Abstract
The limited water solubility of pharmacologically active molecules poses a constraint on their pharmacological potential. Despite the non-negotiable solubility parameter, various strategies are employed to augment bioavailability. Solubility and bioavailability are pivotal for optimal therapeutic effects at the target site. Overcoming the challenge of enhancing drug bioavailability and solubility is a significant focus in pharmaceutical formulations. In order to enhance the solubility of low water-soluble medicinal products, by means of a variety of techniques including particle size reduction, nanonization, pH correction, solid dispersion, complexation, cosolvency and hydrotropy. The objective is to elucidate techniques for effective absorption and heightened bioavailability.
Keywords
Bio-availability, solubility, bioequivalence, hydrophobic drug.
Introduction
Bioavailability refers to the rate at which absorption occurs and extent of the drug remains in its unaltered form. It’s a crucial parameter for achieving optimal drug concentration in systemic circulation, leading to a pharmacological response1 The efficacy of a drug depends not just on its bioavailability, but also on the solubility of its molecules1. Solubility, which according to various concentration expressions like parts, percentage, molarity, molality, volume fraction and mole fraction are the highest quantities of solutes that can be dissolved in a given solvent.2.
Solubility plays a essential role in drug liberation, impacting bioavailability. For effective drug absorption, the drug must be in solution form at the site of absorption. Drugs with poor bioavailability often exhibit inadequate aqueous solubility, slow dissolution rates, instability in physiological pH, limited permeation through biological membranes, and extensive first-pass metabolism3. Enhancing the bioavailability of drugs with low water solubility, Higher doses may be required, leading to adverse reactions and increased therapy costs, without achieving the desired pharmacological response4.
Bioavailability enhancement strategies involve alterations in disintegration and dissolution. Factors like incomplete absorption and first-pass metabolism contribute to the general decrease in bioavailability, which can vary among patients. Poor solubility of Hydrophilic solid formulations can lead to capacity limitations in dissolution and absorption process5. Numerous investigations have focused on Improving the bioavailability of inadequately soluble substances or absorbed drugs, particularly when administered orally. Natural and synthetic lipids are commonly explored to enhance the oral bioavailability of a substance. Drugs with low solubility can be formulated in three distinct ways to overcome the challenges of poor absorption6.
- Crystalline solid formulations
- Amorphous formulations
- Lipid formulations
Crystalline solid formulations
Enhancing the dissolution rate of a drug can be achieved through various strategies, including altering physiochemical properties like salt formation, and micronization of the crystalline compound. Micronization, performed with an air-jet mill, can yield a particle size of 2-5, while nanocrystal technology, using methods like ball-milling and dense gas technologies, can further reduce particle size to 100-200nm. However, these approaches have limitations, such as the impracticality of salt formation for neutral compounds and potential challenges with very fine powders due to poor wettability and handling difficulties.6
Amorphous formulations
Amorphous formulations, such as “solid solutions”. Can be created through techniques like spray drying and melt extrusion. These formulations often incorporate surfactants and polymers to enhance surface activity during dispersion enhancing the bioavailability of these drugs typically employs various approaches, including formation of complexes with cyclodextrins, creating polymeric conjugates, and utilizing nanoparticles such as solid lipid nanoparticles (SLN) employing permeation enhancers, and incorporating surfactants6.
Lipid formulations
Recent attention has been directed towards lipid-based carrier systems, with a prevalent method involving the integration of limited aqueous soluble active components into inert vehicles. These vehicles include oils, surfactant dispersions, solid dispersions, emulsions, micro emulsions, Nano emulsions, self-emulsifying formulations (SEF), and liposomes6.
Biopharmaceutical Classification System (Bcs)
BCS serves as a scientific framework designed to categorize drug substances by evaluating their aqueous solubility and membrane permeability. The USFDA introduced BCS classification guidance to enhance the efficiency of API
Purpose and Goals of BCS
- BCS recommends methods for classifying drugs
- Supports the granting of a biowaiver for in vivo bioavailability and bioequivalence studies, based on the approach of BCS
- Optimizing formulation development
- Regulatory decision making
- Resource efficiency.
When integrating the dissolution behaviour of a drug in an in vitro setting., BCS considers three primary factors: solubility, penetrability, and disintegration rate. A drug substance is deemed highly permeable if its degree of absorption in the intestines is 90% or greater. The dissolution properties of the drug product observed in an in vitro setting. it is classified as poorly permeable.
According to BCS, drugs are organized into four main classes based on their solubility and permeabilitybility
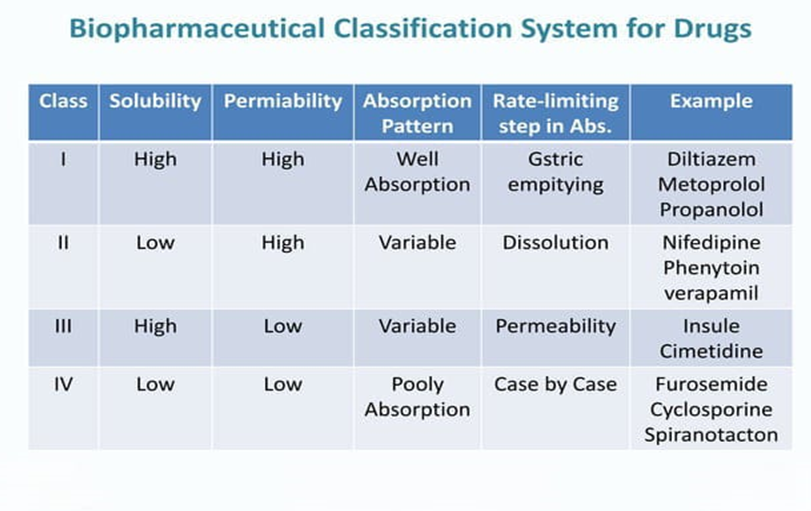
Lack of solubility and permeability may lead to a bioavailability problem. There are solubility problems with most of these compounds. An important parameter of absorption is the rate at which the drug breaks down from its dosage form. Overall, BCS serves as a valuable tool in pharmaceutical industry for rational drug development, regulatory decision support and resource optimization7.
Solubility Enhancement Techniques
The pharmaceutical industry faces challenges in developing various drug formulations due to poor dissolution profiles of certain compounds. Numerous solubilisation techniques exist to enhance both solubility and permeability7.
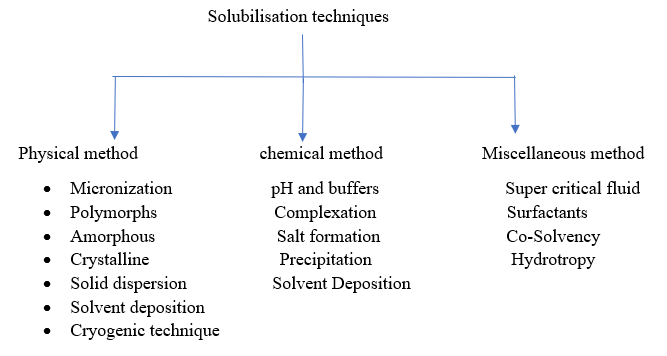
Physical methods
a) Micronization
Reducing particle size, it is a technique known as micronization or micro-milling, which increase the solubility rate of less water soluble drug by creating significant surface area. This process, often achieved through methods like Spray drying or air attrition for example, fluid energy mill, jet mill, it targets the particle sizes from 1 to 10 microns. However, micronization may not be suitable for high dose drugs as it does not alter the drugs saturation solubility. Additionally, there’s a drawback as micronized products tends to agglomerate, reducing their effective surface area for dissolution7.
b) Polymorphs
Polymorphs play a crucial role in improving the bioavailability of barely soluble drugs. Different crystalline forms of drug can exhibit distinct solubilities and dissolution rates. By selecting or engineering polymorphs with improved solubility characteristics, it is possible to enhance the drug’s bioavailability. The choice of polymorph can influence factors such as stability, dissolution kinetics and overall performance in the body, providing opportunities to optimize drug formulations for better absorption and therapeutic efficacy. Polymorphs exhibit varying melting points, influencing their solubility. The solubility differences among polymorphs. Typically range only 2-3 times, attributed to slight variations in free energy8.
c) Amorphous
In amorphous forms, atoms or molecules are randomly arranged exhibiting higher thermodynamic energy compared to their crystalline counterparts. This increased energy typically results in greater solubility and faster dissolution rated8.
d) Crystalline
Crystalline compounds can contain either stoichiometric or non- stoichiometric adducts, su ch as inclusion that involve trapping solvent molecules within the crystal lattice. Stoichiometric adducts are commonly known as solvates, form molecular complexes where crystallizing solvents molecules are incorporated into specific sites within the crystal lattice. When water is the incorporated solvent, the complex is referred to as hydrate. A compound lacking water within its crystal structure is termed anhydrous. Anhydrous forms generally exhibit higher aqueous solubilities compared to hydrate forms9.
e) Solid dispersion
Solid dispersion stands out as a widely employed approach to enhance drug solubility in water. This process involves dispersing one or more drugs into an inert polymer or solid matrix. Polymers play a crucial role by ensuring drug stability during storage, preventing crystallization, and maintaining saturation in the dissolution media. The selection of polymers is a crucial step, considering the factors like glass transition temperature, hygroscopicity, solid capacity of solutions, and solubilisation. The chosen polymer must be inert, non-toxic, drug compatible and thermostable particularly for fusion based solid dispersions with a low melting point. The primary methods employed for creating solid dispersions are melting and solvent evopuration9.
Advantages of solid dispersions include
Enhanced dissolution rate and absorption, along with decreased pre systemic metabolism. It facilitates the transformation of drugs from liquid to solid form, as seen in the incorporation of substance like clofibrate and benzyl benzoate in to PEG6000 to create solid dispersions. Additionally, it prevents polymorphic changes, averting bioavailability issues observed in cases like nabilone and PVP dispersions9.
Disadvantages a significant drawback is instability, leading to changes in crystallinity and a decline in dissolution rate overtime. An is the crystallisation of Ritonavir from a supersaturated solution within a solid dispersion system9
f) Cryogenic Technique
The cryogenic method involves creating nanostructured amorphous drug particles with high porosity at extremely low temperatures. Various factors, such as the type of injection device, nozzle location, and cryogenic liquid composition (hydro?uoroalkanes, N2, Ar, O2, organic solvents), characterize cryogenic inventions. Post cryogenic processing, drugs are powdered through drying methods like spray freeze drying and atmospheric freeze drying10.
In the "Spray Freezing onto Cryogenic Fluids" technique, drug and carrier are dissolved in water, atomized above a boiling fluorocarbon refrigerant, and sonication enhances dispersion. "Spray Freezing into Cryogenic Liquids (SFL)" forms nanostructured drug aggregates with good surface area and wettability, employing liquid-liquid encroach and intense atomization. "Spray Freezing into Vapor over Liquid (SFV/L)" uses cryogenic fluid vapours to freeze the drug solution, producing fine particles with high wettability. "Ultra-Rapid Freezing (URF)" involves using solid cryogenic substances for rapid freezing and subsequent lyophilization, resulting in micronized drug powder10. The "Lyophilisation monophase solution technique" is an alternative involving TBA Utilized as an organic cosolvent for the dissolution of hydrophobic drugs, TBA is combined with a hydrophilic carrier in water, followed by lyophilization. TBA, characterized by its high vapor pressure, melting point, and low toxicity, proves to be an excellent medium for freeze-drying. This method effectively improves the dissolving rate of inadequately miscible drugs such as budesonide, salmeterol, ketoprofen, and
nitrendipine by forming complexes with ?CD and HP?CD10.
Spray Freezing onto Cryogenic Fluids
The process, pioneered by Briggs and Maxwell, involves dissolving drugs and carriers (mannitol, maltose, lactose, inositol, or dextran) in water. The mixture is atomized above a boiling fluorocarbon refrigerant, with optional sonication for improved dispersion.
Cryogenic liquid spray freezing (CLS-F)
SFL particle engineering creates amorphous nanostructured drug aggregates with featuring a substantial surface area and wettability. It involves the collision of solution with the cryogenic liquid in a liquid –liquid impingement, resulting in rapid freezing. The lyophilization is applied to freeze dried particles for obtaining a dry form, micronized powders.
Spray Freezing into Vapor over Liquid (SFV/L)
SFV/L freezes Generating finely divided drug particles with elevated wettability. is achieved by exposing drug solutions to cryogenic fluid vapours. Automized droplets are freezed in the vapour phase prior to reaching the cryogenic liquid, facilitating nucleation and the growth of fine drug particles as the solvent solidifies.
Ultra-Rapid Freezing (URF)
URF is an innovative cryogenic technology creating nanostructured drug particles with enhanced Improved surface area and morphology are achieved by applying drug solutions to a solid cryogenic substrate, leading to instantaneous freezing. Subsequent lyophilization results in the formation of micronized drug powder with enhanced solubility10.
g) Solvent deposition
Solvent deposition is a technique used in pharmaceuticals to enhance the bioavailability of drugs. By dissolving a drug in a suitable solvent and then depositing it onto a carrier material, the drug's solubility and dissolution rate can be improved. This method aids in creating formulations with increased absorption and bioavailability, ultimately enhancing the therapeutic effectiveness of the substance in the body8. This approach involves dissolving poorly water-soluble drugs in an organic solvent, such as alcohol, and placing them on an inert, water attracting solid matrix like starch or microcrystalline cellulose. Subsequently, the solvent is evaporated. An instance of this technology is the enhanced dissolution speed of piroxicam achieved through liquid-solid compacts. Likewise, the dissolution speed of the inadequately soluble drug indomethacin was increased using the same liquid-solid compacts method.10
2.Chemical methods
pH and buffers
Altering the pH is a potential method for improving the dissolution rate of poor aqueous soluble drugs. It's crucial to consider the drug's nature and the capacity of buffer of the chosen pH. Excipients that raise the environmental pH above the pKa of weakly acidic drugs can improve their solubility. Similarly, alkaline excipients Weakly basic drugs' solubility can be increased by alkalizing agents. (Is possible through methods such as utilizing buffers. Example, like Flufenamic acid, Mefenamic acid, Niflumic acid, Diclofenac sodium, and Meclofenac sodium, illustrates this approach. Buffers are essential for elevating the bioavailability poorly soluble drugs, as they help to maintain a specific pH in the gastrointestinal tract. This controlled pH environment contributes to improving the drugs solubility, leading to improved absorption. For instance, if a drug is weakly acidic, a buffer with a pH higher than its pKa can increase its solubility. Conversely, for weakly basic drugs, an alkaline buffer may enhance solubility. The use of buffers helps optimize conditions for drug dissolution, absorption, and ultimately, bioavailability11.
Complexation
This is the formation of an un-bonded entity with a specific stoichiometry as molecules come together. Stacking and inclusion are two forms of complexation that improve drug solubility in water. Non-polar components, counteracting water's strong hydrogen bonding, may aggregate to reduce contact, forming homogenous self-associations or mixed complexes. In an inclusion complex, a non-polar molecule (guest) is enclosed in the non-polar cavity of another molecule or group (host), minimizing water contact. Cyclodextrins are frequently employed as host molecules in inclusion phenomena11.
Salt Formation
Salts exhibit enhanced solubility and dissolution compared to the original drug. Typically, a stable alt formation requires a minimum 3-unit difference between the pKa values of the functional group and its counter-ion. Drugs with a mild acidic or basic nature, like penicillin or atropine, often undergo salt formation, a straightforward chemical modification impacting various drug properties without altering the core structure. An ideal salt is chemically stable, non-hygroscopic, poses no processing challenges, dissolves readily in pharmaceutical formulations with a solid dosage presentation. (unless a delayed dissolution is intentional), and offers good bioavailability. Salt formation, incorporating proton exchange or neutralization reactions, is theoretically applicable to any compound with acidic and/or basic properties12.
Precipitation
This method involves dissolving a poorly water-soluble drug, like cyclosporine, in an organic solvent and rapidly mixing it with a non-solvent to induce the drug precipitation into nano-sized particles. The resulting product, known as a hydrosol, is utilized to create Nano-suspensions, such as those of Danazol and Naproxen, through a precipitation technique. The drug solution is injected into water, serving as a bed solvent, with efficient stirring to ensure the substance precipitates as nanocrystals. These nanocrystals can be separately extracted from the solution through filtration and subsequently air-dried13.
3.Miscellaneous methods
Hydrotropy
Hydrotropy serves as an effective approach to elevate the solubility of substances by introducing a substantial amount of a secondary solute, known as a hydro trope. Unlike co-solvency, where polarity is minimized, Hydrotropy concentrates drug molecules, showcasing its mechanistic distinction. Different drugs with limited water solubility, including ketoprofen, aceclofenac, and amoxicillin, benefit from hydrotropic solubilization without necessitating chemical alterations or the use of organic solvents. Hydro-tropes, comprising hydrophilic and hydrophobic groups, play a crucial role in this process, with their efficacy determined by the balance between these components. Nicotinamide and its derivatives, like N, N-diethyl nicotinamide, exemplify notable hydrotropes. These organic amphiphilic molecules, akin to surfactants, can aggregate in aqueous solutions above the minimum hydrotropic concentration (MHC). Hydrotropy, recommended for its pH-independent solvent characteristics and high selectivity, stands out compared to methods like co-solvency and micellar solubilization. However, a drawback is the potential for hydrotropes to aggregate in solution, diminishing their ability to improves the drug solubility in water14.
Cosolvency
The addition of a water-miscible solvent, known as a cosolvent, is a common method Enhancing the solubility of pharmaceutical compounds. with low water solubility. Co-solvents, such as PEG 300, propylene glycol, or ethanol, create solutions with improved solubility for substances with low water solubility. This technique is widely employed in various formulations, including solids and liquids. Concentrations ranging from 5-40% of solid binary systems with polyethylene glycol 6000 have been utilized to improve the solubility of meloxicam. Cosolvent formulations can be administered orally or parenterally, with parenteral forms often requiring dilution with an aqueous medium. While Cosolvency is effective, it is mainly utilized in parenteral dosage forms due to surfactant irritation and the low toxicity of many cosolvents. Frequently employed low toxicity cosolvents for parenteral use encompass Propylene glycol, ethanol, glycerine, and polyethylene glycol. Despite its widespread use, cosolvency may not dramatically increase bioavailability as poorly soluble drugs can precipitate upon dilution, requiring dissolution for oral absorption. By combining cosolvents with additional solubilization techniques and pH adjustment can further enhances the dissolution characteristics of substances with low solubility15.
extent of intestinal absorption is 90% or higher otherwise Surfactants
Surfactants exhibit a molecular structure with both hydrophilic (polar) and hydrophobic (nonpolar) components, making them amphiphilic. These molecules operate at interfaces, enhancing the solubility of challenging compounds. Usually, surfactants comprise hydrocarbon segments connected to polar groups, which may include heteroatoms like N, P, S, or O, along with functional groups like sulphates, amides, amines, alcohols, thiols, esters, acids, sulfonates, and phosphates15. Surfactants can be graded into different types:
- Anionic: Dissociates in water into amphiphilic anions and cations, commonly exemplified by SLS.
- Cationic: Capable of dissociating into five amphiphilic cations and anions, these substances are commonly employed as disinfectants and preservatives, with compounds such as cetrimide and benzalkonium chloride being notable examples.
- Non-ionic: Does not undergo dissociation in water, possessing a non-dissociable hydrophilic groups like amide, ester, ether, alcohol, and phenol. Examples include poloxamer and poly-sorbate, known for their lower irritation compared to anionic or cationic surfactants.
- Amphoteric: Can exhibit non-ionic, anionic, or cationic behaviour depending on water pH conditions, with alkyl betaine as an example18.
Surfactants, when present, decrease surface tension and enhance drug solubility in organic solvents by being attracted to the liquid's surface area, altering surface tension17.
Supercritical fluids
Supercritical fluids (SCFs) display characteristics of both liquids and gases due to exceeding their critical temperature (Tc) and critical pressure (Tp). Supercritical fluid (SCF) techniques enable the micronization of drug particles to sub-micron levels (4). Exceeding critical temperature (Tc) and pressure (Tp), these fluids become highly compressible, in close proximities to their critical temperature19. These compressibility enables even slight pressure variations to notably affect the fluids density and mass transport properties, influencing its solvent power. After drug particles dissolve in supercritical fluid (SCF), they can undergo recrystallization at considerably smaller sizes. Carbon dioxide and water are commonly employed as supercritical fluids, and this process can yield nanoparticulate suspensions ranging from 5 to 2,000 nm in diameter. For instance, the water solubility of itraconazole can be enhanced with the use of the water-soluble polymer HPMC through supercritical fluid processing20.
CONCLUSION .
This article emphasizes that the solubility of a drug is a pivotal factor influencing both its formulation and therapeutic effectiveness. The article underscores that solubility significantly impacts the development of drug formulations, especially for poorly water-soluble drugs. The oral absorption of a drug is significantly influenced by its dissolution, and the article discusses various techniques that can be used, either independently or in combination, to improve the solubility of the drug. The challenges posed by the solubility of many drugs can affect their bioavailability, making solubility enhancement imperative. The article suggests that various techniques mentioned can now be utilized to increase the dissolution characteristics of substances with low solubility.
REFERENCE
- Ramesh V, Meenakshi S, Jyothirmayee N, Bullebbai M, Noorjahan SK, Rajeswari G, Babu NG, Madhavi D. Enhancement of solubility, dissolution rate and bioavailability of BCS Class II Drugs. International Journal of Pharma and Chemical Research. 2016 Apr;2(2):2395-3411.
- Mehta S, Joseph NM, Feleke F, Palani S. Improving solubility of BCS class II drugs using solid dispersion: a review. Journal of drug delivery and therapeutics. 2014 May 15;4(3):7-13.
- Gupta KR, Dakhole MR, Jinnawar KS, Umekar MJ. Strategies for improving hydrophobic drugs solubility and bioavailability. Int J Pharm Chem Anal [Internet]. 2023;10(3):164–74.
- Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. The AAPS journal. 2009 Dec;11:740-6.
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. International Scholarly Research Notices. 2012;2012
- Sikarra D, Shukla V, Kharia AA, Chatterjee DP. Techniques for solubility enhancement of poorly soluble drugs: an overview. JMPAS. 2012;1:1-22.
- Ainurofiq A, Putro DS, Ramadhani DA, Putra GM, Santo LD. A review on solubility enhancement methods for poorly water-soluble drugs. Journal of Reports in Pharmaceutical Sciences. 2021 Jan 1;10(1):137-47.
- Gaikwad SS, Mhalaskar RS, Mahale YD, Jain NP. Review on: solubility enhancement of poorly water soluble drug. Indo Am J Pharm Res. 2014;4(11):5530-41.
- Khan AD, Singh L. Various techniques of bioavailability enhancement: a review. Journal of Drug Delivery and Therapeutics. 2016 May 15;6(3):34-41
- Gupta AK, Sehrawat SK. Bioavailability enhancement of poorly water soluble drugs. IJRLS. 2011 Mar;2(3):640-50
- Bhakay A, Rahman M, Dave RN, Bilgili E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation–Processing aspects and challenges. Pharmaceutics. 2018 Jul 8;10(3):86
- Devhare L, Kore PK. A recent review on bioavailability and solubility enhancement of poorly soluble drugs by physical and chemical modifications. Research chronicle in health sciences. 2016 Sep 30;2(5):299-308.
- Gupta S, Kesarla R, Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. International Scholarly Research Notices. 2013;2013
- Ramesh V, Meenakshi S, Jyothirmayee N, Bullebbai M, Noorjahan SK, Rajeswari G, Babu NG, Madhavi D. Enhancement of solubility, dissolution rate and bioavailability of BCS Class II Drugs. International Journal of Pharma And Chemical Research. 2016 Apr;2(2):2395-3411.
- Mehta S, Joseph NM, Feleke F, Palani S. Improving solubility of BCS class II drugs using solid dispersion: a review. Journal of drug delivery and therapeutics. 2014 May 15;4(3):7-13
- Kesarwani P, Rastogi S, Bhalla V, Arora V. Solubility enhancement of poorly water soluble drugs: a review. International Journal of Pharmaceutical Sciences and Research. 2014 Aug 1;5(8):3123.
- Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. International Scholarly Research Notices. 2012;2012.
- Chaudhary A, Nagaich U, Gulati N, Sharma VK, Khosa RL, Partapur MU. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: A recent review. J Adv Pharm Educ Res. 2012;2(1):32-67.
- O'driscoll CM, Griffin BT. Biopharmaceutical challenges associated with drugs with low aqueous solubility—the potential impact of lipid-based formulations. Advanced drug delivery reviews. 2008 Mar 17;60(6):617-24.


 Adithi P*
Adithi P*
 Chaithra K
Chaithra K
 Chandana N
Chandana N
 Deepthi R
Deepthi R
 Dhruva R Nadig
Dhruva R Nadig


 10.5281/zenodo.13293111
10.5281/zenodo.13293111