Abstract
Plants have a great number of chemicals that can be exploited as valuable sources of natural antibiotics and pesticides. Lantana camara has numerous potent phytochemicals that could be exploited in plant disease control and protection thereby reducing the indiscriminate use of synthetic pesticides. Little research has documented the antimicrobial properties of Lantana camara plant varieties against soil phytopathogens such as Pseudomonas syringae and Phytopthora infestans. This study was carried out to investigate on the antimicrobial potential of Lantana camara on the growth of Pseudomonas syringae and Phytopthora infestans. Roots and leaves were collected, shade dried and ground into fine powder and extracted by cold extraction method with 70% ethanol. Disc diffusion method was used to assess antimicrobial activity of this plant by measuring the inhibitory growth zones formed around paper discs. The leaves extract treatment significantly reduced the growth of Pseudomonas syringae and Phytopthora infestans with increase in extract concentration, even though there were no significant differences between the two test microbes. Therefore, it can be ascertained that certain active ingredients are present in the roots and leaves ‘crude extract of Lantana camara and have the potential to be exploited for manufacture of pesticides for control of diseases associated with Pseudomonas syringae and Phytopthora infestans.
Keywords
Plants have a great number of chemicals that can be exploited as valuable sources of natural antibiotics and pesticides. Lantana camara has numerous potent phytochemicals that could be exploited in plant disease control and protection thereby reducing the indiscriminate use of synthetic pesticides. Little research has documented the antimicrobial properties of Lantana camara plant varieties against soil phytopathogens such as Pseudomonas syringae and Phytopthora infestans. This study was carried out to investigate on the antimicrobial potential of Lantana camara on the growth of Pseudomonas syringae and Phytopthora infestans. Roots and leaves were collected, shade dried and ground into fine powder and extracted by cold extraction method with 70% ethanol. Disc diffusion method was used to assess antimicrobial activity of this plant by measuring the inhibitory growth zones formed around paper discs. The leaves extract treatment significantly reduced the growth of Pseudomonas syringae and Phytopthora infestans with increase in extract concentration, even though there were no significant differences between the two test microbes. Therefore, it can be ascertained that certain active ingredients are present in the roots and leaves ‘crude extract of Lantana camara and have the potential to be exploited for manufacture of pesticides for control of diseases associated with Pseudomonas syringae and Phytopthora infestans.
Introduction
An antimicrobial is a substance that kills or inhibits the growth of microorganism. Antimicrobial drugs either kill microbes or prevent the growth of microbes. Disinfectants are antimicrobial substances used on non-living objects. Antimicrobial agents are the drugs which are having selective toxicity against various pathogens like bacteria, fungi, virus, protozoa. Chemical agents that destroys the disease-causing microorganisms with minimal damage to the host. Contrast with “antimicrobial therapy”. There are substances produced by microorganism which selectively suppress the growth of or kill other microorganism at very low concentration. Plants have a great number of chemicals that provide valuable sources of natural antibiotics. Plant parts possess antimicrobial agents. Plants have natural antimicrobial Substances have no health environmental residual problems. Many pesticides Sold in the markets are toxic and have adverse effects on soil ecosystems. There is development of resistant strains of pathogens due to continued use of synthetic pesticides and drugs. indicates that the use of soil fumigants such As Methyl bromide in control of soil borne disease pathogens is not a safe method due to residual Toxicity on the environment. Indiscriminate use of pesticides adversely affects soil ecosystems.
LANTANA CAMARA: [3]
Lantana camara is a common weed in most agricultural lands. This plant can grow as a Compact clumps and dense thickets. The plant is an invasive species to around fifty different Countries. The plant has been used to cure many health problems.
The genus Lantana (Verbenaceae) as described by Linnaeus in 1753 contained seven species, six from South America and one from Ethiopia. Lantana from the Latin lento, to bend, probably derives from the ancient Latin name of the genus Viburnum. Lantana is mostly native to subtropical and tropical America, but a few taxa are indigenous to Tropical Asia and Africa. It is a genus of about 150 species. L. camara Linn., commonly known as wild or red sage, is the most widespread Species of this genus. It is planted as an ornamental plant and is now A highly invasive weed in many parts of the world. L. camara is found at altitudes from sea level upto 2000 m and can thrive very well under Rainfall ranging from 750 to 5000 mm per annum and it grows up to 3 m Height. It is a woody straggling evergreen, aromatic wild shrub. The stems and branches are sometimes thorny. The leaves are arranged in opposite pairs and are broadly oval, bright green, rough with short Hairs, with finely toothed edges along with a number of veins giving A wrinkled appearance. Flower heads contain 20-40 flowers, usually 2.5 cm across; the color of flowers varies from white, cream or yellow to orange pink, purple and red with small rounded heads, often in two Colors. The fruits are fleshy berries in clusters, shiny and globose in Shape, green in color which on ripening turns to black. The root system Is very strong with a main taproot and a mat of many shallow side Roots. L. camara is known by different names in different languages in India, viz., Raimuniya (Hindi), Chaturangi and Vanacehdi (Sanskrit) and Kakke, Natahu and Unnigida (Kannada), etc. Chemical constituent of L. camara is a rich source of bioactive compounds, viz., flavones, Isoflavones, flavonoids, anthocyanins, coumarins, lignans, catechins, Isocatechins, alkaloids, tannin, saponins, and triterpenoid.

Fig. No. 01: Lantana camara (a) Leaves; (b) Flowers; (c)Fruits
Table no. 01: Plant profile of Lantana camara
Biological classification:
Table no. 02: Biological classification of Lantana camara
MICROBIAL ACTIVITY OF LANTANA CAMARA: [6]
Antimicrobial activity-
In general, microorganisms are divided into bacteria, fungi, viruses, protozoa. All four groups can cause infectious diseases in animals and humans, though bacteria cause the majority of infections. Most antibiotics are active against bacteria Although for the proper treatment of serious infections cultures antibiotic sensitivities are required, antibiotic therapy is often empiric, with etiology being inferred from the clinical features of a disease.
Bacteria:
Bacteria are divided by a staining reaction into the gram positive and the gram negative; each group comprises a wide variety of different species. Tubercle bacilli are the most important acid-fast organisms. Certain antibiotics, such as erythromycin and vancomycin, are effective only against gram-positive bacteria. Others, such as cephalosporins, quinolones, tetracyclines, and chloramphenicol, are effective against both gram-positive and gram-negative bacteria and are referred to as broad-spectrum antibiotics. According to gram stain it is divided into gram positive and gram negative. Gram-negative bacteria are those that do not retain crystal violet dye in Gram staining protocol. Gram-positive bacteria will retain the dark dye after an alcohol wash. In a Gram stain test, a counter stain (commonly safranin) is added after the crystal violet, colouring all Gram-negative bacteria a red or pink colour. The test itself is useful in classifying two distinctly differently types of bacteria based on structural differences in their cell walls.
Fungi:
Fungi may be divided on the basis of their pathogenicity into true pathogens, such as Histoplasma, and opportunistic pathogens, such as Candida, Aspergillus, and Cryptococcus. The opportunistic occur mainly in debilitated and immunocompromised patients. Although hundreds of antibiotics active against fungi have been isolated, clinically useful ones are amphotericin B, nystatin, griseofulvin and the azole antifungals. As broad-spectrum antifungals, amphotericin is active against systemic infections and nystatin against local infections. Griseofulvin is active against fungi that cause skin diseases Azoles, which constitute perhaps the largest group of antifungals, are broad- spectrum and synthetic.
MATERIAL AND METHOD:
Material:
The leaves and roots of Lantana camara were collected from the Medicinal garden of Charak institute. Ethanol is purchased from Rankem laboratory, Sodium hypochlorite is purchased from Prime Dental and Nutrient agar is purchased from Sigma Aldrich.
Method:
Extraction:
The leaves and roots of Lantana camara were shade dried for two weeks at room temperature Conditions. They were then grind separately using an electrical grinder to obtain fine powder and finally kept in separate plastic bags at room temperature till the day of extraction.
Ethanol extraction:
Cold extraction method was used for extraction. 100g of leaf powder was soaked in 400 ml of 70% ethanol for one week: the separated extract was then filtered through whatmans No. 1 filter Paper. The filtrate was then concentrated to obtain the paste using rotary evaporator. The thick Extracted mass was then dried at room temperature. Thereafter, 0.25g. 0.5g and Ig respectively of the paste were weighed and each dissolved in 10 ml of tap water to make 2.5%, 5% and 10% Respectively, and kept in a refrigerator until use.
Preparation of test microorganisms:
The bacterial and fungal strains, Pseudomonas syringae and Pythopthorainfestans were used for Testing antibacterial and antifungal activity. The microorganisms used in this investigation were isolated from diseased tomato fruits exhibiting the pathological symptoms and identified.
Isolation of test microorganisms:
Pseudomonas syringae was isolated from a tomato fruit exhibiting its symptoms; slightly raised black specks surrounded by a green-yellow halo. 5mm by 5mm pieces were cut from the diseased parts of tomato, thereafter they were dipped into 1% Sodium hypochlorite, followed by 70% ethanol and lastly washed in distilled water three times. The sterile plant parts were then inoculated in Nutrient agar and incubated for fortyeight hours at 24oC. After 48 hours of incubation, bacterial colonies were observed on the medium; a loopful was scooped and streaked on the Nutrient agar plates for sub culturing and incubation. Pure culture was made by re-streaking a loopful of bacteria colony on the Nutrient agar and incubated for 48 hours. The same methodology for bacteria was adopted when isolating Phytopthorainfestans except that PDA plates were used instead of nutrient agar and incubated at temperature of 240 for two days.
Determination of antimicrobial activities:
Disc diffusion method was used and the susceptibility test determined by measuring the growth inhibitory zone on the nutrient agar and Potato dextrose agar around the conventional paper disc.
Antibacterial assay:
Twelve Nutrient Agar plates were prepared by pouring 20 ml of molten media into sterile petri
dishes. After solidification of media 100 ul of P. syringae suspensions were dispensed to each plate using a micropipette and spread with the aid of a sterile spreader to achieve uniform growth. The plates were then allowed to dry. Four Whatman No.1 filter paper discs each 6mm in diameter were impregnated with leaf extracts of different concentrations (2.5%, 5% and 10%) and introduced into the plates at equidistant. The sterile disc impregnated with distilled water was used as control. All the plates were incubated at 28oC for 24 hours under static conditions. After 24 hours the zone of growth inhibition around the paper discs were measured and recorded in millimeters.
Antifungal assay:
Twelve PDA plates were prepared by pouring 20 ml of molten media into sterile petri dishes. After solidification of the media 100 ul of P. infestans suspension was dispensed to each plate using a micropipette and it was spread with the aid of a sterile spreader to achieve uniform growth, the plates were allowed to dry. Thereafter, four Whatman No.1 filter paper disc 6mm diameter, that were impregnated with different concentrations of leaf extract were introduced to each plate at equidistant. The sterile disc impregnated with distilled water was used as control. All the plates were incubated at 28oC for 24 hours under static conditions and the zones of growth inhibition were measured and recorded in millimeters. All the assays were performed.
RESULT AND DISCUSSION:
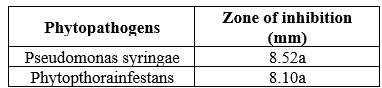
The results presented in table 1.1 show that different concentrations of leaf
extract of Lantana camara inhibited the growth of Pseudomonas syringae and Phytopthora
infestans; however, there was no significant difference between the two tested microorganisms.
Table 1 Antimicrobial effects of Ethanol leaf extracts of lantana camara on growth of Pseudomonas syringae and Phytopthora infestans
Plates 1, 2, 3 and 4 show the inhibition of growth diameter formed on plates inoculated with Pseudomonas syringae and Phytopthora infestans around paper discs impregnated with different concentrations of leaf extract of 2.5%, 5% and 10% respectively. The zone of inhibition was highest at 10% leaf extract concentrati
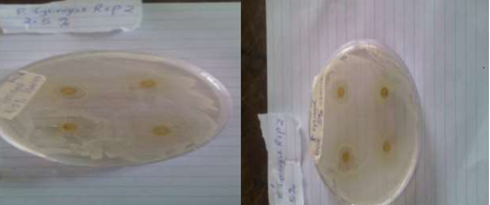
Plate 1: Zone of inhibition of leaf ethanolic extracts on P.syringae at A 2.5% concentration and B 5% concentration.

Plate 2: Zone inhibition of leaf ethanolic extract on P. syringae at 10% concentration.
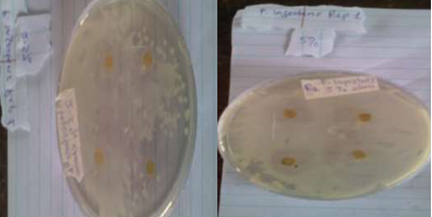
Plate 3: Zone inhibition of leaf ethanolic extract on P. infestans at A- 2.5% concentration and B- 5% concentration.

Plate 4: Zone inhibition of leaf ethanolic extract on P. infestans at 10 concentration.
CONCLUSION:
The present experimental findings have revealed that the leaf extract of the Lantana camara plant possesses inhibitory and antimicrobial activities with a more pronounced effect on the growth of Pseudomonas syringae than Phytopthora infestans. The study has also revealed that this variety of Lantana camara plant contains phytochemical compounds such as saponin, flavonoids, tannins, and cardiac glycosides, which may be responsible for the growth inhibitory effects exhibited by the leaf extracts of this plant. This study provides the basis for developing the plant products into useful pesticides that may be used to control soil pathogenic bacteria and fungi. We are successfully done study on LANTANA CAMARA for antimicrobial activity using different Bacteria and Fungi. We have found the zone of inhibition is high in bacteria rather than fungi. So, it proves that Lantana camara shows Antimicrobial Activity.
REFERENCES:
- Ashish, S., Sadaf, Q., Kavita S., & Noor, A.K. (2011). Antimicrobial Agrios, G. N. (1997) Plant Pathology. 4th edition. Academic press, New york, pp, 226.
- Sukul S, Chaudhuri S. Antibacterial natural products from leaves of Lantana camara L. Indian J Pharm Sci 2001;63:20-3
- Barreto, F.S, Sousa, E.O., Campos, A.R., Costa, J.G.M, & Rodrigues, F.F.G. (2010).Antibacterial Activity of Lantana camara Linn and Lantana montevidensis Brig Extracts from Cariri-Ceará, Brazil. Journal of Young Pharmacists, 2(1), 42-44.
- Sharma P, Shrivastava B, Sharma GN, Jadhav HR. Phytochemical and pharmacological profile of Lanata camara: An overview. J Adv Pharm Educ Res 2013;3(4):294-305.
- Sharma B, Kumar P. Bioefficacy of Lantana camara L. Against Some Human Pathogens. Indian J Pharm Sci 2009;71(5):589-93.
- Lonare MK, Sharma M, Hajare SW, Borekar VI. Lantana camara: Overview on toxic to potent medicinal properties. Int J Pharm Sci Res 2012;3(9):3031-5.
- Ashwini S, Girish K. Phytochemical screening and antibacterial activity of methanolic leaf extract of Coleus aromaticus Benth. Int J Res Pharm Sci 2014;5(4):270-4.
- Elkhalfi, B., Essari, A., Serrano, A., & Soukri, A. (2013). Antibacterial activity of plant methanolic extracts on a field isolate of Pseudomonas syringaepv tomato from the Casablanca region (Morocco). Journal of Advances in Bioscience & Biotechnology, 4, 1-10.
- Sanjeeb, K., Guarav, K., Loganathan, K., Kokati, V., & Bhastara, R. (2012). A Review onmedicinal properties of Lantana camara. Research Journal of Pharmacy and Technology,5(6), 711-715


 Anshika Rathore *
Anshika Rathore *
 B. N. Birla
B. N. Birla
 Vivek Yadav
Vivek Yadav
 Aman Karma
Aman Karma
 Sonali Patidar
Sonali Patidar








 10.5281/zenodo.11098200
10.5281/zenodo.11098200