Abstract
The emergence of parasite resistance and the low oral bioavailability of antimalarial drugs are the two main problems that hinder the clinical success of antimalarial chemotherapy. The prophylactic efficacy of 17C91, a carbamate prodrug of atovaquone (ATQ), was studied in a severe combined immunodeficient mouse model of Pneumocystis carinii pneumonia (PCP) at an oral dose equivalent to 100 mg ATQ per kg body weight per day. The activity of atovaquone in combination with another antimalarial agent should be studied. The aim of this study was to improve the solubility and bioavailability of the Atovaquone tablet, using an internal biosynthesizer in a ternary solid dispersion system containing hydrophilic polymers with different concentrations of biosurfactant. Atovaquone is an antimalarial agent and belongs to the class IV biopharmaceutical classification system. The developed method is simple, sensitive and reproducible and can be used for routine analysis of atovaquone in bulk and tablet dosage forms. Solid nanoparticles of atovaquone were successfully produced by electrospray and characterized for their particle size and flow properties. Atovaquone is measured at 251 nm in methanol. The Beer’s law was observed in the drug concentration range of 1–10 ?g/mL. Food likely increasing its absorption of Atovaquone by increasing its solubility in the gut lumen. These results suggest that liposome loaded ATV is more efficacious than the free drug against Leishmania infantum in this murine model.
Keywords
Atovaquone (ATQ), Solid dispersion, Biosurfactant, Bioavailability, Ternary system, Pneumocystis carinii pneumonia (PCP).
Introduction
Atovaquone is a hydroxynaphthoquinone originally developed for its potent antimalarial activity. Since these agents are often effective against other protozoa, atovaquone has also been evaluated for use against P.Carinii. Its mechanism of action against P.Carinii is not fully understood. In Plasmodium species, atovaquone appears to block electron transport in the cytochrome complex (Complex III) [1]. Atovaquone is very lipophilic and, therefore, shows a low solubility in water [2]. The current commercial formulation of atovaquone, a fine particle suspension, and shows limited gastrointestinal absorption, resulting in plasma concentrations that are not proportional to the administered dose, and must be taken with meals to maximize absorption [3]. In asymptomatic HIV-infected patients receiving atovaquone with food, there was considerable within-subject and between-subject variation in plasma concentrations of the drug. An initial pharmacokinetic study in healthy volunteers under fasting conditions with a tablet formulation of atovaquone showed that absorption was slow and erratic and that AUC increased less proportionally with doses greater than 450 mg (unpublished data)[4]. This review covers its pharmacology, mechanism of action, clinical trials and current applications. Thus, atovaquone will be a useful option for the treatment of patients with mild to moderate PCP who are intolerant or insensitive to cotrimoxazole, especially if the increase in the plasma concentration of the first drug with the suspension improves the rates of answer Atovaquone should also be considered as a promising agent for the treatment of toxoplasmosis. The assays published for atovaquone are limited to complex gas chromatographic methods and high-performance liquid chromatography (HPLC) methods with multiple sample preparation and extraction procedures [5, 6]. Non-ionic surfactants were used for enhancement of solubility of poorly water-soluble drug in the range of 5%w/w to 20%w/w. Synthetic surfactants are non-biodegradable. In recent years novel biotechnological products are produced from various microorganisms through fermentation. Bio surfactants are produced from specific microorganisms with economic substrates such as waste fried oils, molasses etc., in a suitable media and environmental conditions [7, 8]. Atovaquone, whose chemical name is 5-2-[4-(4-chlorophenyl) cyclohexyl]-3-hydroxy-1, 4-naphthoquinone, is a hydroxy-1,4-naphthoquinone, an analogue of ubiquinone, with antipneumocystic activity . Atovaquone is extremely active (in animals and in vitro) against Pneumocystis carinii, Plasmodia and tachyzoites and cystic forms of Toxoplasma gondii. Atovaquone is the Trans isomer of 2-[4-(4-chlorophenyl) cyclohexyl]-3-hydroxy-1, 4-naphthoquinone, whose synthesis, activity and uses are described in patent no. US5053432 and EP0362996 [9].
2. Classification
- Antiprotozoal agent
- Antimalarial agent
2.1 Antiprotozoal agents: Antiprotozoal agents are a class of drugs used to treat infections caused by protozoa, which are single-cell organisms, belonging to a group of parasites. Protozoans typically are microscopic and similar to plants and animals as they are eukaryotes, thus having a clearly defined cell nucleus. Antiprotozoal are effective in treating diseases such as Afric Trypanosomiasis (sleeping sickness) and Chagas disease (an inflammatory, infectious disease caused by the parasite Trypanosoma cruzi).
Symptoms:
- Fever
- Severe headaches
- Irritability
- Extreme fatigue (tiredness)
- Swollen lymph nodes
Side Effects:
- Diarrhoea
- Nausea
- Vomiting
- Abdominal pain
- Anorexia
Use: Antiprotozoal agents are used in conditions such as:
African Trypanosomiasis (sleeping sickness)
Chagas disease (caused by a parasite known as Trypanosoma cruzi, is a vector-transmitted disease affecting animals and humans, commonly known as American Trypanosomiasis)
Refractory prostate cancer.
2.2 Antimalarial drugs: Antimalarial agents are drugs used to prevent or treat malaria, a disease caused by Plasmodium parasites transmitted to humans through the bites of infected Anopheles mosquitoes. These agents work by targeting different stages of the parasite's lifecycle, including those in the liver and blood.
Types:
1. Chloroquine
- Mechanism: Interferes with the parasite's ability to digest haemoglobin in red blood cells.
- Uses: Primarily used for the treatment and prevention of malaria caused by Plasmodium falciparum and other Plasmodium species.
2. Artemisinin and its derivatives (Artemether, Artesunate, Artemotil)
1. Mechanism: Acts by generating reactive oxygen species that damage the parasite's Proteins and lipids.
2. Uses: Artemisinin-based combination therapies (ACTs) are the standard treatment for P. falciparum malaria, especially in areas with resistance to chloroquine.
3. Quinine and Quinidine
- Mechanism: Disrupts the parasite's ability to digest haemoglobin by inhibiting heme polymerization.
- Use: Often used in severe malaria or in combination with other drugs.
4. Mefloquine :
- Mechanism: Interferes with the parasite's ability to degrade haemoglobin and affects the parasite's membrane.
- Use: Often used for prophylaxis and treatment of Plasmodium falciparum malaria.
Fig no1. Structure of Atovaquone
These methods are analogous to the methods described in the Journal of American Chem. Society, 1948, gives the condensation product in only about 1 to 6% [Feser, J. American Chemical Society, vol. As mentioned, the production yield of Atovaquone using the process of Patent No. 432 is very low, practically in the order of only 3 to 5% [9].
3. Advantages
3.1 Therapeutic Advantages
1. Effective against opportunistic infections: Atovaquone tablets are effective in preventing and treating opportunistic infections such as Pneumocystis jirovecii pneumonia (PCP) and toxoplasmosis in HIV/AIDS patients.
2. Broad-spectrum antiprotozoal activity: Atovaquone tablets have broad-spectrum activity against various protozoa, including Plasmodium, Toxoplasma, and Babesia.
3. Alternative to sulfonamide-based regimens: Atovaquone tablets provide an alternative treatment option for patients who are intolerant to sulfonamide-based regimens.
3.2 Safety and Tolerability Advantages
1. Well-tolerated: Atovaquone tablets are generally well-tolerated, with a low incidence of adverse effects.
2. Few drug interactions: Atovaquone tablets have few drug interactions, making them a suitable option for patients receiving multiple medications.
3. No requirement for renal dose adjustment: Atovaquone tablets do not require dose adjustment in patients with renal impairment.
3.3 Convenience and Practicality Advantages
1. Oral administration: Atovaquone tablets are administered orally, making them easy to take and convenient for patients.
2. Once-daily dosing: Atovaquone tablets can be taken once daily, improving patient compliance and adherence.
3. Availability in various formulations: Atovaquone tablets are available in various formulations, including suspension and tablets, making them suitable for patients with different needs.
4. Disadvantages
4.1. Therapeutic Disadvantages
1. Variable absorption: Atovaquone tablets have variable absorption, which can affect their efficacy.
2. Resistance development: Resistance to Atovaquone has been reported, particularly in Pneumocystis jirovecii.
3. Limited activity against certain pathogens: Atovaquone tablets have limited activity against certain pathogens, such as Mycobacterium tuberculosis.
4.2 Safety and Tolerability Disadvantages
1. Gastrointestinal side effects: Atovaquone tablets can cause gastrointestinal side effects, such as nausea, vomiting, and diarrhea.
2. Hepatotoxicity: Atovaquone tablets can cause hepatotoxicity, particularly in patients with pre-existing liver disease.
3. Allergic reactions: Atovaquone tablets can cause allergic reactions, such as rash, pruritus, and anaphylaxis.
4.3 Convenience and Practicality Disadvantages
1. High cost: Atovaquone tablets can be expensive, particularly for long-term treatment.
2. Limited availability: Atovaquone tablets may not be widely available in all countries or regions.
3. Requires food for optimal absorption: Atovaquone tablets require food for optimal absorption, which can be inconvenient for some patients.
4.4 Other Disadvantages
1. Limited pediatric data: There is limited data on the use of Atovaquone tablets in pediatric patients.
2. Limited data in pregnancy and lactation: There is limited data on the use of Atovaquone tablets in pregnancy and lactation.
3. Requires monitoring: Atovaquone tablets require monitoring of liver function, renal function, and CD4 cell count in HIV patients.
5. History
Atovaquone is an antiprotozoal medication primarily used to treat and prevent certain infections caused by protozoa, such as Pneumocystis jirovecii pneumonia (PCP) and Toxoplasma Gondii. It was developed in the late 20th century and is notable for being effective against these infections, particularly in immunocompromised patients, such as those with HIV/AIDS.
5.1 Development and Approval:
The 1980s by researchers at the pharmaceutical company Glaxo (now GlaxoSmithKline). The drug was initially explored for its
1. Discovery and Research: Atovaquone was synthesized in potential against malaria and other protozoal infections.
2. Mechanism of Action: Atovaquone works by inhibiting the mitochondrial electron transport chain in protozoa, leading to a lack of ATP production, which is crucial for their survival.
3. Clinical Trials: Following its discovery, extensive clinical trials were conducted to assess its safety and efficacy. It was found to be particularly effective against Pneumocystis pneumonia, a common opportunistic infection in HIV/AIDS patients.
4. FDA Approval: Atovaquone was approved by the U.S. Food and Drug Administration (FDA) in the mid-1990s, initially as a treatment for PCP and later for Toxoplasmosis. It is often used in combination with other medications, such as proguanil, for malaria prevention.
Formulations and Use.
- Tablets and Suspension: Atovaquone is available in tablet form and as an oral suspension, making it accessible for various patient populations, including those who may have difficulty swallowing tablets.
- Dosage and Administration: The dosage varies based on the condition being treated and the patient's health status. It's important for patients to follow their healthcare provider's instructions closely.
5.2 Current Use: Atovaquone remains an essential medication in the treatment and prevention of specific protozoal infections. Its role is particularly critical in populations at risk for opportunistic infections due to immunocompromising conditions. Research continues into its potential uses and combinations with other therapies.Bottom of Form
6. Physicochemical Properties
Atovaquone is an antiprotozoal agent used primarily to treat infections such as Pneumocystis pneumonia and malaria. Here are some of its main physical-chemical properties:
1. Chemical structure: Atovaquone is a derivative of hydroxynaphthoquinone. Its chemical formula is C18H16ClO3.
2. Molecular Weight: About 338.78 g/mol.
3. Solubility: Atovaquone is poorly soluble in water, which can affect its bioavailability. It is more soluble in organic solvents such as methanol and ethanol.
4. pH stability: The stability of atovaquone can be affected by pH; it is generally stable in acidic to neutral conditions, but can be degraded in alkaline environments.
5. Melting point: The melting point of atovaquone is about 180-182°C.
6. Polymorphism: Atovaquone can be polymorphic, which can affect its digestion and absorption characteristics.
7. Partition Coefficient: Atovaquone has high lipophilicity, as indicated by its log P value, which suggests that it partitions well in the lipid environment, which provides insights into the formulation and effective delivery of atovaquone tablet forms.
MATERIALS AND METHODS
7.1 Materials [10]:
ATV was kindly provided by the Wellcome Foundation. Soy lecithin (S75, 70% phosphatidylcholine) was purchased from LipoõÈd. Cholesterol was supplied by Sigma Aldrich Chemie. ATV was diluted in a mixture composed of cremophor, DMSO and water (0.16/1/24, respectively) [10]. Atovaquone (566c80) was a gift from Burroughs Wellcome Co., Research Triangle Park, North Carolina, 5-hydroxy-6-desmethylprimaquine (5H6DP) was a gift from Mohamed Nasr, Division of AIDS, and National Institute of Allergy and Infectious Diseases, Bethesda, Maryland. All other chemicals were purchased from Sigma Chemistry Co. (St. Louis, Missouri), unless otherwise noted [11]. Renal osmolarity was 600 mosmoles/l. Four concentrations were studied: 50, 100, 200 and 400 mg/ml, administered at doses of 0.2, 0.4, 0.8 and 1.6 mg/kg of body weight, respectively [10]. The organic solvents used were of HPLC grade and all other reagents were of analytical grade [12]. ATQ was provided ex gratia by Glenmark Pharmaceuticals Ltd. (Mumbai, India). ART was obtained from Cipla Ltd. (Mumbai, India). Proguanil was received from Ipca Laboratories Ltd. (Mumbai, India). Field’s stain A and B, absolute methanol, sodium carboxy methylcellulose, glycerine, and ethylenediaminetetra acetic acid (EDTA) were purchased from Loba Chemie, India [13]. The nanocapsule-encapsulated ATV was prepared as previously described. Briefly, 100 mg poly (d, l-lactide) (PLA; molecular weight=200,000; Boehringer Ingelheim, Germany) and 125 mg soybean lecithin (70% phosphatidyl choline; Lipoid, Ludwigshafen, Germany) were dissolved in 25 ml acetone. ATV (10 mg) was then dissolved in 0.5 ml benzyl benzoate (density at 20°C=1.118 g/ml; Cooper, Melun, France) and added to the acetone solution [12]. Atovaquone was supplied as shown by Glen Mark Pharmaceutical Ltd., Mumbai and used without additional cleaning. AR Grade Methanol was purchased from Rankem Chemicals. All solvents used in spectrophotometric analysis were of analytical grade [14]. Micronized ATQ (batch ref., Q20M; Wellcome) was suspended in 0.25% (wt/vol) celacol (methylcellulose) in distilled water and ball milled for 48 h prior to dosing to ensure even suspension. ATQ oral microparticulate suspension (ATQ suspension) (batch ref., WPDL/92/0036/86) (median particle diameter, 1.4 mm, with 90% of the particles ,2.3 mm) was diluted with an inert suspending vehicle and briefly mixed prior to use[15]. Atovaquone possesses a distinct double-peak plasma concentration – time profile, in which the first peak occurs between 1 and 8 h and the second betwwen 24 and 96 h after ingestion.The mean half life of Atovaquone in normal volunteers,is 2.9 days and in AIDS patients, 2.2 days[16].
7.2 Plasma ATQ concentration:
At the end of some studies (24 h after the last dose), mice anesthetized with halothane were exsanguinated by cardiac puncture,and plasma was collected. Plasma was stored frozen before quantitation of the ATQ levels. Following solvent extraction from the plasma, the ATQ concentration was determined by a reversed-phase high-pressure liquid chromatography method, involving UV detection[17].
7.3 Description of biological material:
Peripheral blood samples preserved in Whatmanw Chr filter paper were obtained from microscopically and PCR confirmed P. falciparum carriers, upon informed consent. These comprised 131 samples from Guinea Bissau[18], 30 samples from the Zanzibar Islands, and three cases from a clonal epidemic Cape Verde[19]. The two samples referred as Malaronee clinical failures were obtained from two patients in the Gothenburg Hospital[20].
8. Animal study
8.1 Animals:
Female albino Swiss rats (12 to 14 weeks old, weighing 30 to 35 g) and female Sprague-Dawley rats (weighing 180 to 210g) from Bombay Veterinary College, Mumbai, India. The animals were placed in polypropylene cages located in the animal room maintained at a controlled temperature and a relative humidity of 60 ±5%, in a cycle of 12 h light / 12 h dark [21]. Mice were housed under barrier conditions with autoclaved chow, acidified water, and bedding in sterilized shoe cages equipped with sterile equipment [22]. Initial evaluation of Atovaquone was done using the rat model of PCP .This model was chosen because the infection was found to be remarkably similar to that in humans [23,24,25] and because drugs that are effective against PCP in immunosuppressed rats have been shown to be effective in humans [26,27].The rat model has also been used to investigate possible interaction between atovaquone and other drugs. To date, only one drug, erythromycin, has been shown to interfere with the activity of Atovaquone[16].
8.2 Parasite:
Chloroquine-sensitive rodent malaria parasite Plasmodium berghei ANKA strain, obtained from the Tata Institute of Fundamental Research ([TIFR], Mumbai), was used to induce infection in mice. This strain has a life cycle similar to that of the human parasite [21]. The strain of Leishmania used was isolated in Greece from a dog and identified as L.infantum MON 1 (WHO reference: MCAN/GR/94/CRE 69).The zymo- deme of this strain is usually responsible for visceral leishmaniasis [28]. Plasmodium berghei ANKA strain was employed for evaluation of anti-malarial activity. This strain was tested and found to be free of contamination with Eperythrozoon coccoides [29]. The parasites were maintained by continuous intraperitoneal passages in the mice once per week [21]. The infective promastigotes so produced were centrifuged down (1200×g, 10 min), resuspended in normal saline and vortexed. For use as an inoculum, more normal saline was added to this suspension, to give 2×10 8 promastigotes/ml [30].
METHODS
9.1 Preparation of ATQ S and ATQ NS:
ATQ (3% w/v) NS prepared by Kathpalia et al. The combination of pH-based precipitation method, sonication and micro fluidization was used for this study. This Nano suspension, unlike the previously reported ATQ NS, was prepared without the use of organic solvents or methods such as high-pressure homogenization [13].
9.2 Media Dissolution:
Sodium hydroxide solution (0.1 M) was prepared from sodium hydroxide pellets obtained from BDH (Poole, UK). Deionized water was used throughout [31].
9.3 Therapeutic Efficacy
Although new formulations of atovaquone are being developed and a suspension with improved bioavailability is now available (section 2), the drug was administered orally in tablet form in all clinical trials.
9.3.1 Treatment of P carinii Pneumonia
P. carinii is almost exclusively' a pulmonary pathogen, although it does disseminate to other sites including the reticuloendothelial system, skin, pararespiratory structures and CNS in some patients. Infection with P. carinii is thought to occur earlyin life and remain latent until immunodeficiency (primarily cellular) allows reactivation, which results in clinical disease[32]. PCP was the initial AIDS-related illness in 46% of patients receiving no anti-PCP prophylaxis in 1 study, although 14.5% of mY-infected patients who received prophylaxis before a diagnosis of AIDS was made still developed this pneumonia as the initial AIDS-related illness[33].
9.3.2 Clinical Trials
Patients included in most trials of Atovaquone had AIDS and mild or moderate PCP, except some with severe PCP who were enrolled in 1 noncomparative study[34]. In other studies, trial protocol was modified to allow the use of these agents in patients with moderately severe disease[35,36] following a consensus report on corticosteroid use in patients with PCP[37]. Clinical response was defined for only 3 clinical trials[35,36,38]. Atovaquone has shown efficacy in patients with PCP. 57% of 123 patients with severe PCP responded to treatment with atovaquone 750mg 3 times daily; patients who had failed to respond to conventional therapy were less likely to respond to atovaquone than patients who were intolerant of these therapies[34].
9.4 Tolerability
Atovaquone, most commonly administered at a dosage of 750mg 3 times daily, as tablets, demonstrated an acceptable tolerability profile in patients with AIDS and mild to moderate PCP enrolled in the clinical trials reviewed in section 3.1. The incidence of adverse events occurring in patients treated with the suspension appears similar to that seen with the tablet formulation[39]. When all patients enrolled in this trial were considered, 9 and 24% of atovaquone and cotrimoxazole recipients, respectively, experienced treatment-limiting effects. Rash (4%) and liver dysfunction (3%), as measured by increases in aspartate aminotransferase, alanine aminotransferase and! or bilirubin levels, most commonly caused Atovaquone therapy to be withdrawn. Treatment with cotrimoxazole was most frequently stopped because of vomiting (7%), fever (6%), nausea (5%) and neutropenia (3%), as well as rash and liver dysfunction (8 and 7%, respectively»)[36]. Adverse events which occurred in patients with PCP who received atovaquone in noncomparative trials were similar to those reported in comparative trials. In addition, bilateral lower leg paraesthesias were reported by 1 patient who received a full course of treatment with atovaquone 750mg 3 times daily for 21 days; this symptom improved after therapy was completed[40].
Table.No.1 Clinical trials evaluating the efficacy of atovaquone 750mg (A) administered as tablets with food in patients with AIDS and mild to moderate Pneumocystis carinii pneumonia (PCP)
|
Reference Treatment No. of Number of Reason for treatment failure No of patients with
(duration in patients patients no adverse not recurrent PCP
days) successfully response event evaluable (time from com-
treated (%) (%) (%) (%) pletion of therapy)
|
|
Noncomparative trials
Dohn et al. Atid (42) 10(previously 8 (80) 1 1 2 (within 6mo)
untreated)
Epstein Atid (21) 18(prior adverse 15 (83) 2 2 (4, 5mo)a
et al. reaction to
sulphonamides)
Falloon A tid (5) then 34(0·3 previous 27(79) 5 2 4 (6·10wk)
etal.[I7] bid (16) episodes) 3 (20·23wk)
orA tid (21)
orAqid (21)
White et al. Atid (21) 612 (intolerant or 474 (77)
unresponsive to
cotrimoxazole
Comparative randomised multicentre trials
Hughes Atid(21)C 160 (some had 99 (62) 28 (17.5) 11 (7)t 22 (14)
et al.[34]b previous episodes
of PCP)"
Cotrimoxazole 162 (some had 103 (64) 10(6j1 33 (2O) 16 (10)
d tid (21) previous episodes
of PCP)"
Dohn et al. Atid (21) 56 (75% had history 32(57) 16 (29) 2 (4)t 6 (11)
of or current
intolerance to
sulfonamides or
trimethoprim)"
IV pentamidine 53 (75% had history 21 (40) 9 (17) 19 (36)t 4(8)
3-4 mg/kg od of or current
(21) intolerance to
sulfonamides or
trimethoprim
|
10. Procedure [14]
10.1 Preparation of the standard solution:
Approximately 10 mg of the drug was accurately weighed and transferred into a 100 mL volumetric flask and dissolved in approximately 25 mL of methanol. The volume was made up to the mark with methanol. Ten millilitres of this drug solution was transferred to a 100 mL volumetric flask and diluted to the mark with methanol.Top of Form
10.2 Determination of the wavelength of maximum absorption:
Five millilitres of Atovaquone solution was transferred into a 10 ml volumetric flask. Dilute to point with methanol. The absorbance of the final solution was measured in the range of 200-400 nm, compared to methanol used as a control. Atovaquone showed an absorption maximum at 251 nm.
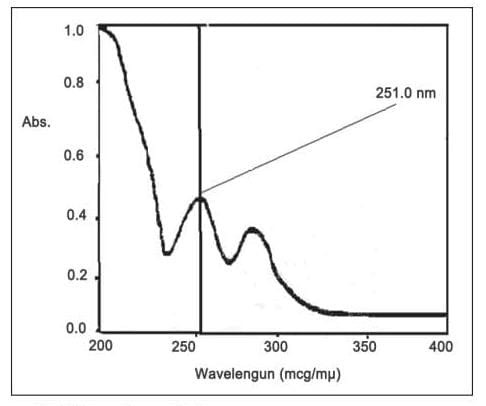
Fig No. 2. UV spectrum of atovaquone
10.3 Preparation of calibration curve for Atovaquone
Stock solutions of atovaquone (1–10 ml) were pipett ed out in to a series of 10 volumetric flask of 10 ml. The volume in each volumetric flask was made up to the mark with methanol and the mixer was shacked. That produced the concentration range of 1–10 ?g/ml of Atovaquone. The absorbances of solutions were measured at 251 nm against methanol as blank.

Fig No .3. Calibration curve of atovaquone at 251 nm
10.4 Preparation of atovaquone tablet with the dispersed solid product [41]
Compressed tablets containing physical mixture and dispersed solid product with and without surfactants were prepared separately by direct compression method, dry binder polyvinyl pyrrolidone, and talc lubricants, magnesium stearate were used as excipients mixed with lubricants and compressed on a 10 stations rolling machine (M/s Karnavati) to a hardness of 6 kg/cm2 using 9mm flat punches.
Table No. 2. Formulation of Atovaquone tablets:
|
Ingredients(mg)
|
Formulation code
|
|
F1
|
F2
|
F3
|
F4
|
F5
|
F6
|
|
Atovaquone
Solid dispersion system
Bio surfactant
Poloxamer407
Lactose
PVP
Mg Stearate
Talc
Total weight
|
62.5
-----
-----
-----
159.5
5
1
2
230
|
62.5
-----
4
-----
155.5
5
1
2
230
|
62.5
-----
-----
30
129.5
5
1
2
230
|
-----
S1
-----
-----
34.5
5
1
2
230
|
-----
S7
-----
-----
30.5
5
1
2
230
|
-----
S7
-----
-----
4.5
5
1
2
230
|
11. Estimation of Atovaquone tablet [14]
For analysis of the commercial formulation, 20 tablets were accurately weighed and crushed into a fine powder. A portion equivalent to 10 mg of Atovaquone was weighed and transferred into a 100 mL volumetric flask. The volume was made up to the mark with the same solvent and filtered through Whatmann filter paper No. 42. The volume was adjusted to the mark and absorbance was recorded at 251 nm.
12. Evaluation Parameters
1. Physical Characteristics
1. Appearance: The tablets should be uniform in color, size, and shape. No visible defects such as cracks or discoloration should be present.
2. Hardness: The tablet should have adequate hardness to withstand physical stress during handling but should also be easily disintegrated in the stomach.
3. Friability: The tablet should not crumble or break under normal conditions (e.g., during transport or handling).
4. Weight Uniformity: The tablet weight should be consistent across batches. Significant variations in weight can indicate manufacturing issues or inconsistent drug content.
5. Disintegration Time: The tablet should disintegrate within the specified time frame in an aqueous medium, ensuring the drug is available for absorption in the body.
2. Chemical Characteristics
1. Content Uniformity: Atovaquone content should be uniform within each tablet and across a batch. This ensures each tablet delivers the correct dose.
2. Potency: The tablet should contain the exact amount of atovaquone as specified by the manufacturer (typically within ±10% of the labeled dosage).
3. Dissolution Profile: The tablet must dissolve in a specific time frame (usually within 30 minutes) to ensure that atovaquone is properly absorbed into the bloodstream. The dissolution rate is typically tested in a simulated gastric fluid (SGF) or simulated intestinal fluid (SIF).
3. Stability
1. Shelf-life and Storage Conditions: The tablet's stability over time should be evaluated under different environmental conditions (e.g., temperature, humidity, and light exposure). Stability testing ensures that atovaquone remains effective and safe during its shelf life.
2. Degradation Products: The potential for degradation over time should be monitored, ensuring that no harmful or ineffective degradation products form.
4. Microbial Quality
1. Sterility: If the tablet is intended for use in immunocompromised patients, sterility testing may be required.
2. Microbial Limits: The tablets should meet microbial limit specifications to ensure there is no microbial contamination.
5. Bioavailability and Pharmacokinetics
1. Bioequivalence: When evaluating generic formulations, bioequivalence testing is important to ensure that the generic atovaquone tablet performs similarly to the branded version in terms of absorption and efficacy.
2. Plasma Concentration Profiles: The rate and extent of absorption of atovaquone from the tablet into the bloodstream must be evaluated to ensure therapeutic efficacy.
13. Quality control tests
1. Physical Characteristics Tests
1. Appearance: Visual inspection of the tablet's color, shape, and size.
2. Weight variation: Determination of the tablet's weight to ensure consistency.
3. Thickness: Measurement of the tablet's thickness to ensure uniformity.
4. Hardness: Determination of the tablet's hardness to ensure it can withstand handling.
2. Identification Tests
1. Infrared spectroscopy: Identification of the active ingredient (Atovaquone) using infrared spectroscopy.
2. High-performance liquid chromatography (HPLC): Identification and quantification of Atovaquone using HPLC.
3. Assay Tests
1. HPLC assay: Quantification of Atovaquone in the tablet using HPLC.
2. UV-Visible spectroscopy assay: Quantification of Atovaquone in the tablet using UV-Visible spectroscopy.
4. Impurity Tests
1. Related substances test: Detection and quantification of related substances (impurities) in the tablet using HPLC.
2. Unknown impurities test: Detection and identification of unknown impurities in the tablet using HPLC and mass spectrometry.
5. Dissolution Tests
1. Dissolution test: Determination of the rate and extent of dissolution of the tablet in a simulated gastric fluid.
2. Disintegration test: Determination of the time taken for the tablet to disintegrate in a simulated gastric fluid.
6. Stability Tests
1. Accelerated stability test: Evaluation of the tablet's stability under accelerated conditions (e.g., high temperature, high humidity).
2. Long-term stability test: Evaluation of the tablet's stability over a longer period (e.g., 12 months).
7. Microbiological Tests
1. Microbial limit test: Determination of the presence and amount of microorganisms in the tablet.
2. Sterility test: Determination of the presence or absence of microorganisms in the tablet.
8. Packaging Tests
1. Packaging integrity test: Evaluation of the packaging material's integrity and ability to protect the tablet.
2. Labeling test: Verification of the label's accuracy and compliance with regulatory requirements.
14. Evaluation of atovaquone tablet
14.1 Pre-compression: The entire formulation of the atovaquone lubricated powder mixture was subjected for micromeritics properties using conventional methods. Bulk density and tapped density was determined by using measuring cylinder method. Hausner's ratios, Carr’s index, were determined using the bulk density and tapped density data. A shear angle was determined by conventional funnel method [42].
14.2 Post-compression: All atovaquone tablets were subjected to non-pharmacological and pharmacological tests. Tablet thickness, hardness, was determined using a calliper and a Monsanto hardness tester, respectively. Friability and disintegration tests were performed as per Indian pharmacopeia (IP 2014) [43].
15. Statistical evaluation
The survival probability and statistical significance were determined using Kaplan–Meier log-rank survival test (Graph Pad Instat 6.0 software, San Diego, CA). Data on survival time, percent parasitaemia, and percent suppression are represented as mean ± standard deviation (SD) [13].
16. Evaluation of the effective doses
The mean effective dose of ATV bound was 0.172 ± 0.05 mg/kg. The average effective dose of free ATV cannot be determined in our model because a plateau was reached for 33.0% parasite suppression. The ED25, at 0.46 ± 0.15 mg/kg and 0.022 ± 0.01 mg/ kg for free and bound ATV, respectively, indicated that liposome loaded with ATV were 23 times more effective than the free drug (p < 0>
1. Malaria Treatment (Uncomplicated)
Indication: Atovaquone is used in the treatment of Plasmodium falciparum and Plasmodium vivax malaria.
Standard Dose:
Adults and Children ? 40 kg: 750 mg of atovaquone once daily for 3 days.
Children 5-14 kg: 250 mg once daily for 3 days.
Children 15-24 kg: 375 mg once daily for 3 days.
Children 25-39 kg: 500 mg once daily for 3 days.
2. Malaria Prophylaxis (Prevention)
Indication: For prevention of malaria caused by P. falciparum and other Plasmodium species in areas where malaria is endemic.
Standard Dose:
Adults and Children ? 40 kg: 250 mg once daily.
Children 5-9 kg: 62.5 mg once daily.
Children 10-19 kg: 125 mg once daily.
Children 20-29 kg: 187.5 mg once daily.
Children 30-39 kg: 250 mg once daily.
3. Treatment of Pneumocystis jirovecii Pneumonia (PJP)
Indication: Atovaquone is an alternative treatment for PJP, particularly in patients with HIV/AIDS or those with immunosuppression.
Standard Dose:
Adults: 750 mg twice daily with food for 21 days.
Children (? 4 years): Dosing is weight-based:
5-14 kg: 250 mg twice daily.
15-24 kg: 375 mg twice daily.
25-39 kg: 500 mg twice daily
.? 40 kg: 750 mg twice daily.
17. Applications
1. Prophylaxis and Treatment of Opportunistic Infections
1. Pneumocystis jirovecii pneumonia (PCP) prophylaxis and treatment: Atovaquone tablets are used to prevent and treat PCP in HIV-infected patients.
2. Toxoplasmosis prophylaxis and treatment: Atovaquone tablets are used to prevent and treat toxoplasmosis in HIV-infected patients.
2. Treatment of Malaria and Babesiosis
1. Malaria treatment: Atovaquone tablets, in combination with proguanil, are used to treat uncomplicated malaria caused by Plasmodium falciparum.
2. Babesiosis treatment: Atovaquone tablets, in combination with azithromycin, are used to treat babesiosis.
3. Treatment of Other Infections
1. Cryptosporidiosis treatment: Atovaquone tablets are used to treat cryptosporidiosis in HIV-infected patients.
2. Cyclosporiasis treatment: Atovaquone tablets are used to treat cyclosporiasis.
4. Pediatric and Geriatric Use
1. Pediatric use: Atovaquone tablets are used to prevent and treat PCP in HIV-infected children.
2. Geriatric use: Atovaquone tablets are used to prevent and treat PCP and toxoplasmosis in elderly HIV-infected patients.
5. Other Applications
1. Traveler's diarrhea prevention: Atovaquone tablets, in combination with proguanil, are used to prevent traveler's diarrhea.
2. Immunocompromised patient care: Atovaquone tablets are used to prevent and treat opportunistic infections in immunocompromised patients.
CONCLUSION:
A stable solid dispersion of ATQ was successfully developed using HME technology. The developed formulation showed better in vitro release compared to the marketed formulation. Each marketed tablet of ATQ-PG (Malarone1) contains 250 mg of ATQ and 100 mg of PG. The method was linear in the concentration range from 200 to 1200 /band with r2 of 0.998. The analysis percentage was found to be 102.8 ± 1.845%. Clinical trials for PCP prophylaxis with ATQ suspension are currently on going. At 15 minutes, the concentration of sodium hydroxide in the dissolution medium, the speed of peristaltic pump and flow rate were evaluated as statistically significant. A method was developed for the evaluation of tablet form. The drug shows an absorption maximum at 251 nm. Atovaquone tablets prepared from physical mixture and dispersed solid products. The evaluated results show that addition of biosurfactant to the ternary system was better than the physical mixture.
REFERENCES
- Gutteridge WE. 566C80, an antimalarial hydroxynaphthoquinone with broad spectrum: experimental activity against opportunistic parasitic infections of AIDS patients. J Protozoal 1991; 38:141S-3S.
- Information for investigators: Mepron brand atovaquone tablet, intravenous and oral suspension formulations. Burroughs Wellcome Co. Aug. 31, 1992.
- Falloon J, Sargent S, Piscitelli SC, et al. Atovaquone suspension in HIV-infected volunteers: pharmacokinetics, pharmacodynamics, and TMP-SMX interaction study. Pharmacotherapy 1999; 19:1050-6.
- Hughes WT, Kennedy W, Shenep JL, et al. Safety and pharmacokinetics of 566C80, a hydroxynaphthoquinone with anti-Pneumocystis carinii activity: A Phase I study in human immunodeficiency virus (HIV)-infected men. J infects Dis 1991; 163: 843-848.
- Doig MV, Jones AE. High-performance liquid chromatographic assay for the measurement of atovaquone in plasma. Biochem Anal 1990; 20:157-62
- Shah YI, Pradhkar AR, Dhayagude MG. Introduction to Biostatistics and Computer sciences. Pune: Nirali Prakashan. 1996. p. 53
- Venkatram, Roger JA. Characteristics of drug-phospholipid coprecipitates I: physical properties and dissolution behavior of griseofulvin-dimyristoyl phosphatidylcholine systems. J Pharm Sci 1984; 73:757-61.
- Vishal J, Parekh, Patravale VB, Aniruddha B, Pandit. Mango kernel fat: a novel lipid source for the fermentative production of sophorolipid biosurfactant using starmerella bombicola NRRL-Y 17069. Ann Biol Res 2012; 3:1798-803.
- Kumar,Ashok [IN/IN]; 123/AB,CRD,IPCA Laboratories Ltd., Kandivli Industrial Estate, Charkop, Kandivli (West) ,Mumbai 400067,Maharashtra (IN).
- Cauchetier E, Fessi H, Boulard Y, Deniau M, Astier A, Paul M. Preparation and physicochemical characterization of atovaquone-loaded liposomes. Drug Dev Res 1999; 47(4):155±61.
- ITTARAT I, JAMES W S. Effect of atovaquone and other inhibitor on Pneumocystis carinii Dehydrogenase 2; 1995:39(2):325-328.
- Cauchetier, E., Deniau, M., Fessi, H., Astier, Az Paul, M. (2003). Atovaquone-loaded nanocapsule: influence of the nature of the polymer on their in vitro characteristics. International Journal of Pharmaceutics, 250, 273–281.
- Kathpalia H, Juvekar S, Shidhaye S. Design and in vitro evaluation of atovaquone nanosuspension prepared by pH based and anti-solvent based precipitation method. Colloid Interface Sci Commun 2019; 29:26–32.
- K N Patel, J K Patel. A validated method for development of Atovaquone as API and tablet dosage forms by UV spectroscopy, 385-315.
- W.Comley, Cliev. L. Antipneumocystis activity of 17C91, a prodrug of Atovaquone 10;1995:vol 39(10):2217-2219.
- Information for investigators : Mepron brand Atovaquone tablet, intravenous and oral suspension formulations. Burroghs Wellcome co. Aug 31, 1992.
- Wellcome Foundation. Unpublished method.
- Arez AP, Palsson K, Pinto J, et al. Transmission of mixed malaria species and strains by mosquitoes, as detected by PCR, in a study area in Guinea-Bissau. Parassitologia 1997;39:65–70.
- Arez AP, Snounou G, Pinto J, et al. A clonal Plasmodium falciparum population in an isolated outbreak of malaria in the Republic of Cabo Verde. Parasitology 1999;118:347–55.
- Farnert A, Lindberg J, Gil JP, et al. Plasmodium falciparum malaria resistance to atovaquone-proguanil treatment. BMJ 2003; in press.
- Borhade V, Pathak S, Sharma S, Patravale V. Formulation and characterization of atovaquone nanosuspension for improved oral delivery in the treatment of malaria. Nanomedicine 2014; 9:649–66.
- Melanie C, P Kumar. A Novel encochleated formulation improves Atovaquone activity in a murine model of Pneumocystis Pneumonia. 1997; 95.
- Gutteridge WE 566C80, an antimalarial hydroxynaphthoquinone with broad spectrum: experimental activity against opportunistic parasitic infections of AIDS patients J protozool 1991;38:141S-3S.
- Hudson AT, Dickins M, Ginger CD, et al.566C80: a potent broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drug Exp Clirt Res 1991;17:427-35.
- Hughes WT, Gray VL, Gutteridge WE, et al. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental Pneumocystis Carinii Pneumonitis .Antimicrob agents Chemother 1990;17:427-35.
- Hughes WT, Kuhn S, Chaudhary S, et al. Successful Chemo- Prophylaxis for Pneumocystis Carinii Pneumonitis N Engl J Med 1997;297:1419-26.
- Hughes WT, McNabb Pc, Makres TD, Feld Man S. Efficacy of trimethoprim and Sulfamethoxazole in the prevention and treatment of Pneumocystis Carinii Pneumonitis. Antimicrob Agents Chemother 1974;5:289-93.
- E Cauchetier, M Paul. Therapeutic evaluation of free and liposomes-encapsulated atovaquone in the treatment of murine leishmaniasis. 30(2000):777-783.
- L Kate, V Gokarna. Bioavailability enhancement of atovaquone using hot melt extrusion technology, (2016) doi: 10.1016/j.ejps.2016.03.00
- CIOMS. Council for international organization of medical sciences. 1985; Guiding principles for biomedical research, Geneva, Switzerland.
- M S Bloomfield, W C Butler, Robustness testing using experimental design, of flow- through dissolution method for a product where the actives have markedly differing solubility properties.2000;4:55-61.
- Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part II. Am Rev Respir Dis 1990; 141: 1582-98
- Hoover DR, Saah AJ, Bacellar H, et aI. Clinical manifestations of AIDS in the era of pneumocystis prophylaxis. N Engl J Med 1993 Dec; 329: 1922-6
- White A, Rogers M, Andrews E, et aI. Results of a treatment IND for Mepron (atovaquone) theapy in patients with acute PCP [abstract WS-B 14-1]. IXth International Conference on AIDSIIVth STD World Congress; 1993 Jun 6-11; Berlin, Germany.
- Falloon J, Kovacs J, Hughes W, et al. A preliminary evaluation of 566C80 for the treatment of pneumocystis pneumonia in patients with the acquired immunodeficiency syndrome. N Engl J Med 1991 Nov 28; 325: 1534-8
- Hughes W, Leoung G, Kramer F, et al. Comparison of Atovaquone (566C80) with trimethoprim-suifamethoxazole to treat Pneumocystis carinii pneumonia in patients with AIDS. N Engl J Med 1993 May 27; 328: 1521-7.
- Masur H, Meier P, McCutchan JA, et al. Consensus statement on the use of corticosteroids as adjunctive therapy for Pueumocystis pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 1990; 323: 1500-4.
- Dohn MN, Weinberg WG, Torres RA, et al. Oral Atovaquone compared with intravenous pentamidine for Pneumocystis carinii pneumonia in patients with AIDS. Ann Intern Med1994 Aug 1; 121: 174-80.
- Wellcome. Mepron (atovaquone) suspension. England: Wellcome, 1995. Scientific brochure. (Data on file).
- Epstein LJ, Mohsenifar Z, Daar ES, et al. Clinical experience with atovaquone: a new drug for treating Pneumocystis carinii pneumonia. Am J Med Sci 1994 Jul; 308: 5-8
- U B Bolmal, Pramod H J. Formulation of atovaquone tablet using biosurfactant in a ternary solid dispersion system: IN VITRO & IN VIVO Evaluation.2;2019(11).
- Eraga SO, Arhewoh MI, Okunzuwa JI, Iwuagwu MA. Preliminary investigation of the mucoadhesive properties of thermally modified mucin on metronidazole tablets. Int J Pharm Sci 2015; 7:96-00.
- Kishore BA, Ramana MV. Development and in vivo evaluation of gastroretentive floating tablets of antipsychotic drug risperidone. Int J Pharm Sci 2016; 8:43-52.
- Ling J, Baird JK, Fryauff DJ, et al. Randomized, placebo-controlled trial of atovaquone/proguanil for prevention of Plasmodium falciparum or Plasmodium vivax malaria among migrants to Papua, Indonesia. Clin Infect Dis 2002;35:825–33.
- Hughes WT. Limited effect of trimethoprim-su1famethoxazole prophylaxis on Pneumocystis carinii. Antimicrob Agents Chemother 1979; 16 (3): 333-5.
- Falloon J, Boenning C, Pagano G, et al. The pharmacokinetics of atovaquone suspension in patients with HIV infection [abstract242]. In: Program and Abstracts of the 1st National Conference on Human Retroviruses and Related Infections;1993 Dec 12-16; Washington DC, 1993.
- Hughes WT, Lafon SW, Scott JD, et al. Adverse events associated with trimethoprim-sulfamethoxazole and Atovaquone during the treatment of AIDS-related Pneumocystis carinii pneumonia. J Infect Dis 1995; 171: 1295-301
- Smith NA, Nelson MR, Gazzard BG, et al. The use of Atovaquone in HIV positive patients [abstract]. AIDS 1994 Nov; 8 Suppl. 4: S37.
- Kovacs JA, NIAID-Clinical CIAIDSP. Efficacy of Atovaquone in treatment of toxoplasmosis in patients with AIDS. Lancet 1992 Sep 12; 340: 637-8.
- Araon LB, David RH. Antiparasitic agent atovaquone. Antimicrob Agents Chemother 2002;46:1163-73.
- Patil AN, Shinkar DM, Saudagar RB. Review article: solubility enhancement by solid dispersion. Int J Curr Pharm Res 2017; 9:15
- Borhardev, Patak S, Sharma S, Patravale V. Formulation and characterization of atovaquone nanosuspension for improved oral delivery in the treatment of malaria. Nanomedicine 2014;5:649-66.
- Hammond DJ, Burchell JR, Pudney M. Rapid high-performance liquid chromatographic assay for atovaquone. Mol Biochem Parasitol 1985;17:97-109.
- Torres RA, Weinberg W, Stansell J, et al. Atovaquone for salvage treatment and suppression of toxoplasmic encephalitis in patients with AIDS. Atovaquone/Toxoplasmic Encephalitis Study Group. Clin Infect Dis 1997; 24:422-9.
- Durand R, Paul M, Rivollet D, et al. Activity of pentamidine- loaded poly (D, L-lactide) nanoparticles against Leishmania infantum in a murine model. Parasite 1997;4(4):331±6.
- Dalencon F, Amjaud Y, La€orgue C, Derouin F, Fessi H. Atovaquone and rifabutine-loaded nanocapsules: formulation studies. Int J Pharm 1997;153:127±30.
- Sabchareon A, Attanath P, Phanuaksook P, et al. Efficacy and pharmacokinetics of atovaquone and proguanil in children with multidrugresistant Plasmodium falciparum malaria. Am J Trop Med Hyg 1999;60:533–41.
- Schöler N, Krause K, Kayser O, Müller RH, Borner K, Hahn H, et al. Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis. Antimicrob Agents Chemother 2001;45:1771–9.
- Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother 2000;44:2100–8.
- Chavada VD, Bhatt NM, Sanyal M, Shrivastav PS. The first normal-phase high-performance thin-layer chromatographic method for the simultaneous determination of the antimalarial drugs atovaquone and proguanil from Malarone tablets 2016; 29(2): 140–144.


 Tejaswini Gedam*
Tejaswini Gedam*
 Dr. Nilesh Chachda
Dr. Nilesh Chachda
 Gaurav Meshram
Gaurav Meshram



 10.5281/zenodo.14451886
10.5281/zenodo.14451886