to a Dietary Approaches to Stop Hypertension (DASH)-style Diet in Relation to Preeclampsia: A Case-Control Study. Sci Rep 2020;10:9078.
- Vogt TM, Appel LJ, Obarzanek E, et al. Dietary approaches to stop hypertension: rationale, design, and methods. DASH Collaborative Research Group. J Am Diet Assoc 1999;99:S12–18.
- Arvizu M, Bjerregaard AA, Madsen MTB, et al. Sodium intake during pregnancy, but not other diet recommendations aimed at preventing cardiovascular disease, is positively related to risk of hypertensive disorders of pregnancy. J Nutr 2020;150:159–66.
- Slavin JL. Dietary fiber and body weight. Nutrition 2005;21:411–8.
- Slavin JL. Position of the American dietetic association: health implications of dietary fiber. J Am Diet Assoc. 2008;108:1716–31.
- Brown L, Rosner B, Willett WW, et al.. Cholesterol-Lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42.
- Lopez-Miranda J, Williams C, Lairon D. Dietary, physiological, genetic and pathological influences on postprandial lipid metabolism. Br J Nutr. 2007;98:458–73.
- Qiu C, Coughlin KB, Frederick IO, et al.. Dietary fiber intake in early pregnancy and risk of subsequent preeclampsia. Am J Hypertens. 2008;21:903–909.
- Whelton SP, Hyre AD, Pedersen B, et al.. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–81.
- Brantsaeter AL, Haugen M, Samuelsen SO, et al. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr. 2009;139:1162–1168.
- Borgen I, Aamodt G, Harsem N, et al. Maternal sugar consumption and risk of preeclampsia in nulliparous Norwegian women. Eur J Clin Nutr. 2012;66:920–925.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC). Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press (US);2011. PMID: 21796828.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. Efsa J. 2016;14:e04547.
- Perry A, Stephanou A, Rayman MP. Dietary factors that affect the risk of pre-eclampsia. BMJ Nutr Prev Health. 2022 Jun 6;5(1):118-133.
- Serrano NC, Quintero-Lesmes DC, Becerra-Bayona S, et al.. Association of pre-eclampsia risk with maternal levels of folate, homocysteine and vitamin B12 in Colombia: a case-control study. PLoS One 2018;13:e0208137.
- Liu C, Liu C, Wang Q, et al. Supplementation of folic acid in pregnancy and the risk of preeclampsia and gestational hypertension: a meta-analysis. Arch Gynecol Obstet. 2018;298:697–704.
- Yakoob MY, Khan YP, Bhutta ZA. Maternal mineral and vitamin supplementation in pregnancy. Expert Rev Obstet Gynecol. 2010;5:241–56.
- Vande Vusse L, Hanson L, Safdar N. Perinatal outcomes of prenatal probiotic and prebiotic administration. J Perinat Neonatal Nurs. 2013;27:288–301.
- Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565–76.
- Rayman MP. Selenium and human health. Lancet. 2012;379:1256–68


 Pooja Sonawane*
Pooja Sonawane*



 10.5281/zenodo.12800879
10.5281/zenodo.12800879