Abstract
Autoimmune diseases (AIDs) are driven by the immune system targeting the own body’s tissues, with many existing treatments offering limited effectiveness. Recent advances in pharmacology have identified new molecular targets for therapy, including immune checkpoint inhibitors, cytokine-targeting biologics, JAK inhibitors, and immune-modulating small molecules. These advancements aim to improve treatment effectiveness and reduce side effects. Additionally, the function of the gut microbiome in the regulation of the immune system and its potential implications for personalized medicine are opening new therapeutic avenues. While monoclonal antibodies targeting TNF-?, IL-6, and IL-17 have proven effective, challenges like drug resistance persist. Emerging strategies, such as B cell depletion and T cell modulation, along with combination therapies, hold promise for better management of autoimmune diseases.
Keywords
Emerging Trends, Autoimmune Diseases
Introduction
Overview of autoimmune diseases
Immunology is the branch of science that focuses on the body's reaction to antigenic challenges, derived from the Latin term “Immunitas,” meaning freedom from. The concept of 'immunity' typically pertains to the body's ability to resist various pathogens. Immunity will be separated into 2 primary groups: innate (or native) immunity and acquired (or adaptive) immunity. This discipline encompasses a broad array of scientific principles and mechanisms that serve an essential function in the body's. defense system against infectious agents, while also recognizing that these responses can sometimes lead to damage to the host organism, a phenomenon known as autoimmunity. (1) Autoimmune diseases (ADs) are highly prevalent worldwide, affecting both adults and children, with incidence estimates ranging from 5% to nearly 30% and a significant annual increase. These diseases severely impact quality of life and can lead to permanent disabilities. Late diagnoses also strain healthcare systems financially; for instance, in the U.S. in 2013, managing systemic lupus erythematosus (SLE) with complications cost over $30,000 to $32,000 annually, about 6.25 to 6.5 times higher than treating the disease in its early, uncomplicated stages.(2) Autoimmunity refers to immune reactions that target and damage the body's its own cells or tissues.(3)Autoimmunity means to a category of diseases characterized by immune responses directed against specific self-antigens. Autoimmune diseases (ADs) can significantly contribute to ongoing tissue damage associated with these conditions. These diseases may be categorized as tissue-specific, such as those that impact the thyroid or the pancreatic ?-cells, where unique antigens specific to those tissues are targeted. On the other side, there are autoimmune disorders that may spread throughout the body, impacting many tissues and specifically targeting autoantigens that are expressed everywhere. Autoimmune illnesses have complicated and elusive root causes. Researchers believe that both hereditary and environmental variables have a role in setting off an immune response that targets the body's own tissues. “Certain components in the human (major histocompatibility complex) MHC class II and other parts of immune system regulators have been regulated with prominent autoimmune diseases such multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus.” A common genetic abnormality that interferes with immunological tolerance systems may also explain why autoimmune diseases tend to run in families. But it is still not well understood what precise genetic variables cause immune responses to target certain antigens in a way that is organ-or tissue-specific. Among environmental factors, infections have been identified as possible causes or aggravators of autoimmune diseases. (1)A wide variety of disorders known together as “autoimmune diseases” arise when B and T cells in the immune system mistakenly target healthy bodily parts. Although they disproportionately impact females, they may impact any organ system and manifest in people of any age. Their clinical presentations vary greatly, ranging from major organ failure to minor, undetectable laboratory abnormalities, while they share many basic processes. A systemic or organ-specific autoimmune illness begins with an overreaction to self-antigens by the immune system.(4)Disorders distinguish by an overreaction of the immune system to biological tissues, resulting in pathological alterations and tissue destruction, are known as autoimmune diseases (AD). (5)There is a similar underlying etiology for the many clinical presentations of autoimmune disorders. The innate immune response is activated, which involves the generation of self-reactive antibodies and the existence of self-reactive T cells that respond to different stimuli.(6)Healing autoimmune disorders is a formidable medical task. Every patient presents with a unique set of symptoms, and clinicians must identify the underlying cause(s) of these symptoms in order to provide effective treatment. The healthcare system is looking for cost-effective ways to identify and treat clinical syndromes quickly, with an emphasis on prevention, while researchers are trying to understand the function of autoreactivity in these syndromes.(7) Medical professionals often classify autoimmune disorders into two broad categories: those that affect the whole body (systemic lupus erythematosus) and those that affect particular organs (type 1 diabetes mellitus). This categorization works well in a clinical setting, but it may not capture the subtle differences in how they work. Autoimmunity is the result of an abnormal reaction to a specific antigen, whether it is foreign or self-derived. A more sophisticated categorization differentiates between disorders involving a widespread disturbance the involvement in the selection, regulation, or programmed cell death of B cells or T cells.
The lack of the Fas protein or its corresponding receptor, which are critical for cell death, exemplifies a universal deficiency. Demyelination syndrome, which may develop after a Campylobacter jejuni infection, is an example of a distinct antigen-related disorder. Appropriate treatment approaches may vary according to the underlying pathologic processes; this classification helps to guide such therapies Antinuclear and anti-DNA antibodies are commonly found in individuals with systemic lupus erythematosus, are among the autoantibodies that may be generated by alterations that reduce The critical level required for the survival and activation of autoreactive B cells. In most cases, these selfantibodies are present at low levels in the overall population.” Molecular alterations that significantly impact cytokine production or regulatory T cell activity are often involved in the development of inflammatory bowel disease. There are now over 80 known autoimmune disorders, which have historically been categorized as either systemic or organ-specific. Typical immune responses may cause sickness by misdirected assaults on self-antigens or particular organs, as seen in organ-specific autoimmune disorders. “Systemic autoimmune illnesses, on the other hand, are divided by the persistent activation of both innate and adaptive immune cells and the immune system's assault on several organs. An excellent illustration of a systemic autoimmunity illness is systemic lupus erythematosus (SLE). Crucially, the expression profile of the self-antigen that seems to be targeted during the autoimmune response is secondary to clinical results when deciding whether an autoimmune illness is organ-specific or widespread.(1) Autoreactive immune responses cause indirect harm to tissues and organs in a wide variety of autoimmune illnesses. (1)Systemic disorders, such as systemic lupus erythematosus, and organ-specific diseases, such as type 1 diabetes mellitus, are the two primary types of autoimmune diseases.” Although this categorization serves a practical use in therapeutic settings, it fails to adequately represent the variations in their origins. A more detailed distinction can be made between conditions involving broad disruptions in T or B cell regulation and those resulting from abnormal immune responses to specific antigens. For instance, a general defect can occur with the absence of the Fas protein, while an antigen-specific disorder is exemplified by demyelination following Campylobacter jejuni infection. This nuanced classification aids in determining appropriate therapeutic interventions based on the underlying mechanisms. This mechanistic classification applies to animal models, but distinguishing whether a human disease arises from generalized lymphocyte dysfunction or a specific antigen abnormality can be challenging. Autoantibody production may result from changes that lower the survival and activation threshold of autoreactive B cells. This is seen in systemic lupus erythematosus, when antibodies against nuclear and DNA are present. People typically have low amounts of these autoantibodies. Despite general B-cell self-tolerance, some autoantibody-driven disorders like Guillain-Barré syndrome (caused by antiganglioside antibodies) reveal a breakdown of B-cell tolerance to certain antigens. Due to increased T cell activation and heightened sensitivity to gut microbiota, inflammatory bowel disease may be caused by genetic alterations that impact regulatory T cell activity or cytokine production. Diseases affecting the whole body as well as those affecting just certain organs might result from changes in the T cell repertoire. Thymectomy, which involves the removal of the mouse's thyroid gland, might cause wasting syndrome or immunological responses in newborn mice, depending on their genetic makeup. Systemic autoimmune disorders and organ-specific autoimmune diseases become more difficult to distinguish due to this. For example, in polymyositis and Sjögren's syndrome, the patient develops autoreactivity against transfer RNA synthetases, but the illness stays confined. Significant considerations include the patterns of lymphocyte movement and the accessibility of antigens in the afflicted tissues. T cell subgroup-specific transport molecule expression has been the subject of studies conducted by von Andrian and Mackay. For example, antibodies against the Ro (SSA) antigen may induce total heart block in the fetal heart but not in adults because antigen expression is controlled by developmental stages, which makes autoreactivity more harmful during specific growth periods. Adults develop pemphigus from antibodies that target desmoglein, but newborns don't have this illness. This is because these antibodies only target one of the two desmogleins seen in newborn skin.(8)Despite ongoing research, our understanding of the causes and mechanisms of these disorders is limited. What is known is that a delicate balance between tolerance and autoimmunity may be upset by a combination of hereditary factors, environmental factors, psychological factors, and random occurrences. There has been a lot of buzz about Quercetin, also known as 3,3?,4?,5,7-pentahydroxyflavone, a kind of diet flavonoid that may be occur in a number of foods and plants, including asparagus, apples, capers, chokeberries, radish leaves, cilantro, lovage, and other vegetables and fruits. Neuroprotective, antiallergic, antioxidant, anti-inflammatory, immunomodulatory, antibacterial, and anti-tumor properties are only a few of the many biological actions shown by adequate doses of quercetin, according to a plethora of prior research. Research on the uses of natural products as possible treating agents for autoimmune illnesses is now underway in the scientific community. A dietary supplement containing quercetin may be useful in the management or avoidance of autoimmune diseases. (9) Two examples of biliary autoimmune disorders encompass (primary sclerosing cholangitis)PSC and (primary biliary cholangitis) PBC.(10).Preventing infection by eliminating harmful invaders is the major function of the immune system. It activates a specific immune response through the collaboration of cellular and humoral responses, regulated by cytokines. Cellular immunity involves T lymphocytes, while humoral immunity relies on B lymphocytes producing antibodies. Both components work together synergistically for effective protection.(11,12)
Classification of autoimmune disorder
Two main groups of autoimmune illnesses are recognized by medical experts. One group includes systemic diseases like systemic lupus erythematosus, while the other group includes organ-specific disorders like type 1 diabetes mellitus. While helpful in therapeutic contexts, this categorization may miss the mark when it comes to determining the underlying reasons of the discrepancies. A more refined classification distinguishes between disorders caused by an immune response to a specific antigen (either foreign or self) and those resulting from general issues in T or B cell selection, regulation, or apoptosis. Lack of the Fas protein, along with its receptor, serves as a significant example of components that are essential for the process of cell death a generic problem; demyelination syndrome, which may occur after a Campylobacter jejuni infection, is an example of a condition associated to a particular antigen. Therapeutic approaches may vary depending on the underlying disease processes, so This classification is essential for management. In systemic lupus erythematosus, the presence of antinuclear and anti-DNA antibodies indicates that changes lowering the survival threshold of autoreactive B cells often result in the production of various autoantibodies.The general population often has modest concentrations of these autoantibodies. Inflammatory bowel disease (IBD) is associated with a possible overreaction to gut microbiota by T cells, which may be caused by genetic abnormalities that drastically affect regulatory T cell activity or cytokine production.(13)In order to keep the immune system in a steady state, it is necessary for different kinds of cells to interact in a coordinated manner inside certain microenvironments. Cytokines and their receptors are able to selectively and adaptively control cellular mobility in both healthy and diseased conditions due to their varied distribution and controlled expression. Because of their centrality to phenotypic development, cytokines are the targets of therapeutic immune regulation. (14) Autoimmune disorders, including lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease, and thyroid autoimmunity, have been associated with a heightened risk of specific cancers. The involvement of the immune system in controlling the proliferation of cancer cells elucidates this association, while the impact of hormones on cellular growth and immune activity further underscores the interplay between these conditions.(15)
Prevalence and impact on global health
There are two primary types of autoimmune disorders (AD), systemic and organ-specific, and they impact 5-8% of the population, respectively. Some examples of systemic autoimmune illnesses include rheumatoid arthritis (RA), dermatomyositis Mixed connective tissue disease (MCTD) and systemic lupus erythematosus (SLE) are two distinct autoimmune disorders. are both autoimmune disorders characterized by overlapping symptoms and features. Celiac disease (CD) and autoimmune thyroiditis (AIT) are examples of organ-specific autoimmune disorders. Despite frequent overlap, this categorization does not correspond to the underlying pathogenetic processes. Inflammation, muscular and joint discomfort, lethargy, increased antibodies against endocrine glands, and destruction of connective tissue are symptoms of several autoimmune illnesses. Diagnosing them is difficult due to nonspecific symptoms. Their complexity is vast, and while the exact cause is unclear, both genetic and environmental factors play significant roles. One theory suggests that hygiene standards, including drinking water quality and living conditions, may influence the progression of autoimmune diseases. AIDS is defined by specific clinical and biological criteria, with autoantibody titer being crucial. Identifying autoantibodies is important for diagnosing and monitoring autoimmune diseases (AIDs), as their presence or absence can indicate risk factors and guide treatment. It is common practice for clinicians to confirm AIDS diagnoses by testing sera from patients. Despite a lot of study, the frequency of AIDs in those who have symptoms is still not known. In order to improve the Moroccan databases and facilitate diagnosis, this project is to examine the frequency of different autoimmune illnesses a comprehensive evaluation of autoantibody profiles was conducted among a substantial cohort of patients exhibiting symptoms.(17) Patients often bear a heavy burden due to the protracted duration of their disease and the intensive treatment regimens. The root causes of bipolar disorder, a common mental illness, are still largely understood, despite progress in therapy. Researchers have been more interested in the link between autoimmune disorders and bipolar disorder in the last several years. “Among people with bipolar illness, a prior clinical study found an elevated risk of immune-related conditions like rheumatoid arthritis, thyroid disorders, and type 1 diabetes are included in this classification. Those diagnosed with bipolar disorder exhibited an increased likelihood of developing autoimmune diseases in comparison to the control group , a trend consistent across different bipolar subtypes., according to a bigger controlled trial that included structured interviews. In addition, compared to those with healthy immune systems, those who suffer from several autoimmune disorders have a higher risk of developing bipolar disorder. Researchers have known since 1987 that individuals with multiple sclerosis had a considerably greater frequency of bipolar illness compared to the basic population (P<0>
Importance of understanding autoimmune mechanisms
In autoimmune diseases, the immune system erroneously targets healthy tissues, causing a variety of symptoms and complications. These illnesses are complicated, and their causes and development are also. In most cases, doctors would rule out immune system regulatory systems malfunction or immunological tolerance deterioration of body tissues as the cause. (18) Autoimmune diseases share several characteristics, suggesting similar pathogenic mechanisms in genetically predisposed individuals. These diseases often cluster in families, with genetics and environmental factors both playing crucial roles in their onset. For example, arthritis in animal models only develops in genetically predisposed animals when exposed to specific adjuvants. While some cases arise suddenly due to genetic defects, they are not typical of most human autoimmune disorders. Additionally, autoimmune diseases show a notable sex bias, with females more frequently affected by antibody-mediated disorders, while males often experience more severe inflammation. Both endogenous and exogenous sex hormones can influence immune responses and disease progression. Infectious agents are also recognized as significant contributors to autoimmune disorders. While it has been difficult to substantiate the function of infections in people, research in animals has shown that infectious agents may generate autoimmune disorders. For example, coxsackievirus infection can cause inflammatory heart disease. There is evidence that infections may trigger autoimmune diseases through shared pathogenic pathways. For example, inflammatory heart disease is associated with parasitic infections in addition to viral and bacterial infections. Another example is the coxsackievirus infection which is linked to diabetes, thyroiditis, and heart inflammation. Thus, it seems that the inflammatory reaction to an infection, rather than the particular infectious agent, plays a more crucial role in the increment of autoimmune diseases. Cytokines are crucial in progression of autoimmune diseases, as they may influence the atypical immune responses characteristic of these disorders.(21). (22)
2.Pathophysiology of Autoimmune Diseases
In autoimmune illnesses, it has been shown, mostly via animal research, that other effector cells and inflammatory mediators may take the place of the original mechanisms that cause autoreactivity. Activation of naïve lymphocytes occurs at the beginning of the disease and their recruitment by epitope spreading may continue later on; however, it is not yet known whether memory cells or naive cells are accountable for disease activity and flare-ups. The processes underlying an autoimmune illness may change over time, as shown by many cases. Mice deficient in the Fas protein are unable to develop multiple sclerosis when given antibodies that target this protein; nevertheless, these same antibodies, when given to patients after the illness has progressed, prevent the death of activated cells. In addition, cytokines' effects might vary with the progression of the autoimmune illness. For instance, transforming growth factor-beta adds to organ fibrotic damage when the disease has already developed, although suppressing autoreactivity during the beginning of the disease. Significant therapy implications arise from the fact that damaged cells and soluble mediators may alter over time; treatments that work well in the beginning may no longer be helpful or may even be harmful toward the end. The clinical effectiveness of TNF-alpha blocking in RA and Crohn's disease exemplifies the heterogeneity of these effects; nonetheless, it may cause systemic lupus erythematosus in rare instances and antinuclear antibodies in as many as 10% of treating patients. (23)
Mechanism of autoimmunity
The stimulation of CD4 helper T cells, which respond specifically to an autoantigen.is generally acknowledged as the primary initiator of autoimmunity. The “single initiating antigen hypothesis” describes the current state of knowledge on autoimmunity and how it is thought to be triggered by a particular antigenic challenge. One of the processes thought to begin autoimmunity is molecular mimicry, which occurs when an immune response is triggered against both an autoantigen and an exogenous microbial antigen because of their structural similarities. Aside from molecular mimicry, infections may trigger autoimmune reactions by activating polyclonal antibodies and releasing localized autoantigens. Infectious pathogens produce lipopolysaccharide (LPS), which, like viral and bacterial DNA this acts as an adjuvant to boost the immune response by interacting with Toll-like receptors on dendritic cells and macrophages., these compounds boost inflammatory cytokine production and innate immunity. The immune response is enhanced by this mechanism because it increases the production of MHC antigens and co-stimulatory molecules including B7-2 and OX40L. As a whole, these responses activate acquired immunity, but they also have the potential to trigger T cells that might be autoreactive. Therefore, it's conceivable to introduce cryptic epitopes—which are not ordinarily expressed—in order to elicit an autoimmune reaction. Furthermore, it is believed that non-infectious causes might potentially serve as triggers for autoimmunity. Some drugs, including procainamide and hydralazine, may cause an SLE-like clinical state by inducing the development of antinuclear antibodies; estrogen, for instance, has been shown to worsen systemic lupus erythematosus (SLE) in mice models. In addition, environmental factors like iodine consumption play a vast role in autoimmune thyroid disease.
The mechanism through which an autoimmune disorder arises.
It is very unlikely that autoimmunity activation is a static event that might cause autoimmune disease. A large number of autoimmune diseases may proceed in part due to CD4 T cells, according to research by K. Yamamoto in mice models. Research on animals has shown that anti-CD4 monoclonal antibodies may deactivate or suppress CD4 cells, hence preventing the development of autoimmune disorders. Additionally, the production of IgG-type autoantibodies, the infiltration of CD4+ T cells in various organ-specific autoimmune diseases and their association with MHC class II antigens, such as the HLA-DR antigen in humans. have all hinted to the involvement of antigen-specific CD4 cells in autoimmune issues. One important process, epitope spreading, is linked to the development of autoimmune disorders, but there are many more. During the chronic phase of autoimmunity, the number of autoantigens identified by B and T cells continues to rise, a process known as autoantibody accumulation. The idea that a single epitope might trigger a systemic autoimmune response is fundamental to grasping this notion. In this mechanism, B cells and T cells work together; B cells play a key role in delivering antigens to T cells. Contrarily, research in NOD mice, which mimic type I diabetes, found that autoimmune illness might advance by means of the clonal growth and affinity maturation of a particular T cell clone that is specific to an antigen. Also, we found that the responsive epitope isn't always susceptible to expanding as the illness advances, according to our research on T cell clonality in organ infiltration. A key factor in the progression of autoimmune disorder is probably the equilibrium between the immune responses to reactive epitopes can have both beneficial and detrimental effects.(24)”
3.Current Pharmacological Treatments
Medications are used to soothe pain and inflammation or to reduce the immunological response in illnesses that impact the immune system, such rheumatoid arthritis (RA). Even though they have a wide range of negative effects, analgesics like corticosteroids and non-steroidal anti-inflammatory medications (NSAIDs) are efficient in reducing inflammation. Among the many potential side effects of commonly used nonsteroidal anti-inflammatory drugs (NSAIDs) is gastrointestinal toxicity, which may present itself in a variety of ways, including nausea, vomiting, ulcers, heartburn, and stomach discomfort. While corticosteroids like prednisone are an excellent pain reliever, they also have the potential to cause weight gain and even osteoporosis in some people. The alternative therapy options for rheumatoid arthritis (RA) include a range of DMARDs, such as chloroquine and methotrexate, which work by reducing the rate of joint deterioration. Despite the efficacy of these drugs, it takes a few months of treatment before you feel their benefits. On top of that, they don't target inflammation specifically, so they come with a whole host of unwanted side effects, such rashes, stomach pain, and decreased thrombocytes, leukocytes, and erythrocytes counts. Treatments for rheumatoid arthritis (RA) have strong side effects, therefore researchers are focusing on finding ways to cure the illness itself rather than just treating its symptoms. These particular cytokines, including (tumor necrosis factor-alpha)TNF- ?, are vital in the development and severity of RA, and these 'biological response modifiers' (BRMs) aim to reduce their effects. Within this class of drugs, you may find the TNF-? receptor protein Enbrel® as well as the monoclonal antibodies Remicade® and HUMIRA™, which attach themselves exclusively to TNF-?. Clinical experiments have shown that these biological response modifiers (BRMs) may decrease joint deterioration and reduce rheumatoid arthritis complications. As a BRM, antibodies are ideal because they have a longer half-life than small-molecule treatments and can bind selectively and strongly to a wide variety of biological molecules. (25) Constructing hybridoma cell lines is perhaps the most important step in making monoclonal antibodies. (26)
Phytochemicals in the management of autoimmune neurological disorders An aggravation of diseases that cause damage to neurons is a major cause of infections in the nervous system. When microglia secrete certain pro-inflammatory mediators, it causes inflammation in the nervous system, which in turn causes neurodegeneration. Free radicals, cytokines, tumor necrosis factor-alpha, and leukotrienes are some of the pro-inflammatory mediators. “Molecules that promote inflammation activate a number of Signal transduction pathways such as mTOR, MAPK, and PI3K/AKT are crucial in the context of autoimmune diseases. The activation of these pathways initiates inflammatory responses by engaging transcription factors, including HIF-1?, NF-?B, among other signaling transducers.” As neuroprotective drugs, phytochemicals work by focusing on or blocking pathways that are mediated by inflammation.
Phytochemicals: Mechanisms of Action in the Treatment of Immune Neurodegenerative Disorders.
Flavonoids
Quercetin
Apigenin Derivatives
Diosgenin
Rosmanaric Acid.(27)
4.Emerging Therapeutic Targets
Autoimmune diseases present considerable challenges to the advancement of pharmacological treatments. Among these obstacles are the lack of precise biomarkers, the difficulty in recruiting big enough cohorts for reliable clinical trials, the effectiveness of novel therapies in patients who have not responded to previous treatments (clinical equipoise), and the vagueness surrounding disease phenotypes. The subsequent discussion will explore these issues with the objective of identifying possible strategies to address them.(28). As our comprehension of immunopathogenesis improves alongside the increase in autoimmune diseases and continuous advancements in biotechnology, a multitude of medications designed with innovative therapeutic targets and improved efficacy are in development, showing encouraging outcomes. Nonetheless, considerable medical needs persist, particularly regarding variable therapeutic responses and adverse effects linked to immunosuppression. In response to the limitations of current therapies, treatment approaches for inflammatory diseases have evolved to become more varied and personalized for each patient. In this regard, we introduce a fresh perspective on targeted immunotherapy supported by cutting-edge molecular engineering methods. (29)

The variety of biological therapies available for the treatment of autoimmune diseases is expanding swiftly, driven by a deeper comprehension of molecular mechanisms and improvements in production technologies.(31)
5.Gene Therapy and CRISPR Approaches
CRISPR/Cas9 technology exemplifies a groundbreaking method for gene editing, enabling the modification of eukaryotic cell genetics. This technique can be utilized to specifically target and interrupt the genomes of pathogenic organisms, such as viruses and parasites, while also allowing for the alteration of particular genes in host cells that are linked to the progression of both viral and non-viral infections, thereby offering a potential solution to combat these infection.(32)
CRISPR/Cas9 tools for gene editing
The widespread availability of CRISPR/Cas9 reagents, along with its remarkable versatility, has accelerated the rapid development of innovative tools, as documented in the existing literature. This progress has enhanced our understanding and encouraged creative strategies to tackle various genetic mutations linked to primary immunodeficiencies (PIDs). With examples of their use in real-world PIDs, this article gives a rundown of the tools that are presently on the market. the CRISPR/Cas9 system targets a specific genomic DNA sequence by identifying the protospacer adjacent motif (PAM) and using a 20-nucleotide guide RNA (gRNA). Cas9 nuclease undergoes a conformational shift during RNA-DNA pairing formation; this change enhances the nuclease activity of Cas9 and causes a double-strand break to occur three base pair upstream of the PAM region, the first Cas9 nuclease, SpCas9, originates from the class 2 type II CRISPR/Cas system of Streptococcus pyogenes. It recognizes a 5'-NGG-3' PAM sequence, which should be present in the genome every 8-12 base pairs. Notable progress in this area has resulted in the creation of Cas9 variants that recognize different PAM sequences, as well as nucleases obtained from various bacterial sources.”
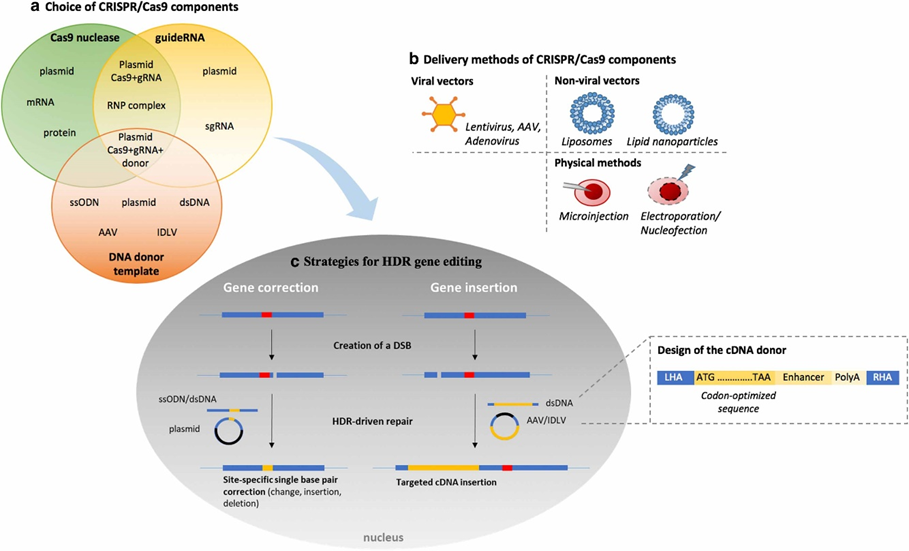
The procedure for choosing the CRISPR/Cas9 gene-editing tool from an array of CRISPR/Cas9 components is outlined in three main areas:
(a) the selection of components
(b) the methods of delivery
(c) gene correction techniques that make use of Homology-directed repair (HDR) consists of two main components: the left homology arm and the right homology arm.
The sequences 50-NGGNG-30 for CRISPR1 and 3, respectively, occurring every 64 base pairs, as well as the sequence from Neisseria meningitidis (PAM = 50-NNNNGATT-30 every 128 base pairs), have significantly broadened the range of genomic sites that can be targeted. Using customized SpCas9 High Fidelity variants is one of many ways to improve the CRISPR/Cas system's safety profile. Further studies have shown that by creating a double-strand break (DSB) between two binding sites, Cas9 nickases, the catalytically inactive form of Cas9, often referred to as dead Cas9, is linked to a FokI nuclease domain. may enhance specificity. There are fewer instances of off-target cleavage when using Cas9n as opposed to wild-type (WT) Cas9, as its specificity is up to 1,500 times higher. Cas9 from Staphylococcus pyogenes isn't the most specific of its class 2 type V CRISPR/Cas system orthologs; other bacterial species' Cpf1/Cas12a and Cas12b nucleases are more specific. The goal of developing novel base editing methods is to reduce the rate of insertions and deletions (indels).at specific places by converting mutant sequences to wild-type ones without causing DNA breakage. The present approach uses adenosine deaminase to convert A:T to G:C and cytidine deaminase to convert C:G to T:A irreversibly, respectively, without DNA breakage. The spectrum of targetable mutations is quite restricted, and the method has limits in terms of repair efficiency. In addition, approaches that are more sensitive have shown off-target effects that are considerable; specifically, the off-target frequency is 20 times higher with cytosine base editors than with adenosine base editors. The guide RNA is an essential part of the CRISPR genomic-editing technology. To begin with, in vitro transcription was the usual method of synthesis for guide RNAs, which were originally about 100 nucleotides long and comprised of a 20-nucleotide spacer that was complementary to the target genomic region. Although using single-guide RNA with shorter spacers of 17, 18, or 19 nucleotides might improve specific, it is important to carefully evaluate each application since it may reduce genome-editing effectiveness. It may be too expensive to produce gRNA on a big enough scale for clinical use, and it may be difficult for certain research organizations to do in vitro transcription. One possible benefit of synthetic sgRNAs is the ability to make chemical alterations to them. Several strategies exist for improving the stability of sgRNA and increasing the frequency of genome editing. A new and promising strategy that allows for early editing actions with less associated toxicity is the creation of ribonucleoprotein (RNP) complexes by combining Cas9 nuclease with sgRNA. This element needs careful examination and will be further developed in connection to particular mutations and patient-induced disorders (PIDs). The choice of donor in the gene-editing system is critical since it directly effects the ultimate result. The DNA used in these experiments can come from a variety of sources, including ssODNs, long strands of single-stranded DNA, linear or circular double-stranded DNA, and complementary DNA (cDNA) delivered through engineered viral vectors like recombinant adeno-associated virus (rAAV) or integrase-defective lentivirus (IDLV), which can only integrate partially and only expresses the gene temporarily. Because of its great infectiousness in non-dividing cells, minimal immunogenicity, and relatively low integration rates within the host genome, the rAAV is an ideal delivery vehicle. According to studies, the ability of rAAV to transport genetic material may be increased to 6.5 kb when two co-transduced AAV vectors are used, in addition to the usual 4.5–5 kb. Serotype 6 rAAV has shown the best affinity for targeting human hematopoietic stem cells (HSCs), and a mutant AAV6 capsid has shown improvements. Donors should have 400 base pair homology arms, have their cDNA optimized for codons, and modify the PAM sequence to prevent the need for re-extraction of donor or genetically modified cells. CRISPR-Cas9 technology and autoimmune diseases. Therapeutic trials conducted in vitro utilizing CRISPR-Cas technology. Autoimmunity typically results from a systemic immune response that occurs due to insufficient regulated of the immunity. In the realm of in vivo genetic correction using these CRISPR-Cas9 technology, it is crucial for the guide RNA (sgRNA) and the Cas9 enzyme to penetrate the cell nucleus to perform edits at designated DNA sites. There are two main delivery systems for CRISPR-Cas9: viral vectors and lipid nanoparticles. The adeno-associated virus (AAV) is the most frequently utilized viral vector, noted for its low risk of integrating into foreign genomic material and its well-established safety profile. Although the SpCas9 protein from Streptococcus pyogenes is very powerful, its size limits its use. As a result, scientists are looking for smaller Cas9 variations from other bacteria. For in vivo gene editing, it is possible to combine Cas9 proteins from Staphylococcus aureus or Campylobacter jejuni with sgRNA and encapsulate them in AAV. The results are impressive. One study used SaCas9 non-homologous end joining facilitates the process of connecting the ends of DNA strands successfully knockout PCSK9 in liver cells. PCSK9 is a protein that modulates low-density lipoprotein (LDL) and greatly contributes to atherosclerosis and coronary heart disease. The knockout resulted in a significant lower in LDL levels. Meganuclease delivered in vivo with AAV targeting PCSK9 decreased blood cholesterol level, according to another study. Also investigated are uncommon metabolic diseases. Additionally, research on Duchenne muscular dystrophy (DMD) has effectively removed the harmful mutation from exon 23, which has improved the function of the heart and skeletal muscles. Inflammatory Mediators, Immunogenetics, and CRISPR-Cas9 Technology. An improper immune response, known as autoimmunity, develops when there is a severe mismatch between signals that promote inflammation and those that inhibit it. “Consequently, lowering the concentrations of inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF).may successfully halt the autoimmune process. An example would be the pro-inflammatory reactions that may be induced in the skin and other tissues by interleukin-36 (IL-36) cytokines, which include IL-36A, IL-36B, and IL-36G, among others belonging to the IL-1 family. Research indicates that IL-36 cytokines could be heavily involved in the onset of autoimmune disorders. When stimulated with IL-36, the adaptor protein known as MyD88 activates the myeloid differentiation main response gene. Deactivating the MyD88 adaptor protein using CRISPR-Cas9 has been shown to boost IL-1B and IL-36G production by reducing the activity of many differ expressed genes. One important function that tumour necrosis factor alpha-induced protein 3, commonly referred to as TNFAIP3.functions to inhibit the activation of NF-kappa B and to avert apoptosis induced by TNF. This protein is activated by TNF-alpha. Researchers have shown that TNFAIP3 variants increase the likelihood of getting RA and systemic lupus erythematosus (SLE). As a possible new treatment target, UBE2L3 was found in a genome-wide association analysis of SLE. The creation of the linear ubiquitin chain assembly complex is crucial for the activating of NF-kB and UBE2L3 is the principal E2 enzyme implicated in this process. New evidence also suggests a connection between infantile-onset IBD and familial Behçet-like autoinflammatory illness. Correcting pathogenic mutations of TNFAIP3 may be able to reverse related autoimmune symptoms, according to research using transcription activator-like effector nucleases can be rephrased as nucleases that resemble transcription activators and are designed to target specific DNA sequences. (TALENs) to delete TNFAIP3.the autoimmune response might be impacted by the elimination of the putative causative mutation, rs6927172, by CRISPR-Cas9 gene editing, which also affects the expression of the IL-20RA and TNFAIP3 genes. In addition to cytokines, T cells are linked to other genetic variations that have a role in the onset of autoimmune disorders. One example is the importance of FoxP3+ T cells in maintaining immunological tolerance. Multiple autoimmune diseases can appear in early infancy in individuals Immuno-dysregulation polyendocrinopathy, enteropathy X-linked (IPEX) syndrome arises from mutations in the FoxP3 gene. In individuals affected by IPEX, the function of regulatory T cells is significantly compromised due to a substantial reduction in FoxP3 levels.. An intriguing treatment strategy for controlling IPEX instances might be CRISPR-Cas9-mediated epigenetic editing of the FoxP3 gene's promote or conserved non-coding sequence 2, which has been shown to reduce effector T cell proliferation by 20-30%. CRISPR-Cas9 or induced pluripotent stem cells (iPSCs). The area of autoimmune diseases has explored many treatment options that make use of CRISPR-Cas9 technology. Utilizing CRISPR-Cas9 gene editing technology, it is possible to incorporate the interleukin-1 receptor antagonist (IL1Ra) or the gene encoding the soluble TNF receptor into induced pluripotent stem cells (iPSCs). These cells were then differentiated into articular cartilage.” The inflammatory response was reduced by this method, which, when exposed to inflammatory cytokines, built up a responsive negative feedback system. A number of further investigations have shown that by deleting the interleukin-1 receptor 1 (IL1r1) in mouse iPSCs, articular cartilage can withstand tissue deterioration caused by IL-1alpha.Further, CRISPR-Cas9 gene editing was used in MSCs to improve MSC chemokine receptors and intrinsic activation of pancreatic transcription factors. We drove these engineered MSCs to develop into surrogate insulin-producing cells; these cells retain their immunomodulatory characteristics and have the potential to be transplanted following ex vivo growth. A combination of sgRNAs targeting specific insulin promoters and a nuclease-deficient Cas9-virion protein 160 HEK293T, HeLa, and fibroblast cells has been successfully used to stimulate the natural transcription of human insulin, according to recent research. (34)
Mechanism
The CRISPR-Cas system functions as a natural immune defense in prokaryotic organisms, allowing them to recognize and eliminate invading viruses during subsequent encounters. This mechanism involves the integration of a segment of viral DNA into their own genetic framework within a specific area referred to as CRISPR. The target DNA is precisely cleaved by the Cas protease with the help of the carefully engineered single-guide RNA (sgRNA). Double-Strand Breaks and the DNA repair mechanisms that follow are essential to the CRISPR/Cas9 genome editing process. Cellular DNA maintain pathways, like Non-Homologous End Joining, are triggered by CRISPR/Cas9-induced DSBs. However, NHEJ is error-prone and can result in insertions and deletions (indels), which disrupt or eliminate the function of genes or genomic elements, including regulatory regions, by creating small mutations at the target site. Homology-Directed Repair is an alternate repair procedure that may fix hereditary DNA errors without adding new mutations. It can be engaged as necessary. In the therapy of autoimmune illnesses, there has been significant practical value in genetically modifying immune cells or expressing relevant proteins. Applying this technique to study non-viral infections associated with autoimmune diseases (ADs) improves our knowledge of the pathogenic processes driving these conditions, according to recent research and clinical trials. In addition, research and treatment techniques for ADs may be further advanced via the use of these system to create more accurate procedures. As part of the three-stage CRISPR/Cas9 immune response, foreign sequences are added to the proximal end of the CRISPR array during adaptation to make it more adaptable.A brief crRNA is generated by enzymatic cleavage of precursor CRISPR RNA molecules containing components located inside the repeat spacer, as described in the method. These crRNAs can pair with complementary protospacer sequences present in invading viruses or plasmids. The crRNA forms a complex with trans-activating CRISPR RNA (tracrRNA), resulting in a double-stranded RNA structure that is subsequently processed into mature crRNA-tracrRNA complexes. The spacer region of the crRNA serves as a memory, containing segments derived from viral or plasmid DNA. The crRNA-tracrRNA complex interacts with Cas proteins to form ribonucleoprotein (RNP) complexes, which guide the Cas proteins to silence foreign sequences, thereby triggering an immune response. The SpCas9 nuclease has been shown in studies to bind and cleave DNA sequences independently of an RNA complex. Inducing double-strand breaks (DSBs) is as simple as scripting a single guide RNA (sgRNA) to target a certain genome. Most of the time, processes like homology-directed repair or error-prone nonhomologous end joining are used for fix these DSBs. Even with the repair of DSBs, small insertion or deletion mutations at the breakpoint can disrupt the gene's open reading frame. Gene knockout can be accomplished by generating indels within the coding exons. If the targeted protein domain is a crucial functional region, the indels introduced by CRISPR/Cas9 will result in a high frequency of mutations. The CRISPR/Cas9 gene editing technology presents considerable potential and opportunities for clinical validation. Its high specificity can offer a more efficient and safer treatment approach for various autoimmune diseases with distinct pathogenesis.(35)
6.Personalized Medicine in Autoimmune Disease Treatment
Autoimmune diseases represent complex conditions influenced by a combining of genetic predispositions and its environmental factors, and currently, there is no definitive cure available. Various treatment strategies can be utilized to attain remission or, at the very least, alleviate the symptoms associated with these conditions. To foster the development of personalized medicine, it is essential to accurately identify patient groups that are relatively homogeneous and demonstrate similar pathogenic signaling pathways. As a result, the important goals of autoimmunity is research are to find new biomarkers, discover new therapeutic goals and agents, and understand the mechanisms that cause the various types of autoimmune diseases. For personalized medicine to work, it's necessary to analyze population-level data and modify it so it takes into account each patient's specific biological, genetic, and clinical characteristics. Factors such as race, lifestyle variances, and environmental impacts add to the complexity of the problem and need careful consideration. In this regard, there is a noticeable disparity between non-Caucasian and Caucasian populations; this must be recognized in order to open the door to individualized care for more people. “Using this model, Vetchinkina et al. found variations in genetices associated with demographic and clinical character of Russian rheumatoid arthrisis patients. By investigating many known risk variables within an understudied ethnic group, our research adds to what is already known about RA genetics. In a Latin American cohort with substantial Amerindian origin, Díaz-Peña et al. investigate the impact of environmental factors, genetic ancestry, and single nucleotide polymorphisms on chronic obstructive pulmonary disease. They discovered that the PPP1R12B gene is associated with chronic obstructive pulmonary disease (COPD), and that PRDM15 gene variations that impact COPD risk interact with one another and with ethnic background.” A recent review highlighted advancements in understanding genetic factors influencing rheumatoid arthritis (RA) in underrepresented populations, noting that Genome-Wide Association Studies (GWAS) have improved insights into autoimmune disease mechanisms. However, representation of African and Latin American individuals in GWAS is still lacking, which may limit the benefits of research findings for these groups. Future advancements in personalized medicine are expected to enhance predictions of treatment responses and disease outcomes in autoimmune conditions, though not all findings may apply to non-Caucasian populations. These findings highlight the urgent need for further GWAS involving underrepresented groups. Unfortunately, the variability among patients with autoimmune diseases results in differing responses to specific treatments. Three review articles address this complexity from various angles. A review of the current state of selective inhibitors targeting epigenetic modulators, with an emphasis on inhibitors of histone deacetylase (HDAC) and bromodomain and extraterminal (BET), as well as their prospective applications in inflammatory and autoimmune disorders, was provided by Ghiboud et al. In addition, several autoimmune diseases have shown substantial improvement after using corticosteroids may provide a potential therapeutic strategy to increment the survival rates in patients with sepsis, according to Son et al., who studied the clinical results of corticosteroid therapy in patients with sepsis or septic shock. Finally, Mazini et al. investigated several mechanisms and possible consequences of adipose-derived stem cells' (ADSCs) interactions with their surroundings. A increasing number of genes and proteins have been identify by the authors as being associated with cell migratory pathways.” These pathways may control the trafficking of CD4+CD28null lymphocytes to certain organs, where they might cause inflammatory damage. Patients suffering from rheumatoid arthritis might benefit from further investigation into the discovered potential biomarkers as possible treatment targets. (36)
7.Future direction and challenges
Rebuilding the immune system with autologous or allogenic stem cells, altering immune activation thresholds, modulating antigen-specific responses, and protecting target organs are the four main therapeutic approaches that are presently being researched. Restoring immune system equilibrium and lowering the autoimmune response are the goals of interfering with costimulation, signaling pathways, chemokines, and other crucial chemicals involved in immunological activation. This approach is predicated on the idea that small alterations in the accessibility of cell-regulating proteins may refocus the immune system away from autoreactivity. This approach is predicated on the idea that when proteins involved in intracellular signaling or cellular connections are slightly changed in availability, the immune system may be redirected away from autoreactivity. Tolerance to certain antigens is the goal of antigen-specific treatment. Oral administration of relevant peptides or autoantigens has shown promise in animal trials, but no such results have been reported in human participants. If this strategy works just when autoreactive cells are first activating, it may not be able to prevent the immune landscape from becoming inflammatory and leading to epitope dissemination once the illness is clinically noticeable. (37)
Future therapeutic targets and maybe new treatments
Reviewing the diagnosis, re-staging the illness, evaluating drug adherence, and ruling out concurrent diseases are all crucial steps before contemplating experimental therapy.
Treatments that deplete B cells
Although T-cells are thought to have a vast role in autoimmune hepatitis (AIH), B-cell regulation has shown great promise in the treatment of this condition. Rituximab, as mentioned earlier, is a medication for B-cell depletion that targets B-cells specifically via their CD20 receptor. Allowing B-cell differentiation and proliferation to take place requires the B-cell activating factor (BAFF). Destroying B-cells via antibody-dependent pathways is the outcome of blocking this pathway. On top of that, BAFF may stimulate a pro-inflammatory Th1/Th17 phenotype by activating CD4+ T-cells. B-cells express CD20 from the very beginning of their development, but mature B-cells are where you'll most often find the BAFF receptor. Restoring numbers of T-regulatory (Treg) cells is the primary goal of anti-BAFF treatment, as opposed to rituximab. A quantitative and functional lack of regulatory T-cells (Tregs) characterizes autoimmune hepatitis (AIH), which disrupts immunological tolerance by creating an imbalance between effector and regulatory T-cells. Therefore, it is crucial for pharmacologists to strike this equilibrium. There has been research on the adoptive transfer of Tregs in relation to transplantation and a range of medical issues. It is possible to alter, enlarge, and stimulate autologous immune cells outside of the body before reinfusing them into patients. Results in mouse models demonstrate that this strategy successfully recruits CXCR3+ Tregs to the liver, where they are helped along by the increased production of CXCR3 ligands (CXCL9/CXCL10) during inflammatory reactions. Although some pharmaceutical drugs may assist in preserving Treg functioning, there is still cause for concern over the possible suppression or dedifferentiation of transplanted regulatory T-cells into effector T-cells. The survival rates and liver-homing capacities of these cells also pose issues. Additional clinical studies are necessary since a proof-of-concept research shown that autologous Tregs may successfully inhibit effector T-cells in autoimmune hepatitis (AIH). Current data on autologous T-cell transfer in AIH are from a phase I/II study (NCT02704338). Tregs are more sensitive to the impact of interleukin-2 (IL-2) on T-cell proliferation than effector T-cells. Tregs in AIH patients have shown decreased IL-2 responsiveness and increased IL-10 production, while Treg survival is enhanced by low-dose IL-2. Hepatic inflammation diminished in two AIH patients treated with low-dose IL-2; nevertheless, one patient did not attain full biochemical remission. Two individuals with AIH were among forty-six subjects who received low-dose IL-2 over the course of six months in a prospective phase I/IIa trial. Rather of activating effector T-cells, this therapy caused the growth and activation of regulatory T-cells (Tregs) in every single subject. Improving the selectivity and lifespan of Tregs is the current focus of research on producing modified versions of interleukin-2 (IL-2). Using Monoclonal antibodies that deplete T-cells targeting CD3(OKT3), which are used to manage severe acute rejection in solid organ transplants, effector T-cell depletion may be achieved. Experimental models with type 2 autoimmune hepatitis (AIH) have shown promising outcomes with this approach, including biochemical remission, decreased circulating T-cells and inflammation in the liver, and positive effects on liver function. Additionally, the remaining T-cells showed reduced sensitivity to autoantigen activation, suggesting the potential development of tolerance. Cytokine neutralization is an additional strategy; this method zeroes in on certain cytokines that contribute to Th0 cell development into Th1 and Th17 cells. One possible mechanism by which Th1-mediated inflammation and fibrosis in the liver are facilitated is the targeted recruitment of certain subsets of lymphocytes, which may improve the activity of effector T-cells. Reduced regulatory T-cell (Treg) activity and increased inflammation are associated with increases hepatic Th17 cell populations in autoimmune hepatitis (AIH). Few studies have investigated the possibility of blocking cytokines such as IL-1, IL-6, IL-17, and IL-23 by interventions that suppress Th17 activity in AIH. Reduced Bifidobacterium levels are associated with worse illness severity; patients with AIH often have microbial dysbiosis, decreased intestinal barrier function, and increased bacterial translocation. Antigen exposure occurs in the liver as a result of bacterial lipopolysaccharide translocation. Additional research is needed to determine the efficacy of antibiotics, probiotics, fecal microbiota transplantation, and autoimmune hepatitis. The activation of toll-like receptor-4 on different types of liver cells by lipopolysaccharides in a mouse model results in the secretion of pro-inflammatory cytokines, which exacerbate inflammation and Cirrhotic. Hepatic inflammation and aminotransferases were both markedly decreased by the TLR4 antagonist JKB-122. Results from phase II pilot research assessing JKB-122 for use as a second-line therapy for AIH have not been released at this time (NCT02556372). (38)
8.Future prospects in Combination treatments
The administration of two or more pharmaceutical agents, either singly or in a fixed-dose combination comprising several active ingredients in a single dosage form, is referred to as combination medication therapy. Physicians often prescribe combination therapy to address and manage a vast varieties of medical conditions; however, the absence of careful monitoring can lead to various complications. While there have been limited conclusive clinical studies conducted thus far, existing data strongly indicate that combination therapy may be advantageous for certain patient populations, such as those who have undergone stem cell transplants. This paper reviews evidence from in vitro, experimental, and clinical studies to identify effective clinical strategies for this promising new therapeutic approach.(39) The presence of many immunosuppressive pathways that might hinder an anticancer immune response raises the possibility of combining immunotherapeutic medications, each with its own mechanism of action, to further strengthen immune responses against malignancies. Recent findings from a phase III trial demonstrated that patients with stage III-IV melanoma had significantly higher response rates and progression-free survival when gp100 peptide vaccine and high-dose IL-2 were administered together, as compared to IL-2 alone. The response rate was 16%, the PFS was 2.2 month, and the P-value for 0.008 was 0.6. This provides further evidence that immunizations may enhance the efficacy of cytokine therapy in individuals with metastatic melanoma. It is quite fascinating to note that recent research has shown that antitumor T lymphocytes may express several inhibitory receptors. This finding has also been seen in mice models. (40)
CONCLUSION
The pharmacological landscape for treating autoimmune diseases is rapidly evolving with the identification of new therapeutic targets and innovative treatment strategies. Advances in biologics, immune checkpoint inhibitors, JAK inhibitors, and small molecules are providing more targeted, effective therapies with fewer side effects. Personalized medicine and the exploration of the gut microbiome’s role in immune regulation offer promising new directions. While challenges like drug resistance remain, emerging therapies targeting B cells, T cells, and immune tolerance mechanisms offer hope for improved outcomes and more precise management of autoimmune diseases.
REFERENCES
- Pratab Singh S, Wal P, Wal A, 68 Srivastava V, Tiwari R, Dutt Sharma R. International Journal of 55Pharmaceutical Technology and Biotechnology 51 *Corresponding Author: UNDERSTANDING AUTOIMMUNE DISEASE: AN UPDATE REVIEW [Internet]. Available from: www.ijptb.com
- Pashnina IA, Krivolapova IM, Fedotkina T V., Ryabkova VA, Chereshneva M V., Churilov LP, et al. Antinuclear autoantibodies in health: Autoimmunity is not a synonym of autoimmune disease. Vol. 10, Antibodies. MDPI AG; 2021. p. 1–26. 49
- Wu MY, Wang EJ, Feng D, Li M, Ye RD, Lu JH. Pharmacological insights into autophagy 81 modulation in autoimmune diseases. Vol. 11, Acta Pharmaceutica Sinica B. Chinese Academy of Medical Sciences; 2021. p. 3364–78.
- Khoury MK, Gupta K, Franco SR, Liu B. Necroptosis in the Pathophysiology of Disease. Vol. 190, American Journal of Pathology. Elsevier Inc.; 2020. p. 272–85. 64
- Reddy Bonam S, Muller S. Parkinson’s disease is an autoimmune disease: a reappraisal. 52
- Yang S, Zhao M, Jia S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Vol. 14, Frontiers in Immunology. Frontiers Media S.A.; 2023. 69
- Pisetsky DS. Pathogenesis of autoimmune disease. Vol. 19, Nature Reviews Nephrology. Nature Research; 2023. p. 509–24. 36
- Utoimmune A, Iseases D, Nne A, Avidson D, Iamond ED. Review Articles Advances in 24Immunology The New Eng land Jour nal of Medicine [Internet]. Vol. 345, J Med. 2001. Available from:www.nejm.org 40
- Shen P, Lin W, Deng X, Ba X, Han L, Chen Z, et al. Potential Implications of Quercetin in 92Autoimmune Diseases. Vol. 12, Frontiers in Immunology. Frontiers Media S.A.; 2021. 34
- Gerussi A, Lucà M, Cristoferi L, Ronca V, Mancuso C, Milani C, et al. New Therapeutic Targets in Autoimmune Cholangiopathies. Vol. 7, Frontiers in Medicine. Frontiers Media S.A.; 2020. 31
- George J, Shoenfeld Y. Introduction: The Immune System, the Autoimmune State and Autoimmune Disease. 2008
- Li Z, Guo J, Bi L. Role of the NLRP3 inflammasome in autoimmune diseases. Vol. 130, Biomedicine and Pharmacotherapy. Elsevier Masson SAS; 2020. 59 30
- Viswanath D. Understanding Autoimmune Diseases-A Review [Internet]. Vol. 6, IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN. Available from: www.iosrjournals.orgwww.iosrjournals.org8|
- Botanical immunodrugs. 25
- Losada-García A, Cortés-Ramírez SA, Cruz-Burgos M, Morales-Pacheco M, Cruz-Hernández CD, Gonzalez-Covarrubias V, et al. Hormone-Related Cancer and Autoimmune Diseases: A Complex Interplay to be Discovered. Vol. 12, Frontiers in Genetics. Frontiers Media S.A.; 2022. 23 23
- Snowden JA, Styczy?ski J, Snarski E, Greco R, Badoglio M, Labopin M, et al. Hematopoietic stem cell transplantation in autoimmune diseases: update from the EBMT Autoimmune Diseases Working Party with special reference to Poland. Vol. 52, Acta Haematologica Polonica. Via Medica; 2021. p. 217–24. 21
- Missoum H, Alami M, Bachir F, Arji N, Bouyahya A, Rhajaoui M, et al. Prevalence of autoimmune diseases and clinical significance of autoantibody profile: Data from National Institute of Hygiene in Rabat, Morocco. Hum Immunol. 2019 Jul 1;80(7):523–32. 43
- Chen M, Jiang Q, Zhang L. The prevalence of bipolar disorder in autoimmune disease: A systematic review and meta-analysis. Vol. 10, Annals of Palliative Medicine. AME Publishing Company; 2021. p. 350–61. 1
- Cannas D, Loi E, Serra M, Firinu D, Valera P, Zavattari P. Relevance of essential trace elements in nutrition and drinking water for human health and autoimmune disease risk. Vol. 12, Nutrients. MDPI AG; 2020. p. 1–22. 47
- Vojdani A, Vojdani E, Rosenberg AZ, Shoenfeld Y. The Role of Exposomes in the Pathophysiology of Autoimmune Diseases II: Pathogens. Vol. 29, Pathophysiology. MDPI; 2022. p. 243–80. 27
- Fairweather D. Autoimmune Disease: Mechanisms. In: Encyclopedia of Life Sciences. Wiley; 2007. 22
- Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK–STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Vol. 77, Drugs. Springer International Publishing; 2017. p. 521–46. 36
- Utoimmune A, Iseases D, Nne A, Avidson D, Iamond ED. Review Articles Advances in
- Immunology The New Eng land Jour nal of Medicine [Internet]. Vol. 345, J Med. 2001. Available from: www.nejm.org
- Rethinking mechanisms of autoimmune pathogenesis 2.
- reviews therapeutic focus [Internet]. Available from: www.drugdiscoverytoday.com
- reviews therapeutic focus [Internet]. Available from: www.drugdiscoverytoday.com 50
- Ashraf H, Solla P, Sechi LA. Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases. Vol. 15, Pharmaceuticals. MDPI; 2022. 33
- Van Herle K, Behne JM, Van Herle A, Blaschke TF, Smith TJ, Yeaman MR. Integrative continuum: Accelerating therapeutic advances in rare autoimmune diseases. Annu Rev Pharmacol Toxicol. 2012;52:523–47. 63
- Jung SM, Kim WU. Targeted Immunotherapy for Autoimmune Disease. Vol. 22, Immune Network. Korean Association of Immunologists; 2022. 8
- Boardman DA, Levings MK. Emerging strategies for treating autoimmune disorders with genetically modified Treg cells. Vol. 149, Journal of Allergy and Clinical Immunology. Elsevier Inc.; 2022. p. 1–11.
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: An update. Vol. 11, BMC Medicine. 2013.
- Mohammadzadeh I, Qujeq D, Yousefi T, Ferns GA, Maniati M, Vaghari-Tabari M. CRISPR/Cas9 gene editing: A new therapeutic approach in the treatment of infection and autoimmunity. Vol. 72, IUBMB Life. Blackwell Publishing Ltd; 2020. p. 1603–21. 19
- de Ravin SS, Brault J. CRISPR/Cas9 applications in gene therapy for primary immunodeficiency diseases. Vol. 3, Emerging Topics in Life Sciences. Portland Press Ltd; 2019. p. 277–87. 37
- Lee MH, Shin J Il, Yang JW, Lee KH, Cha DH, Hong JB, et al. Genome Editing Using CRISPR-41Cas9 and Autoimmune Diseases: A Comprehensive Review. Vol. 23, International Journal of Molecular Sciences. MDPI; 2022. 85
- Zhang X. Development and Application of CRISPR Technology in the Treatment of Autoimmune Diseases. 1
- Díaz-Peña R. Personalized medicine in autoimmune diseases. Vol. 11, Journal of Personalized Medicine. MDPI; 2021. 59 30
- Viswanath D. Understanding Autoimmune Diseases-A Review [Internet]. Vol. 6, IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN. Available from: www.iosrjournals.orgwww.iosrjournals.org8| 44
- Chung Y, Rahim MN, Graham JJ, Zen Y, Heneghan MA. An update on the pharmacological management of autoimmune hepatitis. Vol. 22, Expert Opinion on Pharmacotherapy. Taylor and Francis Ltd.; 2021. p. 1475–88.66
- Rahman M, Islam F, Rahman A, Ahmed T, Borhan Uddin M, Mohammad Shaheen S, et al. 13 PRESENT AND FUTURE PROSPECT OF COMBINATION DRUGS THERAPY 58*Corresponding Author. World Journal of Pharmaceutical Research 1625 World Journal of Pharmaceutical Research SJIF Impact Factor 8 [Internet]. 2020;9(3):1625–38. Available from: www.wjpr.net 77
- Drake CG. Combination immunotherapy approaches. In: Annals of Oncology. 2012.


 Dimple Yadav *
Dimple Yadav *
 Unnati Dewangan
Unnati Dewangan
 Krishna Kumar Baghel
Krishna Kumar Baghel
 Abhay Banchhor
Abhay Banchhor
 Sarita Baghel
Sarita Baghel


 10.5281/zenodo.14726841
10.5281/zenodo.14726841