Abstract
Cubosomes are a unique class of nanoparticles formed from the self-assembly of lipids in water. Essentially, they are soft, isotropic particles with a crystalline internal structure. Lipids like monoolein or phytantriol, when exposed to water, naturally form these cubic structures. To stabilize these nanoparticles, surfactants such as Poloxamers (like F127 and F108) are often employed. The distinctive feature of cubosomes is their internal architecture. A complex network of interconnected water channels is formed within a continuous lipid bilayer, creating a three-dimensional cubic structure. This unique structure allows for the encapsulation of a wide range of molecules, from highly lipophilic to hydrophilic substances. One of the significant advantages of cubosomes is their biocompatibility and biodegradability. The lipids used in their formation, such as monoolein and phytantriol, are naturally occurring substances well-tolerated by the body. This makes cubosomes a promising carrier for drug delivery. Compared to other lipid-based drug delivery systems like liposomes or solid lipid nanoparticles, cubosomes offer several benefits. Their cubic structure provides enhanced stability, leading to slower drug release and improved drug retention. Moreover, the internal architecture of cubosomes prevents drug expulsion to the nanoparticle surface, ensuring controlled and targeted drug delivery. Various methods can be used to prepare cubosomes, including solvent evaporation, ultrasonication, hydrotrope addition, spray drying, and melt dispersion emulsification. These techniques allow for the customization of cubosome properties to suit specific drug delivery applications. In conclusion, cubosomes represent a promising platform for drug delivery. Their unique structure, biocompatibility, and ability to encapsulate various drug molecules make them a subject of increasing interest in pharmaceutical research.
Keywords
Cubosomes; drug delivery; nanoparticle; pH-Sensitive.
Introduction
Cubosomes are nanoscale particles characterized by a unique, three-dimensional cubic internal structure. These structures are formed from liquid crystalline materials, typically lipids or surfactants, when hydrated. The resulting cubic phase is then dispersed into smaller particles to create cubosomes. The core of a cubosome is a complex network of interconnected water channels embedded within a continuous lipid bilayer. This intricate architecture imparts distinctive properties to cubosomes, setting them apart from other nanocarriers. Cubosomes are formed through a specific process involving the self-assembly of lipid or surfactant molecules. Hydration the initial step involves mixing surfactants or polar lipids with water. Under suitable conditions, these amphiphilic molecules undergo a self-assembly process, forming a liquid crystalline phase with a cubic structure. This phase is characterized by its high viscosity and unique geometric arrangement. Dispersion the subsequent step involves dispersing the formed cubic phase into smaller particles. This process typically involves high-energy input methods such as homogenization, high-pressure emulsification, or micro fluidization. The dispersion process breaks down the cubic phase into individual cubosomes, which are colloidal particles with a cubic internal structure.
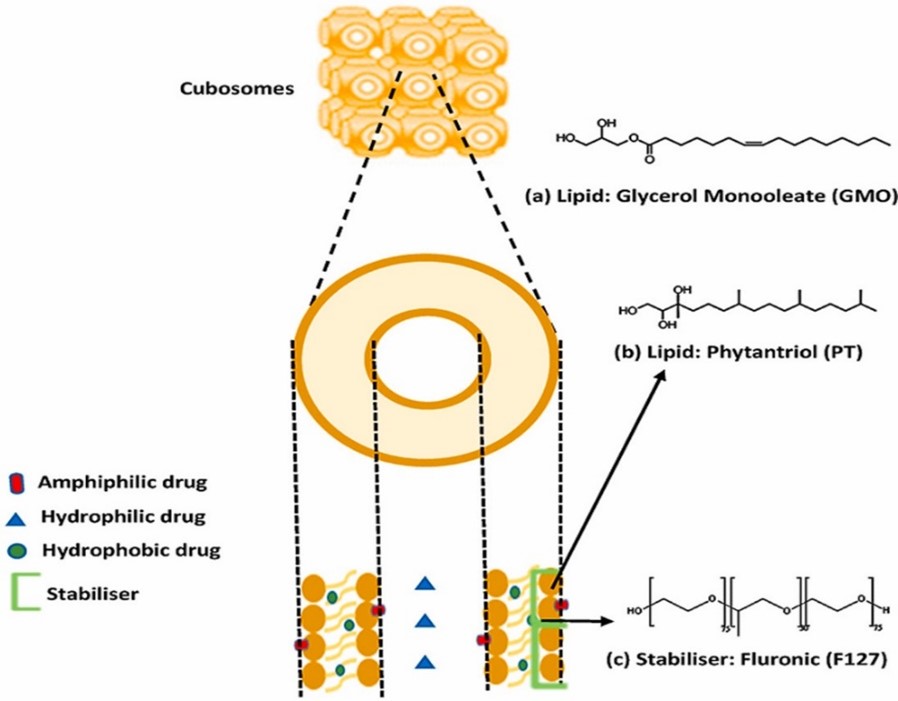
Fig. no. 1 Structure of Cubosomes
cubosomes offer distinct advantages. Compared to liposomes, although both are lipid-based, cubosomes boast a superior internal structure. Their cubic architecture allows for higher drug loading and sustained release compared to the spherical bilayer structure of liposomes. Micelles, on the other hand, are much smaller than cubosomes but this comes at a cost. Their smaller size translates to lower drug loading capacity. Additionally, controlling the release profile of drugs from micelles can be more challenging compared to cubosomes. Finally, nanoemulsions are inherently unstable due to their oil-in-water nature. Cubosomes, however, are thermodynamically stable thanks to their liquid crystalline structure. This stability, combined with their ability to control drug release, makes them a compelling option for drug delivery applications.
Structural Components of Cubosomes
Cubosomes represent a fascinating class of nanostructured liquid crystalline particles that have garnered significant attention in the realm of drug delivery due to their unique properties. Their intricate architecture, composed of specific structural components, is instrumental in determining their behavior and functionality. At the core of a cubosome lies the lipid bilayer, a fundamental component shared with other lipid-based nanocarriers such as liposomes. However, what distinguishes cubosomes is the unparalleled arrangement of this bilayer. Unlike the spherical configuration observed in liposomes, the lipid bilayer in cubosomes curves and folds upon itself to create a three-dimensional, interconnected network. This network forms a cubic lattice, hence the name "cubosomes." The lipids employed in the construction of this lattice are typically amphiphilic molecules, possessing both hydrophilic (water-loving) and hydrophobic (water-repelling) regions. Monoolein and phytantriol are frequently cited examples of lipids used in cubosome formation. [2, 3] The amphiphilic nature of these lipids is crucial for the self-assembly process that gives rise to the cubic structure. When exposed to an aqueous environment, the hydrophilic head groups of the lipids interact favorably with water molecules, while the hydrophobic tails aggregate to minimize their contact with water. This interplay of hydrophilic and hydrophobic forces drives the lipids to adopt a specific geometric arrangement that minimizes the overall free energy of the system. The cubic phase represents one of the possible equilibrium states that can be achieved under appropriate conditions of lipid concentration, temperature, and pH. The lipid bilayer in cubosomes serves as a scaffold, providing structural integrity to the nanoparticles. It also acts as a barrier, controlling the exchange of molecules between the internal and external environment. The specific composition of the lipid bilayer can influence the physical and chemical properties of the cubosomes, including their size, shape, stability, and drug loading capacity. For instance, the inclusion of cholesterol or other sterols can modulate the fluidity and permeability of the bilayer, affecting drug release kinetics.
Intertwined within the lipid bilayer are interconnected aqueous channels, which constitute the second critical component of cubosomes. These channels form a three-dimensional network that permeates the entire structure. The dimensions and topology of these channels can vary depending on the specific lipid composition and formation conditions. The aqueous channels provide a hydrophilic environment that is conducive to the encapsulation of water-soluble drugs. Additionally, they facilitate mass transfer within the cubosome, allowing for the diffusion of molecules between the interior and exterior of the particle. [3, 4] The properties of the aqueous channels significantly impact the behavior of cubosomes. For example, the size and connectivity of the channels can influence the drug loading capacity and release kinetics. Larger channels may accommodate larger drug molecules, while smaller channels can provide a more controlled release profile. The distribution of the aqueous channels within the cubic lattice also affects theoverall stability of the cubosomes.
Properties and Advantages of Cubosomes[5,6]
Properties of Cubosomes
- The distinctive cubic geometry of cubosomes provides a high degree of structural order and stability.
- The intricate network of interconnected water channels within the lipid bilayer results in an extensive interfacial area, allowing for significant drug loading.
- Despite their liquid crystalline nature, cubosomes exhibit solid-like properties, contributing to their stability and potential for controlled release.
- The lipid bilayer composition enables the encapsulation of both hydrophilic and lipophilic molecules, making cubosomes versatile carriers for a wide range of drugs.
Advantages of Cubosomes
- Due to their large internal surface area, cubosomes can accommodate significant amounts of both hydrophilic and lipophilic drugs.
- The cubic structure and the potential for controlled drug release profiles make cubosomes suitable for delivering drugs over extended periods.
- Cubosomes can enhance the solubility of poorly water-soluble drugs, improving their bioavailability.
- The lipid components used in cubosome formation are often biocompatible and biodegradable, reducing the risk of adverse effects.
- Cubosomes exhibit relatively high stability compared to other colloidal systems, which can improve their shelf life and performance.
- The ability to encapsulate a wide range of molecules and the potential for surface modification make cubosomes adaptable to various drug delivery applications.
Disadvantages of Cubosomes
- The formation of cubosomes involves precise control of parameters such as lipid composition, water content, and processing conditions. This can make the formulation process complex and time-consuming.
- The cubic phase from which cubosomes are formed is often highly viscous, which can pose challenges in processing and scale-up.
- While cubosomes exhibit reasonable stability, factors such as temperature, pH, and ionic strength can affect their structural integrity.
Preparation Techniques of Cubosomes [7]
There are range of cubosomes forming techniques are used i.e., melt dispersion emulsifying, sonication, spray drying, solvent evaporation, top-down, bottom-up, or hydrotrope method . Top-down and hydrotrope method are the two most common methods employed in the formation of cubosomes.
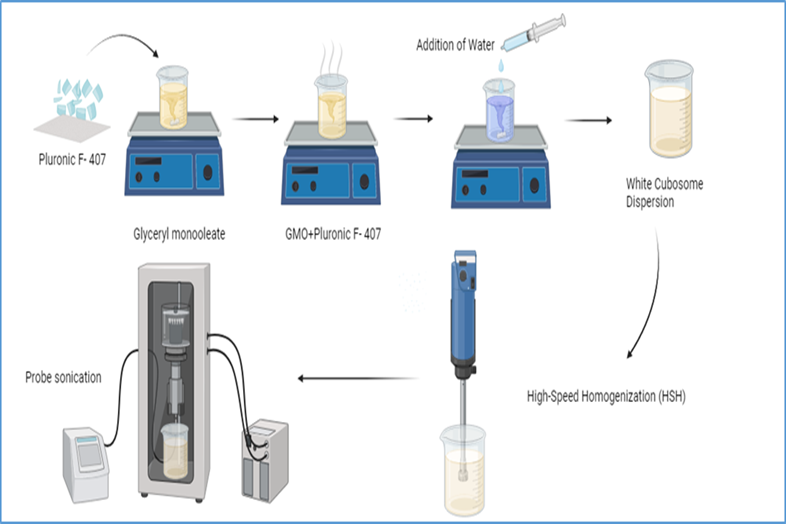
Fig. No. 2 Preparation of Cubosomes
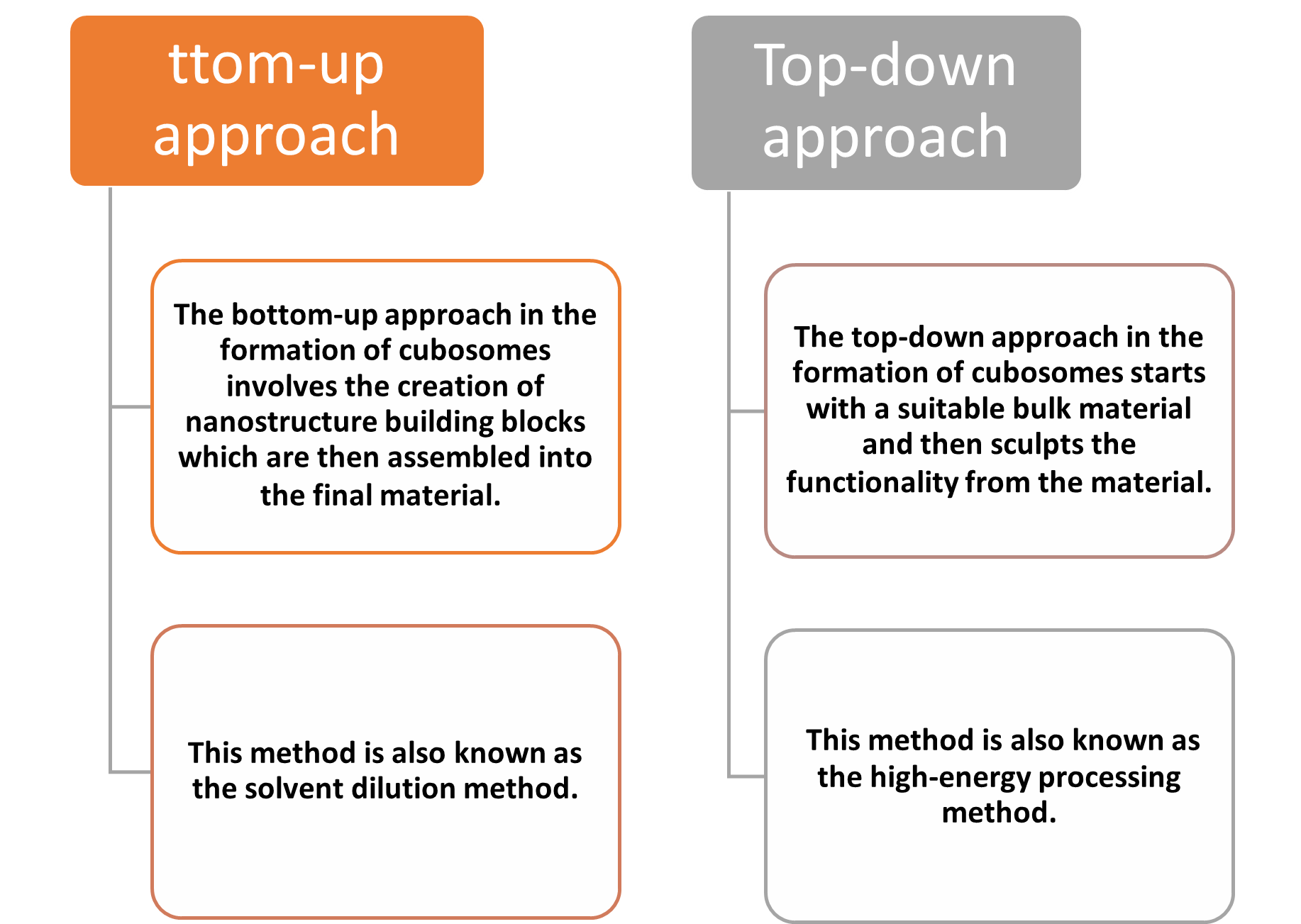
Fig. No. 3 Preparation Techniques of Cubosomes
Melt Dispersion Emulsifying Method for Cubosome Preparation [8]
The melt dispersion emulsifying method is a commonly employed technique for fabricating cubosomes. This approach involves the controlled melting and dispersion of lipid components to create a stable cubic structure. Initially, the amphiphilic lipids, which form the backbone of the cubosome, are melted in conjunction with stabilizing agents. This process typically occurs on a hot plate equipped with a magnetic stirrer, allowing for precise temperature control and homogenous mixing. The drug to be encapsulated can be incorporated into this molten lipid-stabilizer mixture in various ways. One method involves directly dispersing the drug within the molten lipid phase. Alternatively, the drug can be dissolved in an aqueous solution containing a surfactant or a polymer like polyvinyl alcohol, which acts as a dispersion stabilizer. This drug-laden aqueous solution is then introduced into the molten lipid mixture. Subsequently, the molten lipid-drug mixture is injected into a preheated aqueous phase while undergoing vigorous magnetic stirring. This step creates an emulsion, where the lipid phase is dispersed as fine droplets within the aqueous phase. To further refine the droplet size and promote the formation of cubosomes, the emulsion is subjected to homogenization. This high-shear process breaks down the lipid droplets into smaller particles, facilitating the self-assembly of lipids into the characteristic cubic structure. The specific conditions, such as temperature, stirring rate, and lipid-to-water ratio, are critical parameters influencing the final characteristics of the cubosomes. By carefully controlling these variables, researchers can optimize the formation of cubosomes with desired properties, including particle size, drug loading, and stability. The melt dispersion emulsifying method offers several advantages, including its relative simplicity and versatility. It allows for the incorporation of various drugs and lipids, providing flexibility in formulation design. Furthermore, this method can be scaled up for industrial production, making it suitable for large-scale manufacturing of cubosome-based products. However, challenges associated with this method include the potential for drug degradation during the melting process, difficulties in achieving consistent particle size distribution, and the requirement for careful optimization of process parameters.
Solvent Evaporation Method for Cubosome Preparation [9]
The solvent evaporation method is another commonly employed technique for the fabrication of cubosomes. This approach involves the use of organic solvents to solubilize the lipid components, followed by their dispersion in an aqueous phase to induce self-assembly into the cubic structure. Typically, lipids are dissolved in a volatile organic solvent such as ethanol or chloroform. This lipid solution is then added dropwise to an aqueous solution containing a non-ionic surfactant, often a Pluronic-type polymer like F108, F127, F68, or P104. The surfactant acts as a stabilizer, influencing the formation and stability of the cubosomes. The resulting mixture is subjected to magnetic stirring at elevated temperatures to facilitate the evaporation of the organic solvent. The drug can be incorporated into either the lipid phase or the aqueous surfactant phase prior to the formation of the emulsion. To enhance the formation of cubosomes, the mixture is often subjected to further homogenization using techniques like ultrasonication or high-pressure homogenization. This step helps in reducing the particle size and promoting the self-assembly of lipids into the desired cubic structure. Alternatively, the lipid and surfactant mixture can be dissolved in an organic solvent, followed by the complete removal of the solvent under vacuum conditions. The resulting dry lipid-surfactant film is then redispersed in an aqueous phase to form cubosomes. The solvent evaporation method offers several advantages, including the ability to control the composition of the lipid and surfactant phases, as well as the potential for precise drug loading. However, the use of organic solvents raises concerns regarding their toxicity and environmental impact. Furthermore, the complete removal of the solvent is crucial to avoid any residual solvent contamination in the final product.
Ultra-Sonication Method [10,11]
The ultrasonication method involves melting amphiphilic lipids, commonly monoolein, and dispersing them in an aqueous solution containing a surfactant like Pluronic F-108 or F-127. The drug is often incorporated into the molten lipid phase before dispersion. Ultrasonic energy is then applied to the lipid-surfactant mixture to break down the lipid phase into smaller particles and induce the formation of cubosomes.
Spray Drying Method [12]
In the spray drying process, the lipid-surfactantsolvent mixture is atomized with the wave of hot air resulting in the rapid solvent evaporation and formation of dry powder of cubosomes precursor. This technique is simple, cost effective, and normally easy to scale up. Briefly, the amphiphilic lipid with or without Pluronics is dissolved in ethanol or a binary solvent mixture, for example, methanol/chloroform .An aqueous phase consisting of a hydrophilic solid carrier such as dextran or sorbitol is mixed with the lipidethanol solution under stirring which results in the development of a low viscosity emulsion. The drug can be dissolved along with the lipid in the organic solvent or mixed with an aqueous solution of the solid carrier. The lipid-ethanoldextran-water quaternary system can then be efficiently spray-dried to evaporate the organic solvent and water resulting in the formation of dry lipid-coated powder precursor which can be easily redispersed in water to form cubosomes.
Hydrotrope Method for Cubosome Formation [13]
The hydrotrope method, also known as the solvent dilution process, involves the dispersion of molten amphiphilic lipids in a hydrotropic solvent, such as ethanol or chloroform. This lipid-solvent mixture is then gradually added to an aqueous solution containing a surfactant, commonly a Pluronic polymer, under constant stirring. Hydrotropes act as solubilizers, facilitating the dispersion of lipids in the aqueous phase. To determine the optimal conditions for cubosome formation, ternary phase diagrams are often constructed, outlining the relationship between lipid, hydrotrope, and water concentrations. Unlike other methods that require high-energy input, the hydrotrope method generally relies on low-energy techniques like vortexing or magnetic stirring for cubosome formation. However, to achieve the desired particle size reduction, additional processing steps, such as high-pressure homogenization, may be necessary. The hydrotrope method offers advantages such as reduced energy input and the potential for precise control over lipid and surfactant concentrations. However, the use of organic solvents and the need for careful optimization of formulation parameters are important considerations.
APPLICATIONS OF CUBOSOMES [14,15]
Cubosomes, with their unique structural properties and ability to encapsulate a diverse range of molecules, have emerged as a promising platform for a variety of applications. Their potential is particularly evident in the fields of drug delivery, cosmetics, and food science.
Drug Delivery [16]
The capacity of cubosomes to encapsulate both hydrophilic and lipophilic compounds makes them versatile carriers for a wide spectrum of therapeutic agents. This versatility is particularly advantageous for drugs that exhibit poor water solubility, a common challenge in drug formulation. By incorporating these drugs into the lipid bilayer or aqueous channels of cubosomes, their solubility can be significantly enhanced, leading to improved bioavailability. Furthermore, cubosomes offer the potential for controlled drug release. The lipid bilayer acts as a barrier that regulates the diffusion of encapsulated drugs. By manipulating the composition and structure of the lipid bilayer, researchers can fine-tune the release kinetics to achieve desired therapeutic outcomes. Sustained release formulations, for instance, can be developed to prolong drug action, reducing dosing frequency and improving patient compliance. The ability to target drug delivery is another key advantage of cubosomes. By modifying the surface of the particles with specific ligands, they can be directed towards specific cells or tissues. This targeted delivery approach enhances therapeutic efficacy while minimizing side effects. For example, cubosomes can be engineered to target cancer cells, delivering anticancer drugs directly to the tumor site, thereby increasing treatment effectiveness and reducing systemic toxicity. Cubosomes also demonstrate potential in overcoming various challenges associated with drug delivery. They can protect encapsulated drugs from degradation by enzymes or harsh environments, such as the gastrointestinal tract. Additionally, their nanometer size allows for improved penetration through biological barriers and enhanced cellular uptake.
Cosmetics and Personal Care [17,18]
The cosmetic and personal care industry has shown significant interest in utilizing cubosomes as delivery vehicles for active ingredients. Their ability to encapsulate and deliver these components effectively enhances the performance of various products. For instance, cubosomes can be employed to deliver antioxidants, vitamins, and peptides to the skin, promoting hydration, anti-aging, and skin protection benefits. Beyond the delivery of active ingredients, cubosomes can also improve the texture and feel of cosmetic formulations. Their liquid crystalline nature imparts unique sensory properties to products such as creams and lotions. Moreover, cubosomes can contribute to the stability of emulsions, preventing phase separation and extending product shelf life.
Food Industry [19]
The food industry has also recognized the potential of cubosomes to enhance the properties of food products. They can serve as delivery vehicles for functional ingredients like vitamins, minerals, and probiotics, improving their bioavailability and protecting them from degradation during processing and storage. Cubosomes can also contribute to the texture and sensory appeal of food products. Their ability to form stable emulsions and gels allows for the creation of novel food structures with improved mouthfeel and appearance. Furthermore, cubosomes can be used to encapsulate flavors, providing controlled release and enhancing taste perception.
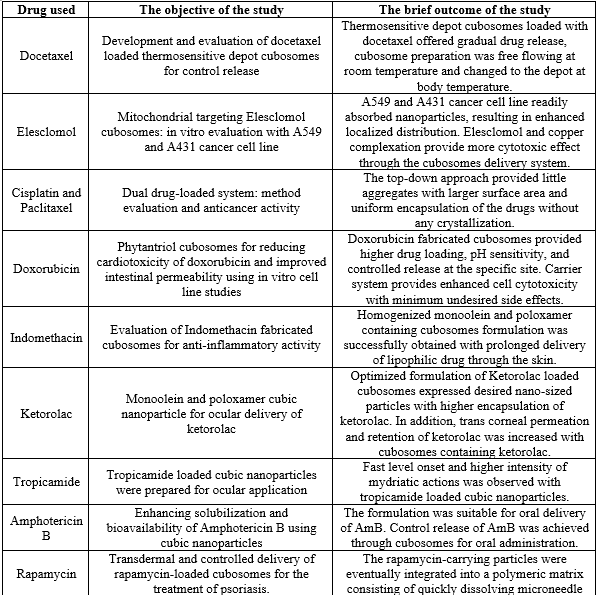
Drugs incorporated in cubosomes [20,21]
Challenges and Future Perspectives of Cubosomes [22,23]
- Complex Formulation and Characterization:
Creating stable and reproducible cubosome formulations often involves intricate optimization of various parameters, including lipid composition, surfactant type, and processing conditions. Characterizing these complex nanostructures requires specialized techniques and expertise.
- Potential Stability Issues:
Cubosomes can be susceptible to factors such as temperature, pH, and ionic strength, which can affect their stability and long-term storage. Ensuring the stability of cubosome-based formulations is crucial for their successful application.
- Limited Clinical Studies:
While preclinical studies have demonstrated the potential of cubosomes, there is a paucity of clinical data to support their efficacy and safety in human subjects.
FUTURE PERSPECTIVES [24]
Exploration of New Lipid and Surfactant Combinations:
Investigating a wider range of lipids and surfactants can lead to the development of cubosomes with improved properties, such as enhanced stability, drug loading capacity, and biocompatibility.
Development of Advanced Characterization Techniques:
Advancements in characterization techniques are essential for gaining a deeper understanding of cubosome structure, dynamics, and interactions with biological systems.
Investigation of Cubosomes for Specific Disease Treatments:
Identifying specific diseases where cubosomes can offer significant advantages over existing therapies is crucial for driving clinical translation.
Characterization of Cubosomes [25]
Characterizing cubosomes is crucial to understand their structure, size, stability, and drug loading capacity.
Particle Size and Size Distribution:
Dynamic Light Scattering (DLS):
This technique measures the size and size distribution of cubosomes. It provides information about the average particle size and polydispersity index (PDI).
Transmission Electron Microscopy (TEM):
TEM offers high-resolution imaging of cubosomes, allowing for direct visualization of particle size and morphology.
Structure and Morphology:
Small-Angle X-ray Scattering (SAXS):
SAXS provides information about the internal structure of cubosomes, confirming the cubic phase and determining lattice parameters.
Cryogenic Transmission Electron Microscopy (Cryo-TEM):
Cryo-TEM allows for the direct visualization of the internal structure of cubosomes in a hydrated state, preserving their native structure.
Surface Properties:
Zeta Potential:
Measuring the zeta potential provides information about the surface charge of cubosomes, which influences their stability and interactions with the surrounding environment.
Drug Loading and Release:
Ultraviolet-Visible (UV-Vis) Spectroscopy:
This technique can be used to quantify the amount of drug loaded into cubosomes.
High-Performance Liquid Chromatography (HPLC):
HPLC is used to determine the drug encapsulation efficiency and release kinetics.
Stability:
Differential Scanning Calorimetry (DSC):
DSC can be used to assess the thermal stability of cubosomes and investigate phase transitions.
Accelerated Stability Studies:
Exposing cubosomes to different temperature and humidity conditions can provide information about their long-term stability.
Other Techniques:
Atomic Force Microscopy (AFM):
AFM can be used to study the topography and mechanical properties of cubosome films.
Nuclear Magnetic Resonance (NMR):
NMR spectroscopy can provide insights into the molecular interactions within the cubosome structure.
CONCLUSION
Cubosomes are unique nanostructures classified as inverse bicontinuous curved cubic phase lyotropic liquid crystals. They are formed when specific amphiphiles, such as glyceryl monooleate, are combined with water under controlled conditions of hydration and temperature. The resulting structure is characterized by a three-dimensional network of interconnected water channels separated by a continuous lipid bilayer. A key advantage of cubosomes lies in their significantly increased membrane surface area compared to liposomes. This ample surface area allows for the encapsulation of a substantial amount of both hydrophilic and hydrophobic drug molecules, as well as amphiphilic compounds. This versatility is a significant improvement over traditional drug delivery systems like liposomes or emulsions, which often exhibit limitations in accommodating diverse drug molecules.The lipid bilayer in cubosomes is characterized by a higher lipid content and stronger electrostatic repulsion between lipid molecules compared to liposomes. This structural feature contributes to enhanced membrane stability, making cubosomes more robust and resistant to degradation.Owing to their inherent physical stability, capacity to deliver multiple drug types, and potential for mucoadhesion, cubosomes have emerged as promising candidates for drug delivery applications. The porous three-dimensional architecture of cubosomes creates a tortuous diffusion pathway for encapsulated drugs, enabling controlled release and prolonging therapeutic effects. These combined properties position cubosomes as a superior nanocarrier compared to conventional drug delivery systems, offering the potential for improved drug efficacy and patient outcomes
REFERENCE
- Date AA, Vador N, Jagtap A, et al. Lipid nanocarriers (GeluPearl) containing amphiphilic lipid Gelucire 50/13 as a novel stabilizer:Fabrication, characterization and evaluation for oral drug delivery. Nanotechnology. 2011;24:275102.
- Salim M, Minamikawa H, Sugimura A, et al. Amphiphilic designer nano-carriers for controlled release:From drug delivery to diagnostics. MedChemComm . 2014;5:1602–1618.
- Li Y, Lin J, Yang X, et al. Self-Assembled Nanoparticles Based on Amphiphilic Anticancer Drug-Phospholipid Complex for Targeted Drug Delivery and Intracellular Dual-Controlled Release. ACS Appl Mater Interfaces. 2015;7:17573–17581.
- Waghule T, Rapalli VK, Singhvi G, et al. Design of temozolomide-loaded proliposomes and lipid crystal nanoparticles with industrial feasible approaches:comparative assessment of drug loading, entrapment efficiency, and stability at plasma pH. J Liposome Res;31. 2020;15:1-1.
- Zhao XY, Zhang J, Zheng LQ, et al. Studies of cubosomes as a sustained drug delivery system. J Dispers Sci Technol. 2004;25:795–799.
- Muller F, Salonen A, Glatter O. Phase behavior of Phytantriol/water bicontinuous cubic Pn3m cubosomes stabilized by Laponite disc-like particles. J Colloid Interface Sci. 2010;342:392–398.
- Huang Y, Gui S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Advances. 2018;8:6978– 6987.
- Caffrey M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr Sect FStructural Biol Commun. 2015;71:3– 18.
- Mariani P, Luzzati V, Delacroix H. Cubic phases of lipid-containing systems. Structure analysis and biological implications. J Mol Biol.1988;204:165– 189.
- Wan Iskandar WFN, Salim M, Hashim R, et al. Stability of cubic phase and curvature tuning in the lyotropic system of branched chain galactose-based glycolipid by amphiphilic additives. Colloids Surfaces A Physicochem Eng Asp. 2021; 623:126697
- Buchheim W, Larsson K. Cubic lipidprotein-water phases. J Colloid Interface Sci .1987;117:582–583.
- Li D, Caffrey M. Lipid cubic phase as a membrane mimetic for integral membrane protein enzymes. Proc Natl Acad Sci U S A. 2011;108:8639–8644.
- Zang J, Feng M, Zhao J, et al. Micellar and bicontinuous microemulsion structures show different solute-solvent interactions:A case study using ultrafast nonlinear infrared spectroscopy. Phys Chem Chem Phys .2018;20:19938–19949.
- Lindblom G, Rilfors L. Cubic phases and isotropic structures formed by membrane lipids - possible biological relevance. BBA - Revw on Biomemb. 1989;988:221–256.
- Khedekar PB. Cubosomes:a vehicle for delivery of various therapeutic agents. MOJ Toxicol. 2018;4:0083.
- Pan X, Han K, Peng X, et al. Nanostructed Cubosomes as Advanced Drug Delivery System. Curr Pharm Des. 2013;1;19:6290- 6297.
- Mohsen AM, Younis MM, Salama A, et al. Cubosomes as a Potential Oral Drug Delivery System for Enhancing the Hepatoprotective Effect of Coenzyme Q10. J Pharm Sci;2021.
- Elgindy NA, Mehanna MM, Mohyeldin SM. Self-assembled nano-architecture liquid crystalline particles as a promising carrier for progesterone transdermal delivery. Int J Pharm. 2016;501:167–179.
- Sarkar S, Tran N, Rashid MH, et al. Toward cell membrane biomimetic lipidic cubic phases: A high-throughput exploration of lipid compositional space. ACS Appl Bio Mater. 2019;2:182– 195.
- Al-mahallawi AM, Abdelbary AA, ElZahaby SA. Norfloxacin loaded nanocubosomes for enhanced management of otitis externa:In vitro and in vivo evaluation. Int J Pharm. 2021;600:120490.
- Peng X, Zhou Y, Han K, et al. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des Devel Ther. 2015;9:4209–4218.
- Wakaskar RR. General overview of lipid– polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J Drug Target. 2018;26:311– 318.
- Chang C, Meikle TG, Drummond CJ, et al. Comparison of cubosomes and liposomes for the encapsulation and delivery of curcumin. Soft Matter. 2021;17:3306– 3313.
- Caffrey M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr Sect FStructural Biol Commun. 2015;71:3– 18.
- Anderson DM, Gruner SM, Leibler S. Geometrical aspects of the frustration in the cubic phases of lyotropic liquid crystals. Proc Natl Acad Sci U S A. 1988;85:5364–5368.


 Prabhakar Madvali*
Prabhakar Madvali*




 10.5281/zenodo.13494538
10.5281/zenodo.13494538