Abstract
NSAIDs, or non-steroidal anti-inflammatory medicines, suppress the inflammatory mediator enzyme cyclooxygenase (COX) in order to reduce inflammation. The development of newer NSAIDs, such as celecoxib, rofecoxib, etoricoxib, lumaricoxib, and valdecoxib, is responsible for the discovery of COX-2-specific inhibitors, or ?oxibs. Their usage is limited to the treatment of rheumatoid arthritis, an inflammatory illness characterized by inflammation of the joint lining, which leads to pain, edema, stiffness, joint degeneration, and loss of joint function. The deterioration of the cartilage that surrounds joints, particularly weight-bearing joints, is known as osteoarthritis and is treated with selective COX-2 inhibitors. This analysis's primary goal is to provide both qualitative and quantitative information about selective COX-2 inhibitors in pharmaceutical and biological formulations. In this review article, we have summarized UV/Vis spectroscopy, high-performance liquid chromatography (HPLC), High-performance thin-layer chromatography (HPTLC), Liquid chromatography-mass spectroscopy-mass spectroscopy (LC-MS/MS), and ultra performance liquid chromatography (UPLC) etc. Based methods for estimation of Selective COX-2 inhibitors. In addition to that, we have discussed the bioanalytical methods for Selective COX-2 inhibitors analysis. In conclusion, this review article will help to research scholars for further method development for drug estimation in pharmaceutical dosage forms and biological fluids.
Keywords
Selective COX-2 Inhibitors, Reumatoid Arthritis, Ostioarthritis, Nsaids, Analytical Method, High-Performance Liquid Chromatography, High-Performance Thin-Layer Chromatography, Ultra Performance Liquid Chromatography, Bioanalytical Methods.
Introduction
In clinical practice, non-steroidal anti-inflammatory medications (NSAIDs) are frequently used to treat pain, inflammation, and fever. Because NSAIDs inhibit the cyclooxygenase (COX) enzyme, they impede prostaglandin synthesis, which accounts for their pharmacological actions. In humans, the enzyme cyclooxygenase (COX) comes in two forms: COX-1 and COX-2. COX-1 is necessary for numerous physiological housekeeping processes, including platelet aggregation, renal homeostasis maintenance, and gastric mucosa protection. Prostaglandins, which mediate reactions to pathologic processes like pain, fever, and inflammation, are synthesized by COX-2.(53) COX is inhibited by NSAIDS. Nevertheless, despite their advantageous benefits, they often conflict with the body's defenses against stomach lining deterioration and platelet dysfunction. As a result, many patients may find that their toxicity-related symptoms are unacceptable. The invention of more recent medications known as COX-2-specific inhibitors (coxibs), such as celecoxib, rofecoxib, etoricoxib, lumaricoxib, and valdecoxib, was made possible by this. They maintain the integrity of the stomach lining or platelet control while inhibiting inflammatory disorders. Selective COX-2 inhibitors are as effective as nonsteroidal anti-inflammatory drugs (NSAIDs), but they have a far better safety record, making it acceptable to use them to treat both acute and chronic pain, with or without inflammatory disorders. It is used to treat the signs and symptoms of osteoarthritis and rheumatoid arthritis. An autoimmune condition called rheumatoid arthritis damages and destroys joints by inflaming the lining of the joints, causing pain, stiffness, swelling, and loss of joint function. The substance that cushions joints wears down over time, usually in weight-bearing joints, and this leads to osteoarthritis.(22)
CELECOXIB:
A specific inhibitor of cyclooxygenase-2 (COX-2) is celecoxib. This medication is licensed to treat the inflammation-related signs and symptoms of osteoarthritis and rheumatoid arthritis. Celecoxib predominantly inhibits COX-2 but not COX-1 in humans at therapeutic levels. Celecoxib has a better safety profile as compared to traditional non-steroidal anti-inflammatory medicines (NSAIDs), which block both cyclooxygenases.First of all Clinical research has shown that celecoxib effectively reduces edema, discomfort, and sensitivity in the joints while also lowering the risk of stomach ulcers. Furthermore, new research has shown that COX-2 inhibitors reduce the growth of colon polyps.(2) The chemical name of celecoxib is (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1yl] benzenesulfonamide).(1)
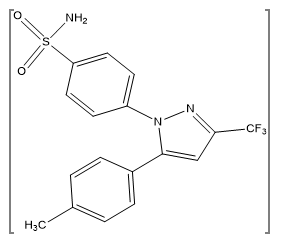
Figure 1: Chemical Structure of Celecoxib
ETORICOXIB:
In the group of nonsteroidal anti-inflammatory drugs (NSAIDs) the newest addition of etoricoxib takes place known as selective cyclooxygenase-2 inhibitors. The chemical name of etoricoxib is {5-chloro-3-(4-methanesulfonylphenyl)-6-methyl-[2,3]-bipyridinyl}. In 38 countries worldwide in Europe, Latin America and the Asia Pacific region ETX has been launched. The new drug application (NDA) has submitted a for ARCOXIA (etoricoxib) to the U.S. Food and Drug Administration (USFDA) by Merck & Co. Inc., for the treatment of osteoarthritis, rheumatoid arthritis, chronic low back pain, acute pain, dysmenorrheal, acute gouty arthritis and ankylosing spondylitis.(54)

Figure 2: Chemical Structure of Etoricoxib
VALDECOXIB:
Valdecoxib is a diaryl substituted isoxazole with the trade name Vx2 (Novartis). The molecular weight of valdecoxib is 314.36. The chemical name of VDX is 4-(5-methyl- 3-phenyl-4-isoxazolyl)benzene sulphonamide. It is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic properties which is use for the treatment of osteoarthritis and Rheumatoid arthritis. Even chronic administration of valdecoxib would not increase the risk of cardiac arrhythmia associated with QT prolongation to patients for the treatment of osteoarthritis and rheumatoid arthritis like disease. Valdecoxib is official only in the martindale extra pharmacopoeia.(103) Valdecoxib was immediately banned by Government decision (GSR NO- 510E) from 28-07-2005 after evidence showed its prolonged used leads to increased risk of heart attacks and stroke.(162)
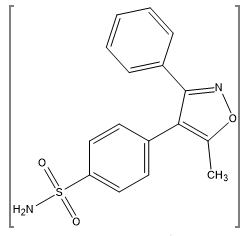
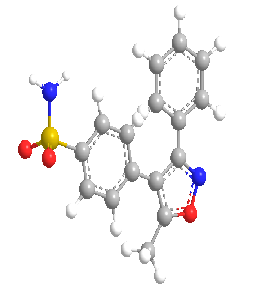
Figure 3: Chemical Structure of Valdecoxib
PARECOXIB:
Parecoxib is a prodrug of valdecoxib. It is a selective cyclooxygenase 2 (COX 2) inhibitor. Parecoxib administered intramuscularly or intravenously in the body.(131) Parecoxib has little or no effect on platelet function. PRX have longer duration of action and it reduced gastrointestinal risk which is considered advantageous in the postoperative repair. Parecoxib can be rapidly hydrolysed into its valdecoxib which is a active metabolite of PRX, and valdecoxib further metabolized by cytochrome P450 enzymes (CYP) into hydroxylated valdecoxib (OH-VX) as the major metabolite. However, the overdosing valdecoxib have been reported for renal safety and high risk of cardiovascular events of concerns. Therefore, it is necessary to monitor the parecoxib and its metabolites concentration in blood in order to control the concentration of valdecoxib in a reasonable range.(132)
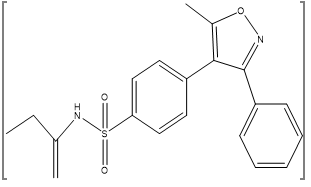
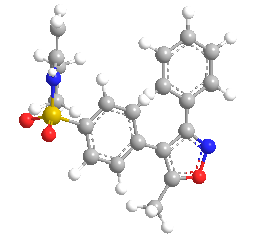
Figure 4: Chemical Structure of Parecoxib
ROFECOXIB:
Rofecoxib belongs to the class of nonsteroidal anti-inflammatory drug (NSAID) called as selective cyclooxygenase-2 inhibitor (COX-2), which gives anti-inflammatory, analgesic, and antipyretic effects. RFX is used for osteoarthritis symptoms, dysmenorrhea, and acute pain.(136) Rofecoxib is chemically known as 4-[4-(methyl-sulfonyl)phenyl]-3-phenyl-2(5H)-furanone.(138) The rofecoxib was voluntarily withdrawn from the global markets because it increased risk of coronary thrombosis and cerebrovascular risk after its chronic use (about 18 months). However, for research purposes comprising characterization studies, preparation of new formulations, and also in clinical studies rofecoxib is currently used. According to Biopharmaceutics Classification System (low solubility and high permeability) RFX is a Class II compound and it has a long half-life (t1/2 = 17 h). Therefore, In the formulation studies of controlled release dosage forms, and also in new drug delivery systems it is used as a model drug.(136) Rofecoxib was immediately banned by Government decision (GSR NO-810E) from 13-12-2004 after evidence showed its prolonged used leads to increased risk of heart attacks.(162)
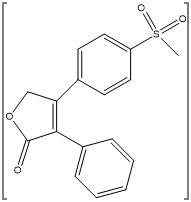
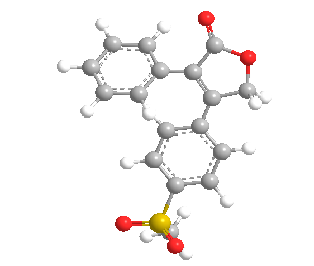
Figure 5: Chemical Structure of Rofecoxib
LUMIRACOXIB:
Lumaricoxib is a selective cyclooxygenase-2 inhibitor developed for the symptomatic treatment of osteoarthritis and acute pain. Lumiracoxib chemically known as 2-[(2-fluoro-6-chlorophenyl)amino]-5-methyl benzeneacetic acid.(157) The molecular weight of LMX is 294 Da. Lumaricoxib is chemically differ from the other COX-2 inhibitors that it lacks a sulfur-containing moiety and possesses a carboxylic group that confers weakly acidic properties (pKa 4.7). It was recently withdrawn from the market in some countries, however it could be available in others.(161)
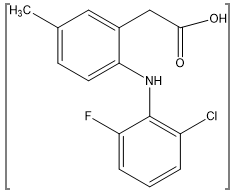
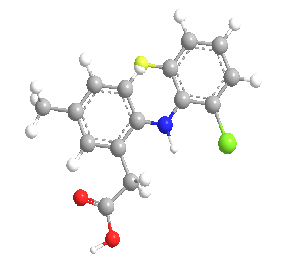
Figure 6: Chemical Structure of Lumiracoxib
Analytical techniques used for determination of Selctive COX-2 inhibitors:
For the determination of Selective COX-2 inhibitors in bulk and pharmaceutical formulations, an exhaustive literature search found numerous analytical techniques such as UV/Visible Spectrophotometry, HPLC, HPTLC, UPLC, LC-MS/MS, and bioanalytical approaches. Figure 7 shows different analytical methods implemented for the estimation of Selective COX-2 inhibitors

Figure 7: Analytical methods of Selective COX-2 Inhibitors
CELECOXIB:
Bio-analytical method for CXB
Bio-analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics (drugs and their metabolites, and biological molecules in unnatural locations or concentrations) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems. The summary of the reported bioanalytical methods is shown in Table 1.
Table 1: Bioanalytical determination of CXB
*** Not Provided
UV-Visible spectroscopy method for CXB
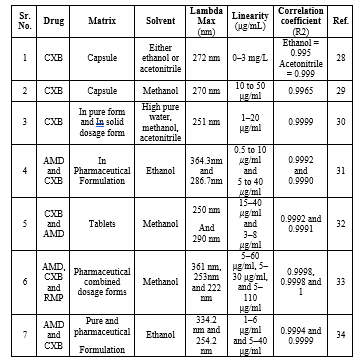
The spectrophotometric methods have been accounted for the determination of CXB. The details of Spectrophotometry determination of basic principle, sample matrix, lambda max, solvent linearity range and the correlation coefficient are summarized in Table 2.
Table 2: Spectrophotometric methods used for determination of CXB
*** Not Provided
Liquid-Chromatography-Mass Spectroscopy methods (LC-MS) for CXB:
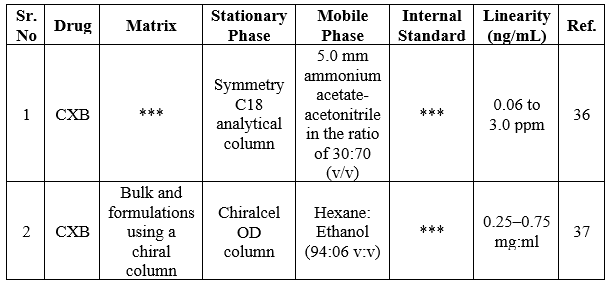
In recent years, the combination of LC/MS has gained a lot of attention for the analysis of interest analytes in complex samples with improved performance. In brief, after a thorough examination, LC/MS interfaces are divided into two categories namely interfaces for indirect and direct input of column effluent. A mechanical mechanism is employed to transmit the column effluent to the MS vacuum at an indirect introduction interface. A classic example of an indirect introduction type of interface is the transportation system. In the case of the direct introduction system, the column effluent flows directly into the mass spectrometric vacuum system via a tube. Mainly, the most straightforward method of linking LC and MS appears to be the direct introduction.33 In this section, we have discussed the LC-MS methods for the determination of CXB in a dosage form Table 3.
Table 3. Summary of LC-MS methods for the determination of CXB in a dosage form
*** Not Provided
HPLC method for CXB
The specificity of the HPLC method is excellent and simultaneously sufficient precision is also attainable. However, it has to be stated that the astonishing specificity, precision, and accuracy are attainable only if wide-ranging system suitability tests are carried before the HPLC analysis. For this reason, the expense to be paid for the high specificity, precision, and accuracy is also high. The summary of the reported HPLC methods is shown in Table 4.
Table 4: Summary of HPLC methods for the determination of CXB in a single and combined dosage form

HPTLC method for CXB
Thin-layer chromatography is a popular technique for the analysis of a wide variety of organic and inorganic materials, because of its distinctive advantages such as minimal sample clean-up, a wide choice of mobile phases, flexibility in sample distinction, high sample loading capacity and low cost. The summary of the reported HPTLC methods is shown in Table 5.
Table 5: Summary of HPTLC methods for the determination of CXB in a single and combined dosage form

ETORICOXIB:
Bio-analytical method for ETX
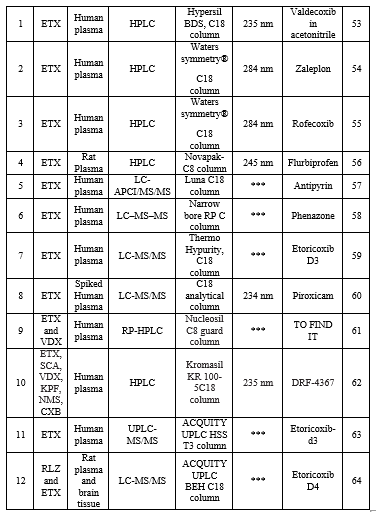
Bio-analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics (drugs and their metabolites, and biological molecules in unnatural locations or concentrations) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems. The summary of the reported bioanalytical methods is shown in Table 6.
Table 6: Bioanalytical determination of ETX
*** Not Provided
UV-Visible spectroscopy method for ETX
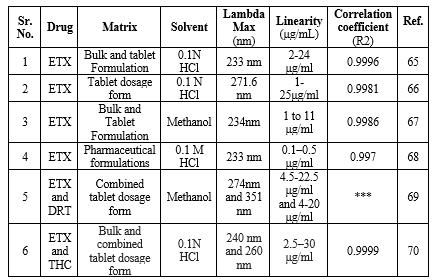
The spectrophotometric methods have been accounted for the determination of ETX. The details of Spectrophotometry determination of basic principle, sample matrix, lambda max, solvent linearity range and the correlation coefficient are summarized in Table 7.
Table 7: Spectrophotometric methods used for determination of ETX
*** Not Provided
Liquid-Chromatography-Mass Spectroscopy methods (LC-MS) for ETX:
In recent years, the combination of LC/MS has gained a lot of attention for the analysis of interest analytes in complex samples with improved performance. In brief, after a thorough examination, LC/MS interfaces are divided into two categories namely interfaces for indirect and direct input of column effluent. A mechanical mechanism is employed to transmit the column effluent to the MS vacuum at an indirect introduction interface. A classic example of an indirect introduction type of interface is the transportation system. In the case of the direct introduction system, the column effluent flows directly into the mass spectrometric vacuum system via a tube. Mainly, the most straightforward method of linking LC and MS appears to be the direct introduction. In this section, we have discussed the LC-MS methods for the determination of ETX in a dosage form Table 8.
Table 8. Summary of LC-MS methods for the determination of ETX in a dosage form
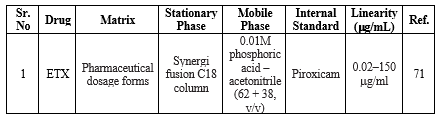
HPLC method for ETX
The specificity of the HPLC method is excellent and simultaneously sufficient precision is also attainable. However, it has to be stated that the astonishing specificity, precision, and accuracy are attainable only if wide-ranging system suitability tests are carried before the HPLC analysis. For this reason, the expense to be paid for the high specificity, precision, and accuracy is also high. The summary of the reported HPLC methods is shown in Table 9.
Table 9: Summary of HPLC methods for the determination of ETX in a single and combined dosage form
*** Not Provided
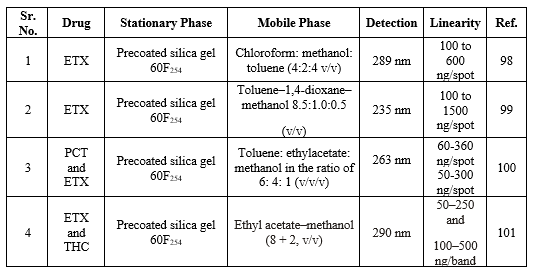
HPTLC method for ETX
Thin-layer chromatography is a popular technique for the analysis of a wide variety of organic and inorganic materials, because of its distinctive advantages such as minimal sample clean-up, a wide choice of mobile phases, flexibility in sample distinction, high sample loading capacity and low cost. The summary of the reported HPTLC methods is shown in Table 10.
Table 10: Summary of HPTLC methods for the determination of ETX in a single and combined dosage form
UPLC methods for ETX
Ultra-performance liquid chromatography (UPLC) is a new category of separation based on well-established principles of liquid chromatography, which utilizes sub-2-mm particles for the stationary phase. The developed UPLC method is validated and therefore could be further used for quantitative analysis of Etoricoxib. Sanjay Shesha Shetgar1*, Ramadevi Dharmasoth2, Bandlamudi Mallikarjuna Rao3, Basavaiah Keloth4 established UPLC method development and validation for simultaneous estimation of Etoricoxib and Thiocolchicoside in tablets. UPLC was carried out in Hibar, C18 column of dimension 100 × 2.1 mm, 1.8 ?m,at 30°C, by using mobile phase 0.1% orthophosphoric acid (pH 2.5) and acetonitrile in a ratio of 90:10 (v/v). The column effluents were monitored at 256 nm using a Acquity Tunable UV detector at a flow rate of 0.3 ml/minute. The linearity of the calibration curve ranged from 1–6 ?g/ml of Thiocolchicoside and 15–90?g/ml of Etoricoxib and the regression coefficient (r2) was 0.999 for both Etoricoxib and Thiocolchicoside drugs.(102)
VALDECOXIB:
Bio-analytical method for VDX
Bio-analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics (drugs and their metabolites, and biological molecules in unnatural locations or concentrations) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems. The summary of the reported bioanalytical methods is shown in Table 11.
Table 11: Bioanalytical determination of VDX

*** Not Provided
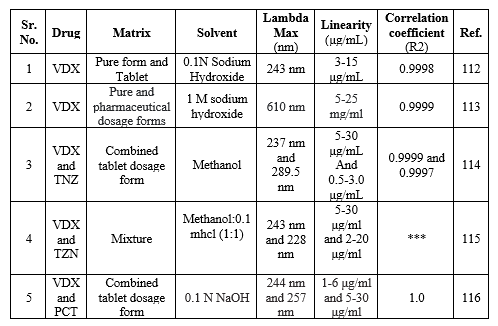
UV-Visible spectroscopy method for VDX
The spectrophotometric methods have been accounted for the determination of VDX. The details of Spectrophotometry determination of basic principle, sample matrix, lambda max, solvent linearity range and the correlation coefficient are summarized in Table 12.
Table 12: Spectrophotometric methods used for determination of VDX
*** Not Provided
Liquid-Chromatography-Mass Spectroscopy methods (LC-MS) for VDX:
In recent years, the combination of LC/MS has gained a lot of attention for the analysis of interest analytes in complex samples with improved performance. In brief, after a thorough examination, LC/MS interfaces are divided into two categories namely interfaces for indirect and direct input of column effluent. A mechanical mechanism is employed to transmit the column effluent to the MS vacuum at an indirect introduction interface. A classic example of an indirect introduction type of interface is the transportation system. In the case of the direct introduction system, the column effluent flows directly into the mass spectrometric vacuum system via a tube. Mainly, the most straightforward method of linking LC and MS appears to be the direct introduction.33 In this section, we have discussed the LC-MS methods for the determination of VDX in a dosage form Table 13.
Table 13. Summary of LC-MS methods for the determination of VDX in a dosage form

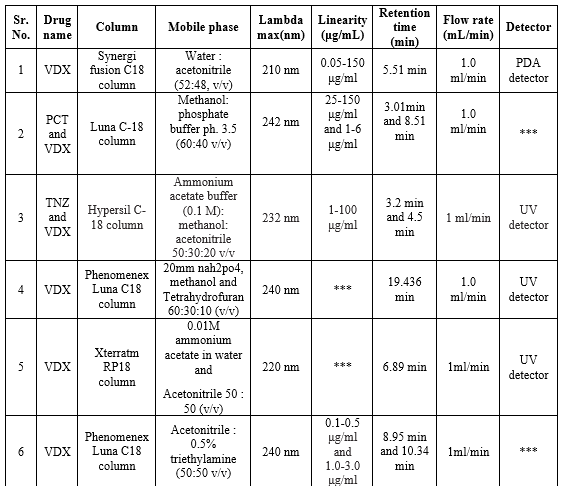
HPLC method for VDX
The specificity of the HPLC method is excellent and simultaneously sufficient precision is also attainable. However, it has to be stated that the astonishing specificity, precision, and accuracy are attainable only if wide-ranging system suitability tests are carried before the HPLC analysis. For this reason, the expense to be paid for the high specificity, precision, and accuracy is also high. The summary of the reported HPLC methods is shown in Table 14.
Table 14: Summary of HPLC methods for the determination of VDX in a single and combined dosage form
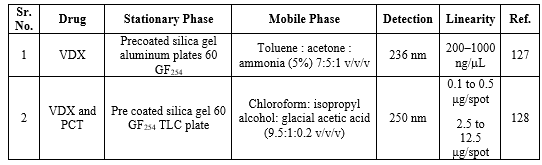
HPTLC method for VDX
Thin-layer chromatography is a popular technique for the analysis of a wide variety of organic and inorganic materials, because of its distinctive advantages such as minimal sample clean-up, a wide choice of mobile phases, flexibility in sample distinction, high sample loading capacity and low cost. The summary of the reported HPTLC methods is shown in Table 15.
Table 15: Summary of HPTLC methods for the determination of VDX in a single and combined dosage form
PARECOXIB:
Bio-analytical method for PRX
Bio-analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics (drugs and their metabolites, and biological molecules in unnatural locations or concentrations) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems. The summary of the reported bioanalytical methods is shown in Table 16.
Table 16: Bioanalytical determination of PRX
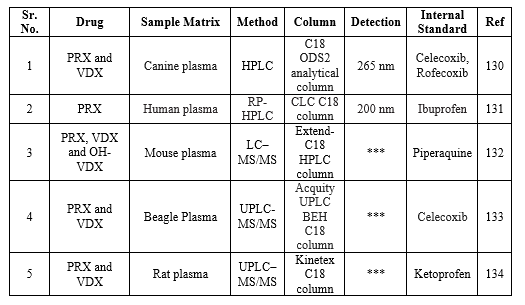
ROFECOXIB:
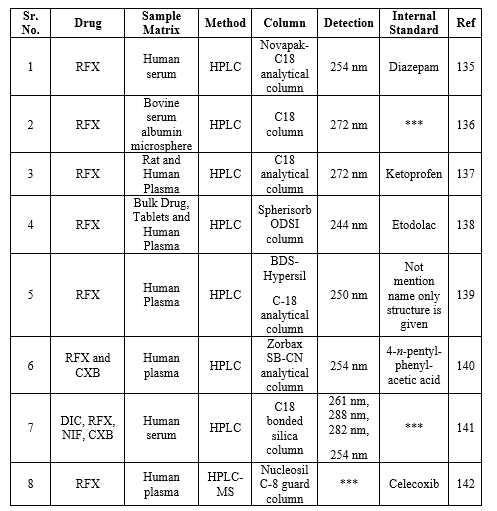
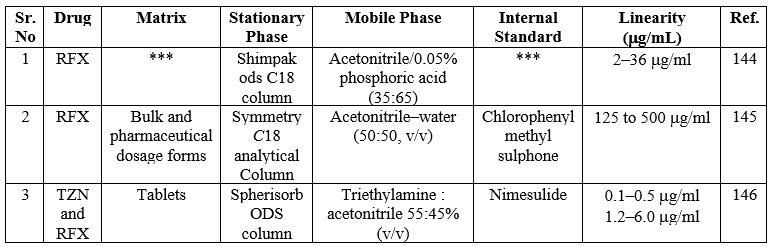
Bio-analytical method for RFX
Bio-analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics (drugs and their metabolites, and biological molecules in unnatural locations or concentrations) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems. The summary of the reported bioanalytical methods is shown in Table 17.
Table 17: Bioanalytical determination of RFX
*** Not Provided
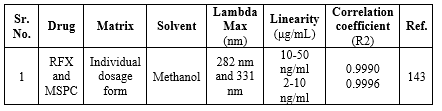
UV-Visible spectroscopy method for RFX
The spectrophotometric methods have been accounted for the determination of RFX. The details of Spectrophotometry determination of basic principle, sample matrix, lambda max, solvent linearity range and the correlation coefficient are summarized in Table 18.
Table 18: Spectrophotometric methods used for determination of RFX
Liquid-Chromatography-Mass Spectroscopy methods (LC-MS) for RFX:
In recent years, the combination of LC/MS has gained a lot of attention for the analysis of interest analytes in complex samples with improved performance. In brief, after a thorough examination, LC/MS interfaces are divided into two categories namely interfaces for indirect and direct input of column effluent. A mechanical mechanism is employed to transmit the column effluent to the MS vacuum at an indirect introduction interface. A classic example of an indirect introduction type of interface is the transportation system. In the case of the direct introduction system, the column effluent flows directly into the mass spectrometric vacuum system via a tube. Mainly, the most straightforward method of linking LC and MS appears to be the direct introduction.33 In this section, we have discussed the LC-MS methods for the determination of RFX in a dosage form Table 19.
Table 19. Summary of LC-MS methods for the determination of RFX in a dosage form
*** Not Provided
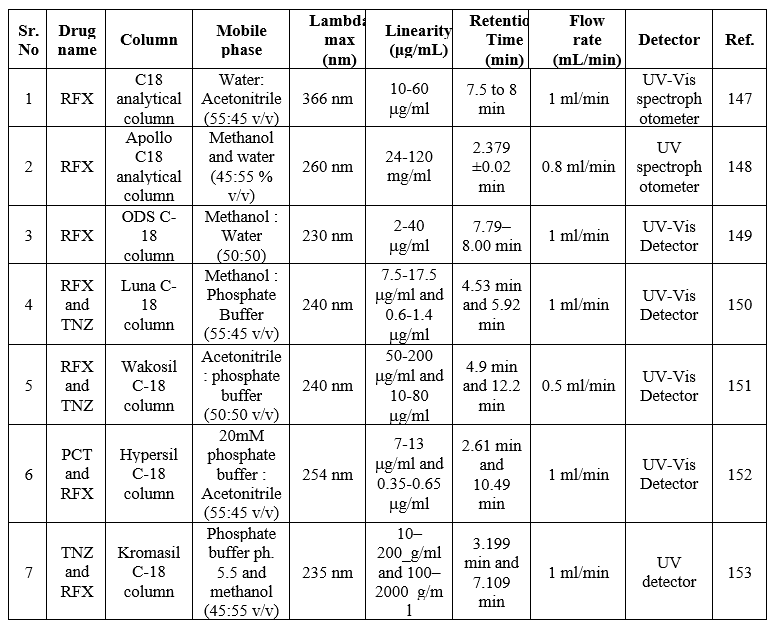
HPLC method for RFX
The specificity of the HPLC method is excellent and simultaneously sufficient precision is also attainable. However, it has to be stated that the astonishing specificity, precision, and accuracy are attainable only if wide-ranging system suitability tests are carried before the HPLC analysis. For this reason, the expense to be paid for the high specificity, precision, and accuracy is also high. The summary of the reported HPLC methods is shown in Table 20.
Table 20: Summary of HPLC methods for the determination of RFX in a single and combined dosage form
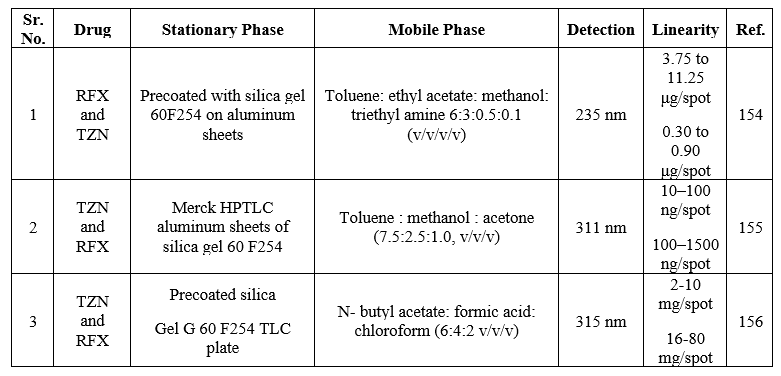
HPTLC method for RFX
Thin-layer chromatography is a popular technique for the analysis of a wide variety of organic and inorganic materials, because of its distinctive advantages such as minimal sample clean-up, a wide choice of mobile phases, flexibility in sample distinction, high sample loading capacity and low cost. The summary of the reported HPTLC methods is shown in Table 21.
Table 21: Summary of HPTLC methods for the determination of RFX in a single and combined dosage form
LUMIRACOXIB:
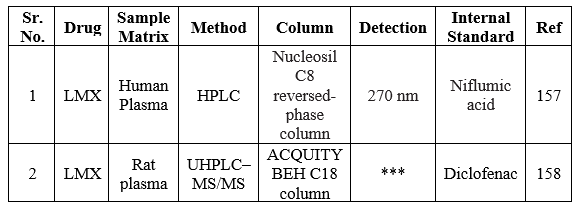
Bio-analytical method for LMX
Bio-analysis is a sub-discipline of analytical chemistry covering the quantitative measurement of xenobiotics (drugs and their metabolites, and biological molecules in unnatural locations or concentrations) and biotics (macromolecules, proteins, DNA, large molecule drugs, metabolites) in biological systems. The summary of the reported bioanalytical methods is shown in Table 22.
Table 22: Bioanalytical determination of LMX
UV-Visible spectroscopy method for LMX
The spectrophotometric methods have been accounted for the determination of LMX. The developed UV spectroscopy method is validated and therefore could be further used for quantitative analysis of lumiracoxib. Moreira, T.S., Pierre, M.B.R. , Fraga, C.A.M. , Sousa, VP established development and validation of HPLC and UV spectrophotometric methods for the determination of lumiracoxib in tablets. The UV method was performed with ethanol as a solvent with the 2-30 ?g/ml linearity. The UV method based on absorbance at 275 nm and the correlation coefficient (r2) is 0.999.(159)
Liquid-Chromatography-Mass Spectroscopy methods (LC-MS) for LMX:
In recent years, the combination of LC/MS has gained a lot of attention for the analysis of interest analytes in complex samples with improved performance. In brief, after a thorough examination, LC/MS interfaces are divided into two categories namely interfaces for indirect and direct input of column effluent. A mechanical mechanism is employed to transmit the column effluent to the MS vacuum at an indirect introduction interface. A classic example of an indirect introduction type of interface is the transportation system. In the case of the direct introduction system, the column effluent flows directly into the mass spectrometric vacuum system via a tube. Mainly, the most straightforward method of linking LC and MS appears to be the direct introduction. In this section, we have discussed the LC-MS methods for the determination of LMX in a dosage form Table 23.
Table 23. Summary of LC-MS methods for the determination of LMX in a dosage form
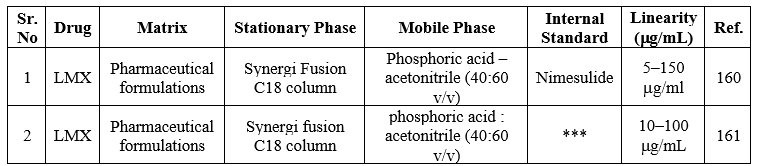
*** Not Provided
HPLC method for LMX
The specificity of the HPLC method is excellent and simultaneously sufficient precision is also attainable. However, it has to be stated that the astonishing specificity, precision, and accuracy are attainable only if wide-ranging system suitability tests are carried before the HPLC analysis. For this reason, the expense to be paid for the high specificity, precision, and accuracy is also high. The developed HPLC method is validated and therefore could be further used for quantitative analysis of lumiracoxib. Moreira, T.S., Pierre, M.B.R. , Fraga, C.A.M. , Sousa, VP established development and validation of HPLC and UV spectrophotometric methods for the determination of lumiracoxib in tablets. The HPLC method was performed on the chromatographic column was packed with propylsulfonic acid bonded with silica gel by using 10 mM phosphate buffer (pH 7.4) - water – acetonitrile (10 : 40 : 50, v/v/v) as a mobile phase at flow rate 1.0 ml/min. The linearity of the drug is 2-30 ?g/ml and the detection of drug at 278 nm by using UV detector.(159)
CONCLUSION
The present review article provides comprehensive data of various analytical and bioanalytical methods developed for Selective COX-2 Inhibitors alone and in combinations. For analysis purpose, different analytical methods have been reported that includes HPLC, HPTLC, UPLC, UV spectroscopy, etc. The method along with their details concerning the mobile phase, stationary phase, retention time, etc., have been summarized in tabular form that will more helpful for the researchers. In the future, enlisted data can be used for the development of analytical methods bio-analysis of Selective COX-2 inhibitors in pharmaceutical and biological formulations. Finally, it presents an opportunity for greater information on what has already been done and what new methods and changes can be developed to get a better estimation of Selective COX-2 inhibitors.
ACKNOWLEDGMENTS
Authors are thankful to VYWS, Institute of Pharmaceutical Education and Research, Borgaon (Meghe), Wardha, Maharashtra, India for providing necessary library facilities.
CONFLICT OF INTEREST
The authors declare that no conflict of interest
ABBREVIATIONS
- UV/VIS - Ultra violet/visible spectroscopy
- HPLC - High-performance liquid chromatography
- HPTLC - High-performance thin layer chromatography
- LC-MS/MS - Liquid chromatography-mass spectroscopy-mass spectroscopy
- UPLC – Ultra performance liquid chromatograpy
- TLC - Thin layer chromatography
- RP - Reverse phase
- nm - Nanometer
- ?g/mL - Micro gram per Milliliter
- PDA - Photo diode array
- CXB – Celecoxib
- ETX – Etoricoxib
- VDX – Valdecoxib
- PRX – Parecoxib
- RFX – Rofecoxib
- LMX – Lamiracoxib
- REP – Repaglinide
- DTX – Docetaxel
- IBU – Ibuprofen
- DIC – Diclofenac
- NIF – Niflumic Acid
- OH-CXB – Hydroxycelecoxib
- COOH-CXB – Carboxycelecoxib
- SCA – Salicylic acid
- KPF – Ketoprofen
- NMS – Nimesulide
- DEZ – Dezocine
- DEX – Dexmedetomidine
- AMD – Amlodipine
- CUR – Curcumin
- ATV-Ca – Atorvastatin calcium
- RMP – Ramipril
- PCT – Paracetamol
- RLZ – Riluzole
- THC – Thiocholchicoside
- PGBN – Pregabalin
- TOP – Tolperisone
- DRT – Drotraverine
- TNZ – Tizanidine
- OH-VDX – Hydroxylated valdecoxib
- MSPC – Mosapride Citrate?
REFERENCES:
- Chow, H.H.S., Anavy, N., Salazar, D., Frank, D.H. and Alberts, D.S., 2004. Determination of celecoxib in human plasma using solid-phase extraction and high-performance liquid chromatography. Journal of pharmaceutical and biomedical analysis, 34(1), pp.167-174.
- Dongari, N., Sauter, E.R., Tande, B.M. and Kubátová, A., 2014. Determination of Celecoxib in human plasma using liquid chromatography with high resolution time of flight-mass spectrometry. Journal of Chromatography B, 955, pp.86-92.
- Zhang, M., Moore, G.A., Gardiner, S.J. and Begg, E.J., 2006. Determination of celecoxib in human plasma and breast milk by high-performance liquid chromatographic assay. Journal of Chromatography B, 830(2), pp.245-248.
- Zarghi, A., Shafaati, A., Foroutan, S.M. and Khoddam, A., 2006. Simple and rapid high-performance liquid chromatographic method for determination of celecoxib in plasma using UV detection: application in pharmacokinetic studies. Journal of Chromatography B, 835(1-2), pp.100-104.
- Jalalizadeh, H., Amini, M., Ziaee, V., Safa, A., Farsam, H. and Shafiee, A., 2004. Determination of celecoxib in human plasma by high-performance liquid chromatography. Journal of pharmaceutical and biomedical analysis, 35(3), pp.665-670.
- Guirguis, M.S., Sattari, S. and Jamali, F., 2001. Pharmacokinetics of celecoxib in the presence and absence of interferon-induced acute inflammation in the rat: application of a novel HPLC assay. inflammation, 1, p.4.
- Reddy, M.N., Sujatha, P., Chauhan, A.S., Ramakrishna, S. and Diwan, P.V., 2003. A Sample And Sensitive Reverse-Phase High Performance Liquid Chromatographic Method For The Determination Of Celecoxib In Rat Plasma. Indian journal of pharmaceutical sciences, 65(3), p.260.
- Emami, J., FALAH, R. and Ajami, A., 2008. A rapid and sensitive HPLC method for the analysis of celecoxib in human plasma: application to pharmacokinetic studies.
- Rose, M.J., Woolf, E.J. and Matuszewski, B.K., 2000. Determination of celecoxib in human plasma by normal-phase high-performance liquid chromatography with column switching and ultraviolet absorbance detection. Journal of Chromatography B: Biomedical Sciences and Applications, 738(2), pp.377-385.
- Schönberger, F., Heinkele, G., Mürdter, T.E., Brenner, S., Klotz, U. and Hofmann, U., 2002. Simple and sensitive method for the determination of celecoxib in human serum by high-performance liquid chromatography with fluorescence detection. Journal of Chromatography B, 768(2), pp.255-260.
- Jayasagar, G., Kumar, M.K., Chandrasekhar, K., Prasad, P.S. and Rao, Y.M., 2002. Validated HPLC method for the determination of celecoxib in human serum and its application in a clinical pharmacokinetic study. Die Pharmazie, 57(9), pp.619-621.
- Arabi, M., Ghaedi, M., Ostovan, A., Tashkhourian, J. and Asadallahzadeh, H., 2016. Synthesis and application of molecularly imprinted nanoparticles combined ultrasonic assisted for highly selective solid phase extraction trace amount of celecoxib from human plasma samples using design expert (DXB) software. Ultrasonics Sonochemistry, 33, pp.67-76.
- Praça, F.S.G., Bentley, M.V.L.B., Lara, M.G. and Pierre, M.B.R., 2011. Celecoxib determination in different layers of skin by a newly developed and validated HPLC?UV method. Biomedical Chromatography, 25(11), pp.1237-1244.
- Ansari, S., 2017. Application of hollow porous molecularly imprinted polymers using K 2 Ti 4 O 9 coupled with SPE-HPLC for the determination of celecoxib in human urine samples: optimization by central composite design (CCD). Analytical Methods, 9(21), pp.3200-3212.
- Werner, U., Werner, D., Pahl, A., Mundkowski, R., Gillich, M. and Brune, K., 2002. Investigation of the pharmacokinetics of celecoxib by liquid chromatography–mass spectrometry. Biomedical Chromatography, 16(1), pp.56-60.
- Han, D.G., Kwak, J., Seo, S.W., Kim, J.M., Yoo, J.W., Jung, Y., Lee, Y.H., Kim, M.S., Jung, Y.S., Yun, H. and Yoon, I.S., 2019. Pharmacokinetic evaluation of metabolic drug interactions between repaglinide and celecoxib by a bioanalytical HPLC method for their simultaneous determination with fluorescence detection. Pharmaceutics, 11(8), p.382.
- Hamama, A.K., Ray, J., Day, R.O. and Brien, J.A.E., 2005. Simultaneous determination of rofecoxib and celecoxib in human plasma by high-performance liquid chromatography. Journal of chromatographic science, 43(7), pp.351-354.
- Ziaei, E., Emami, J., Kazemi, M. and Rezazadeh, M., 2020. Simultaneous Determination of Docetaxel and Celecoxib in Porous Microparticles and Rat Plasma by Liquid-Liquid Extraction and HPLC with UV Detection: in vitro and in vivo Validation and Application. Journal of Pharmacy & Pharmaceutical Sciences, 23, pp.289-303.
- Chamkouri, N., Zare?Shahabadi, V., Niazi, A. and Ramezani, M., 2004. Ibuprophen, diclofenac, and celecoxib quantification in human urine samples with ultrasound?assisted emulsification microextraction–HPLC and chemometrics.
- Navas, N., Urena, R. and Capitan-Vallvey, L.F., 2008. Determination of celecoxib, rofecoxib, sodium diclofenac and niflumic acid in human serum samples by HPLC with DAD detection. Chromatographia, 67(1), pp.55-61.
- Stormer, E., Bauer, S., Kirchheiner, J., Brockmoller, J. and Roots, I., 2003. Simultaneous determination of celecoxib, hydroxycelecoxib, and carboxycelecoxib in human plasma using gradient reversed-phase liquid chromatography with ultraviolet absorbance detection. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 783(1), pp.207-212.
- Pavan Kumar, V.V., Vinu, M.C., Ramani, A.V., Mullangi, R. and Srinivas, N.R., 2006. Simultaneous quantitation of etoricoxib, salicylic acid, valdecoxib, ketoprofen, nimesulide and celecoxib in plasma by high?performance liquid chromatography with UV detection. Biomedical Chromatography, 20(1), pp.125-132.
- Abdel-Hamid, M., Novotny, L. and Hamza, H., 2001. Liquid chromatographic–mass spectrometric determination of celecoxib in plasma using single-ion monitoring and its use in clinical pharmacokinetics. Journal of Chromatography B: Biomedical Sciences and Applications, 753(2), pp.401-408.
- Ma, Y., Gao, S. and Hu, M., 2015. Quantitation of celecoxib and four of its metabolites in rat blood by UPLC-MS/MS clarifies their blood distribution patterns and provides more accurate pharmacokinetics profiles. Journal of Chromatography B, 1001, pp.202-211.
- Zheng, X., Wen, J., Liu, T.H., Ou-Yang, Q.G., Cai, J.P. and Zhou, H.Y., 2017. Genistein exposure interferes with pharmacokinetics of celecoxib in SD male rats by UPLC-MS/MS. Biochemistry Research International, 2017.
- Gika, H.G., Theodoridou, A., Michopoulos, F., Theodoridis, G., Diza, E., Settas, L., Nikolaidis, P., Smith, C. and Wilson, I.D., 2009. Determination of two COX-2 inhibitors in serum and synovial fluid of patients with inflammatory arthritis by ultra performance liquid chromatography–inductively coupled plasma mass spectroscopy and quadrupole time-of-flight mass spectrometry. Journal of pharmaceutical and biomedical analysis, 49(3), pp.579-586.
- Hu, J., Su, X.J., Si, H.L., Song, R.X., Zhang, F., Qiu, X.J. and Chen, X.P., 2021. Simultaneous determination of celecoxib, dezocine and dexmedetomidine in beagle plasma using UPLC-MS/MS method and the application in pharmacokinetics. Drug Design, Development and Therapy, 15, p.2529.
- Damiani, P., Bearzotti, M. and Cabezón, M.A., 2003. A validated spectrofluorometric method for the determination of celecoxib in capsules. Analytical and bioanalytical chemistry, 376(7), pp.1141-1146.
- Karajgi, S.R., Metri, S., Tiwari, V., Hulyalkar, S., Rub, T.A. and Patil, A.S., 2016. UV spectrophotometric method for the quantitative estimation of celecoxib in capsule dosage forms. Der Pharmacia Lettre, 8(10), pp.247-257.
- Saha, R.N., Sajeev, C., Jadhav, P.R., Patil, S.P. and Srinivasan, N., 2002. Determination of celecoxib in pharmaceutical formulations using UV spectrophotometry and liquid chromatography. Journal of pharmaceutical and biomedical analysis, 28(3-4), pp.741-751.
- Attimarad, M., Venugopala, K.N., Aldhubiab, B.E., Nair, A.B., SreeHarsha, N., Pottathil, S. and Akrawi, S.H., 2019. Development of UV spectrophotometric procedures for determination of amlodipine and celecoxib in formulation: use of scaling factor to improve the sensitivity. Journal of Spectroscopy, 2019.
- Pathak, D.S., Pradhan, P.K., Meshram, D.B. and Patel, H.A., 2017. UV spectroscopic method for simultaneous estimation of celecoxib and amlodipine. Pharmawave, 10, pp.48-55.
- Attala, K. and Elsonbaty, A., 2021. Smart UV spectrophotometric methods based on simple mathematical filtration for the simultaneous determination of celecoxib and ramipril in their pharmaceutical mixtures with amlodipine: A comparative statistical study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 244, p.118853.
- Attimarad, M., Narayanswamy, V.K., Aldhubaib, B.E., SreeHarsha, N. and Nair, A.B., 2019. Development of UV spectrophotometry methods for concurrent quantification of amlodipine and celecoxib by manipulation of ratio spectra in pure and pharmaceutical formulation. PloS one, 14(9), p.e0222526.
- Mandale, T.R., Kondawar, M.S. and Kadam, S.D., 2020. Development and validation of analytical method for simultaneous estimation of amlodipine besylate and celecoxib in pure and combined dosage form. Research J. Pharm. and Tech, 13(9), pp.4280-4284.
- Vijaya Bhaskar Reddy, A., Venugopal, N. and Madhavi, G., 2014. A selective and sensitive LC-MS/MS method for the simultaneous determination of twopotential genotoxic impurities in celecoxib. Journal of Analytical Science and Technology, 5(1), pp.1-8.
- Rao, D.S., Srinivasu, M.K., Narayana, C.L. and Reddy, G.O., 2001. LC separation of ortho and meta isomers of celecoxib in bulk and formulations using a chiral column. Journal of pharmaceutical and biomedical analysis, 25(1), pp.21-30.
- Rao, R.N., Meena, S., Nagaraju, D., Rao, A.R. and Ravikanth, S., 2006. Liquid-chromatographic separation and determination of process-related impurities, including a regio-specific isomer of celecoxib on reversed-phase C18 column dynamically coated with hexamethyldisilazane. Analytical sciences, 22(9), pp.1257-1260.
- Chandana, O.S.S. and Ravichandrababu, R., 2017. Stability indicating HPLC method for celecoxib related substances in solid dosage forms. International Journal of Research in Pharmaceutical Sciences, 7(1), pp.10-18.
- Jadhav, A.S. and Shingare, M.S., 2005. A New Stability–Indicating RP-HPLC Method to Determine Assay and Known Impurity of Celecoxib API. Drug development and industrial pharmacy, 31(8), pp.779-783.
- Dhabu, P.M. and Akamanchi, K.G., 2002. A stability-indicating HPLC method to determine celecoxib in capsule formulations. Drug development and industrial pharmacy, 28(7), pp.815-821.
- Baboota, S., Faiyaz, S., Ahuja, A., Ali, J., Shafiq, S. and Ahmad, S., 2007. Development and validation of a stability-indicating HPLC method for analysis of celecoxib (CXB) in bulk drug and microemulsion formulations. ACTA chromatographica, 18, p.116.
- Attimarad, M., Venugopala, K.N., SreeHarsha, N., Aldhubiab, B.E. and Nair, A.B., 2020. Validation of rapid RP-HPLC method for concurrent quantification of amlodipine and celecoxib in pure and formulation using an experimental design. Microchemical journal, 152, p.104365.
- Gugulothu, D.B. and Patravale, V.B., 2012. A new stability-indicating HPLC method for simultaneous determination of curcumin and celecoxib at single wavelength: an application to nanoparticulate formulation. Pharm Anal Acta, 3(4), p.157.
- Abdel Hamid, M.A., Mabrouk, M.M. and Michael, M.A., 2020. A fast and green reversed?phase HPLC method with fluorescence detection for simultaneous determination of amlodipine and celecoxib in their newly approved fixed?dose combination tablets. Journal of Separation Science, 43(16), pp.3197-3205.
- Gadge, M.S. and Jagtap, V.G., Stability Indicating HPLC Method for Development and Validation of Simultaneous Estimation of Amlodipine and Celecoxib from Bulk and Marked Formulation.
- Jadhav, P.S., Jamkar, P.M. and Avachat, A.M., 2015. Stability indicating method development and validation for simultaneous estimation of atorvastatin calcium and celecoxib in bulk and niosomal formulation by RP-HPLC. Brazilian Journal of Pharmaceutical Sciences, 51, pp.653-661.
- Shah, D.B., Patel, D.B.H. and Shah, D.J.S., Response Surface Methodology Based Development and Quantification of Celecoxib and Amlodipine Using RP-HPLC.(2022). Int. J. Life Sci. Pharma Res, 12(4), pp.P75-85.
- Bapatu, H.R., Maram, R.K. and Murthy, R.S., 2015. Stability-indicating HPLC method for quantification of celecoxib and diacerein along with its impurities in capsule dosage form. Journal of chromatographic science, 53(1), pp.144-153.
- Nagamani, P., Manjunath, S.Y. and Kumar, T.H., 2020. Development and Validation of RP-HPLC Method for Estimation of Amlodipine Besylate and Celecoxib in Pharmaceutical Formulation. Journal of Drug Delivery and Therapeutics, 10(6), pp.31-36.
- Sane, R., Pandit, S. and Khedkar, S., 2004. High-performance thin-layer chromatographic determination of celecoxib in its dosage form. JPC-Journal of Planar Chromatography-Modern TLC, 17(1), pp.61-64.
- Attala, K., Eissa, M.S., El-Henawee, M.M. and Abd El-Hay, S.S., 2021. Application of quality by design approach for HPTLC simultaneous determination of amlodipine and celecoxib in presence of process-related impurity. Microchemical Journal, 162, p.105857.
- Rajan, D.S., Bose, A., Gowda, K.V., Ghosh, A. and Pal, T.K., 2006. Development and validation of an HPLC method for analysis of etoricoxib in human plasma. Indian journal of pharmaceutical sciences, 68(4).
- Ramakrishna, N.V.S., Vishwottam, K.N., Wishu, S. and Koteshwara, M., 2005. Validated liquid chromatographic ultraviolet method for the quantitation of etoricoxib in human plasma using liquid–liquid extraction. Journal of Chromatography B, 816(1-2), pp.215-221.
- Shakya, A.K. and Khalaf, N.A., 2007. High performance liquid chromatographic determination of Etoricoxib in human plasma. Asian Journal of Chemistry, 19(7), p.5241.
- Radwan, M.A., Zaghloul, I.Y. and Abd Elbaky, N.A., 2009. Stability indicating high performance liquid chromatographic assay for the pharmacokinetics of cyclooxygenase (COX-2) inhibitor etoricoxib in rats. African Journal of Pharmacy and Pharmacology, 3(7), pp.339-346.
- Dalmora, S.L., Brum Junior, L., Ferretto, R.M., Oliveira, P.R.D., Barth, T. and Sangoi, M.D.S., 2008. Determination of etoricoxib in human plasma using automated on-line solid-phase extraction coupled with LC-APCI/MS/MS. Química Nova, 31, pp.574-578.
- Bräutigam, L., Nefflen, J.U. and Geisslinger, G., 2003. Determination of etoricoxib in human plasma by liquid chromatography–tandem mass spectrometry with electrospray ionisation. Journal of Chromatography B, 788(2), pp.309-315.
- Jalakam, S.P., Waghmode, J., Pawar, P. and Mane, G., 2016. Development of Simple and Rapid LC-MS/MS Method for Determination of Etoricoxib in Human Plasma and its Application to Bioequivalence Study. Biomirror, 7.
- Brum Junior, L., Cátia Ceni, D., Fronza, M., Renato de Oliveira, P. and Luiz Dalmora, S., 2006. Validation of an LC?tandem MS/MS method for the determination of etoricoxib in human plasma and pharmaceutical formulations. Journal of liquid chromatography & related technologies, 29(1), pp.123-135.
- Werner, U., Werner, D., Hinz, B., Lambrecht, C. and Brune, K., 2005. A liquid chromatography–mass spectrometry method for the quanti?cation of both etoricoxib and valdecoxib in human plasma. Biomedical Chromatography, 19(2), pp.113-118.
- Pavan Kumar, V.V., Vinu, M.C., Ramani, A.V., Mullangi, R. and Srinivas, N.R., 2006. Simultaneous quantitation of etoricoxib, salicylic acid, valdecoxib, ketoprofen, nimesulide and celecoxib in plasma by high?performance liquid chromatography with UV detection. Biomedical Chromatography, 20(1), pp.125-132.
- Zhang, X., Guo, N., Ji, W. and Wen, Q., 2019. Rapid quantitative analysis of etoricoxib in human plasma by UPLC?MS/MS and application to a pharmacokinetic study in Chinese healthy volunteers. Biomedical chromatography, 33(2), p.e4414.
- Eure, W.D., Grossman, R.G., Horner, P.J. and Chow, D.S.L., 2021. LC-MS/MS assay of riluzole and etoricoxib in rat plasma and brain tissue with applications for sampling and evaluation in pre-clinical rat model of traumatic brain injury. Talanta Open, 4, p.100052.
- Shahi, S.R., Agrawal, G.R., Rathi, P.B., Shinde, N.V., Somani, V.G., Mahamuni, S.B. and Padalkar, A.N., 2008. Development and validation of UV spectrophotometric method for the determination of etoricoxib in bulk and tablet formulation. Rasayan J Chem, 1(2), pp.390-394.
- Chaple, D.R. and Bhusari, K.P., 2009. Spectrophotometric Methods for the Determination of Etoricoxib in Pharmaceutical Formulations. Research Journal of Pharmacy and Technology, 2(3), pp.597-8.
- Manideep, G., Shane, N.L.J., Pai, G. and Sathyanarayana, M.B., 2018. Development and validation of a UV spectroscopic method to estimate Etoricoxib in bulk and tablet formulation. Research Journal of pharmacy and Technology, 11(2), pp.758-760.
- Singh, S., Mishra, A., Verma, A., Ghosh, A.K. and Mishra, A.K., 2012. A simple Ultraviolet spectrophotometric method for the determination of etoricoxib in dosage formulations. Journal of advanced pharmaceutical technology & research, 3(4), p.237.
- Choudhari, V.P., Parekar, S.R., Chate, S.G., Bharande, P.D. and Kuchekar, B.S., 2011. Development and validation of UV-Visible spectrophotometric baseline manipulation methodology for simultaneous analysis of drotraverine and etoricoxib in pharmaceutical dosage forms. Pharmaceutical methods, 2(4), pp.247-252.
- Acharjya, S.K., Rajesh, Y., Panda, P., Mallick, P. and Annapurna, M.M., 2010. Spectrophotometric methods for simultaneous estimation of etoricoxib and thiocolchicoside in bulk and combined pharmaceutical dosage form. Journal of Pharmaceutical Education and Research, 1(1), p.75.
- Brum Jr, L., Fronza, M., Ceni, D.C., Barth, T. and Dalmora, S.L., 2006. Validation of liquid chromatography and liquid chromatography/tandem mass spectrometry methods for the determination of etoricoxib in pharmaceutical formulations. Journal of AOAC International, 89(5), pp.1268-1275.
- Gangane, P.S., Bagde, S.M., Mujbaile, S.G., Niranjane, K.D. and Gangane, P., 2014. Development and Validation of HPLC assay method for etoricoxib in bulk drug and tablet formulation. Indian J Nat Sci, 4(24), pp.1565-1625.
- Haque, M., Nasrin, S., Monir, M.M., Rahman, M.M. and Chowdhury, S., 2012. Method development and validation of RP-HPLC method of etoricoxib in bulk and its tablet dosage forms. American Journal of PharmTech Research, 2(6), pp.275-283.
- Singh, B., Santhakumar, R., Bala, I., Prasad, S.B. and Verma, S., 2014. Development and validation of RP-HPLC method for the dissolution and assay of etoricoxib in pharmaceutical dosage forms. International Journal of Pharmaceutical Quality Assurance, 6(1), pp.1-7.
- Bhattacharyya, I., Bhattacharyya, S.P., Sen, S. and Laha, T.K., 2009. Reverse Phase High Performance Liquid Chromatographic Method for the Analysis of Etoricoxib in Pharmaceutical Dosage Form. Asian Journal of Research in Chemistry, 2(3), pp.297-299.
- Patel, H.M., Suhagia, B.N., Shah, S.A. and Rathod, I.S., 2007. Determination of etoricoxib in pharmaceutical formulations by HPLC method. Indian Journal of Pharmaceutical Sciences, 69(5), p.703.
- Rao, B.S. and Nagaraju, K.S., DEVELOPMENT, VALIDATION & STRESS DEGRADATION STUDIES OF ETORICOXIB USING DICLOFENAC AS AN INTERNAL STANDARD BY HPLC.
- Shakya, A.K. and Khalaf, N.A., 2007. High Performance Liquid Chromatographic and Ultra Violet Spectroscopic Determination of Etoricoxib in Pharmaceutical Formulations. Asian Journal of Chemistry, 19(3), p.2059.
- Bagade, S.B., Meshram, D.B. and Tajne, M.R., 2011. Estimation of Etoricoxib in tablet Dosage form by RP-HPLC using Internal Standard with Emphasize on Specificity parameter Method. Oriental Journal of Chemistry, 27(2), p.697.
- Topalli, S., Chandrashekhar, T.G. and Annapurna, M.M., 2012. Validated RP-HPLC method for the assay of etoricoxib (a non-steroidal anti-inflammatory drug) in pharmaceutical dosage forms. E-Journal of chemistry, 9(2), pp.832-838.
- Venugopal, S., Tripathi, U.M. and Devanna, N., 2011. Validated Reverse Phase HPLC Method for the Determination of Impurities in Etoricoxib. E-Journal of Chemistry, 8(S1), pp.S119-S126.
- Palte, D. and Kondalkar, A., 2015. Stability studies in combine dosage form of Etoricoxib and Thiocolchicoside using RP-HPLC. Int J Res Stud Biosci, 3(9), pp.163-70.
- Singh, B., Chaudhary, A. and Sharma, A., 2022. RP HPLC Method Development for Simultaneous Estimation of Etoricoxib and Thiocolchicoside. Journal of Pharmaceutical Research International, pp.39-44.
- Zaveri, M. and Khandhar, A., 2010. Quantitative determination of Etoricoxib and Paracetamol in pharmaceutical dosage form and in-vitro comparison by reversed-phase high performance liquid chromatography (RP-HPLC). Asian Journal of Pharmaceutical Research and Health Care, 2(4).
- Kumar, S., Joshi, A., Thakur, R.S., Pathak, A.K. and Shah, K., 2011. Simultaneous estimation of etoricoxib and thiocolchicoside by RP-HPLC method in combined dosage forms. Acta Poloniae Pharmaceutica, 68(6), pp.839-843.
- Narajji, C. and Karvekar, M.D., 2011. Method development and validation for simultaneous estimation of Paracetamol and Etoricoxib in pharmaceutical dosage form by RP-HPLC method. Der Pharma Chem, 3(4), pp.7-12.
- Yeluri, R.R., Reddy, B.S. and Kumari, R.R., 2022. QUANTIFICATION OF PREGABALIN AND ETORICOXIB COMBO IN TABLETS AND BULK WITH DEVELOPED RP-HPLC METHOD: STABILITY INDICATING FEATURE ASSESSMENT. Journal of Advanced Scientific Research, 13(04), pp.31-36.
- Padmavathi, K. and Rao, M.S., 2016. Development and validation of a new stability indicating liquid chromatographic method for the simultaneous determination of thiocholchicoside and etoricoxib in combined dosage form. World Journal of Pharmaceutical Sciences, pp.76-84.
- Chaudhary, A. and Singh, B.K., 2021. Simultaneous Estimation of Pregabalin and Etoricoxib using Novel HPLC Method: An Application in Quantitative Analysis of Pharmaceutical Dosage Forms. INDIAN JOURNAL OF PHARMACEUTICAL EDUCATION AND RESEARCH, 55(3), pp.S837-S843.
- Rao, K.P. and Ramana, G.V., 2014. Cost effective isocratic RP-HPLC method for simultaneous determination of Etoricoxib and Paracetamol in pure and in tablet formulation. J Advan Stud Agric Biol Environ Sci, 1(2), pp.201-209.
- Andraws, G. and Trefi, S., 2020. Ionisable substances chromatography: A new approach for the determination of Ketoprofen, Etoricoxib, and Diclofenac sodium in pharmaceuticals using ion–pair HPLC. Heliyon, 6(8), p.e04613.
- Pattan, S.R., Jamdar, S.G., Godge, R.K., Dighe, N.S., Daithankar, A.V., Nirmal, S.A. and Pai, M.G., 2009. RP-HPLC method for simultaneous estimation of paracetamol and etoricoxib from bulk and tablets. Journal of Chemical and Pharmaceutical Research, 1(1), pp.329-335.
- Rani, K.S. and Parameshwar, P., 2021. METHOD DEVELOPMENT FOR SIMULTANEOUS ESTIMATION OF ETORICOXIB AND THIOCOLCHICOSIDE IN TABLET FORMULATION BY RP-HPLC.
- Goudar, N., Tejas, B., Badamane, M., Sathyanarayana, A.P. and Pai, V., 2022. QUANTITATIVE DETERMINATION AND VALIDATION OF ETORICOXIB AND PARACETAMOL COMBINED TABLET DOSAGE FORM BY REVERSE PHASE-HPLC. Rasayan Journal of Chemistry, 15(3), pp.1702-1708.
- Gupta, K.R., Likhar, A. and Wadodkar, S.G., 2010. Application of stability indicating HPLC Method for quantitative determination of etoricoxib and paracetamol in pharmaceutical dosage form. Eurasian J. Anal. Chem, 5(3), pp.218-226.
- Solanki, R.V., Patel, R.B., Patel, R.K. and Sheth, R.A., 2022. Development and Validation of Fast and Robust Stability Indicating RP-HPLC Method for Simultaneous Estimation of Tolperisone Hydrochloride and Etoricoxib in Pharmaceutical Dosage Form. International Journal of Pharmaceutical Investigation, 12(1), pp.56-61.
- Sumithranandan, E.S.N. and Ajitha, M., 2022. A new validated method for the estimation of pregabalin and etoricoxib an using high performance liquid chromatography and of its degradation: https://doi. org/10.54037/WJPS. 2022.101001. World Journal of Pharmaceutical Sciences, pp.1-11.
- Shah, N.J., Shah, S.J., Patel, D.M. and Patel, N.M., 2006. Development and validation of HPTLC method for the estimation of etoricoxib. Indian journal of pharmaceutical sciences, 68(6), p.788.
- Maheshwari, G., Subramanian, G.S., Karthik, A., Ranjithkumar, A., Ginjupalli, P.M.K. and Udupa, N., 2007. High-performance thin-layer chromatographic determination of etoricoxib in the bulk drug and in pharmaceutical dosage form. JPC–Journal of Planar Chromatography–Modern TLC, 20(5), pp.335-339.
- Dhaneshwar, S.R., Raut, K.O. and Bhusari, V.K., 2011. Validated HPTLC Method for Simultaneous Estimation of Paracetamol and Etoricoxib in Bulk Drug and Formulation. Asian Journal of Pharmaceutical & Biological Research (AJPBR), 1(2).
- Rajmane, V.S., Gandhi, S.V., Patil, U.P. and Sengar, M.R., 2010. High-performance thin-layer chromatographic determination of etoricoxib and thiocolchicoside in combined tablet dosage form. Journal of AOAC International, 93(3), pp.783-786.
- Shetgar, S.S., Dharmasoth, R., Rao, B.M. and Keloth, B., 2022. RP-UPLC method development and validation for simultaneous estimation of Etoricoxib and Thiocolchicoside in tablets. Journal of Applied Pharmaceutical Science, 12(2), pp.144-151.
- Sahu, P.K., Sankar, K.R. and Annapurna, M.M., 2011. Determination of Valdecoxib in Human Plasma Using Reverse Phase HPLC. E-Journal of Chemistry, 8(2), pp.875-881.
- Ramakrishna, N.V.S., Vishwottam, K.N., Wishu, S. and Koteshwara, M., 2004. Quantitation of valdecoxib in human plasma by high-performance liquid chromatography with ultraviolet absorbance detection using liquid–liquid extraction. Journal of Chromatography B, 802(2), pp.271-275.
- Keshetty, S., Venisetty, R.K., Molmoori, V. and Ciddi, V., 2006. Determination of valdecoxib in serum using a HPLC-diode array detector and its application in a pharmacokinetic study. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 61(3), pp.245-246.
- Sane, R.T., Menon, S., Deshpande, A.Y. and Jain, A., 2005. HPLC determination and pharmacokinetic study of valdecoxib in human plasma. Chromatographia, 61(3), pp.137-141.
- Saccomanni, G., Giorgi, M., Del Carlo, S., Manera, C., Saba, A. and Macchia, M., 2011. Simultaneous detection and quantification of parecoxib and valdecoxib in canine plasma by HPLC with spectrofluorimetric detection: development and validation of a new methodology. Analytical and bioanalytical chemistry, 401(5), pp.1677-1684.
- Chen, M., Sun, W., Wang, Z., Huang, C., Hu, G., Chen, Y. and Wang, L., 2020. Determination of parecoxib and valdecoxib in rat plasma by UPLC-MS/MS and its application to pharmacokinetics studies. BMC Pharmacology and Toxicology, 21(1), pp.1-10.
- Hu, J., Lv, B.F., Guo, W.J., Wang, B.W., Miao, D., Qiu, X.J. and Chen, X.P., 2020. Effects of dexmedetomidine on the pharmacokinetics of parecoxib and its metabolite valdecoxib in beagles by UPLC-MS/MS. BioMed Research International, 2020.
- Liu, M., Yu, Q., Li, P., Zhu, M., Fang, M., Sun, B., Sun, M., Sun, Y., Zhang, P., He, Z. and Sun, J., 2016. Simultaneous determination of parecoxib sodium and its active metabolite valdecoxib in rat plasma by UPLC–MS/MS and its application to a pharmacokinetic study after intravenous and intramuscular administration. Journal of Chromatography B, 1022, pp.220-229.
- Li, S.L., Zhu, Y.L., Zhu, C.Y., Li, S.B., Li, Z.H. and Qiu, X.J., 2020. Simultaneous determination of parecoxib and its metabolite valdecoxib concentrations in beagle plasma by UPLC-MS/MS and application for pharmacokinetics study. Drug Design, Development and Therapy, 14, p.1117.
- Sutariya, V.B., Mashru, R., Sankalia, M.G. and Parikh, P., 2004. Spectrophotometric estimation of valdecoxib in pure form and tablets. Indian journal of pharmaceutical sciences, 66(3), p.360.
- Suganthi, A., Sivakumar, H.B., Vijayakumar, S.C., Ravimathi, P. and Ravi, T.K., 2006. Visible spectrophotometric determination of valdecoxib in tablet dosage forms. Indian Journal of pharmaceutical sciences, 68(3).
- Nagulwar, V., Tajne, M.R., Upadhye, K., Bakhle, S. and Wadetwar, R., 2005. Simultaneous estimation of valdecoxib and tizanidine by Vierodt's and Q-analysis UV spectrophotometric method. Indian journal of pharmaceutical sciences, 67(5), p.624.
- Raju, T.S., Raghavachary, K.S.V., Raghupathi Reddy, A., Satish Varma, M., Ravikumar, M. and Yadagiri Swamy, P., 2009. A validated and stability-indicating LC assay method for Valdecoxib. Chromatographia, 69(5), pp.507-511.
- Sankar, A.S.K., An, K., Nagavalli, D., Palaniappan, M.S., Vetrichelvan, T. and Nithyan, K., 2007. Simultaneous spectrophotometric estimation of valdecoxib and tizanidine HCl in mixture. Indian journal of pharmaceutical sciences, 69(1), p.132.
- Nagulwar, V., Dhurvey, Y.R., Upadhye, K., Bakhle, S. and Wadetwar, R., 2006. UV spectrophotometric simultaneous estimation of valdecoxib and paracetamol in combined tablet dosage form. Indian journal of pharmaceutical sciences, 68(5).
- Fronza, M., Junior, L.B., Wrasse, M., Barth, T. and Dalmora, S.L., 2006. Development and Validation of a RP-HPLC Method for the Quantitation and Dissolution Studies of Valdecoxib. Acta Farmaceutica Bonaerense, 25(1), p.117.
- Bhavsar, A.S., Talele, G.S., Fursule, R.A. and Surana, S.J., 2006. RP-HPLC estimation of paracetamol and valdecoxib in combined dosage form. Indian journal of pharmaceutical sciences, 68(5).
- Ramaa, C.S., Shirode, A.R., Wamorkar, V.V., Kakad, A.B. and Kadam, V.J., 2006. Reverse-phase high performance liquid chromatographic determination of Tizanidine and Valdecoxib in tablets. Indian journal of pharmaceutical sciences, 68(4).
- Karthikeyan, K., Saravanan, R., Rajeswari, R. and Pillai, K.C., 2009. Validation of single isocratic HPLC method for the assay of valdecoxib and determination of metaisomer impurity. Journal of chromatographic science, 47(4), pp.309-314.
- Ze?evi?, M., Savi?, G. and Živanovi?, L., 2006. Development and validation of liquid chromatography method for the separation of valdecoxib and its sc?77852 impurity. Analytical letters, 39(9), pp.1875-1890.
- Selvan, P.S., Gopinath, R., Saravanan, V.S. and Gopal, N., 2006. Simultaneous estimation of tizanidine and valdecoxib in combined dosage forms by RP-HPLC method. Asian Journal of Chemistry, 18(4), p.2505.
- Subramanian, G., Faisal, M., Bhat, V., Kumar, A.R. and Udupa, N., 2006. Simultaneous RP-HPLC estimation of Tizanidine and Valdecoxib in tablets. Indian journal of pharmaceutical sciences, 68(3).
- Bhavsar, A.S., Talele, G.S., Fursule, R.A. and Surana, S.J., 2006. RP-HPLC estimation of tizanidine HCl and valdecoxib in combined dosage forms. Indian journal of pharmaceutical sciences, 68(5).
- Puranik, M., Wadher, S.J., Dhole, S. and Yeole, P.G., 2006. Simultaneous estimation of valdecoxib and tizanidine hydrochloride in tablets by RP-HPLC. Indian journal of pharmaceutical sciences, 68(5).
- Ravi, T.K., Gandhimathi, M., Suganthi, A. and Sarovar, S., 2006. Forced?degradation study of valdecoxib as bulk drug and in tablet formulation by HPTLC. Journal of separation science, 29(11), pp.1647-1652.
- Gandhimathi, M., Ravi, T.K., Shukla, N. and Sowmiya, G., 2007. High Performance Thin Layer Chromatographic Method for Simultaneous Estimation of Paracetamol and Valdecoxib in Tablet Dosage Form. Indian journal of pharmaceutical sciences, 69(1).
- Sivasubramanian, L. and Devarajan, K.E.B., 2009. HPTLC for the simultaneous determination of Tizanidine and Valdecoxib in pharmaceutical dosage form. Journal of Pharmacy Research, 2(1).
- Saccomanni, G., Giorgi, M., Del Carlo, S., Manera, C., Saba, A. and Macchia, M., 2011. Simultaneous detection and quantification of parecoxib and valdecoxib in canine plasma by HPLC with spectrofluorimetric detection: development and validation of a new methodology. Analytical and bioanalytical chemistry, 401(5), pp.1677-1684.
- Shaikh, S.M.T., Manjunatha, D.H., Seetharamappa, J. and Kandagal, P.B., 2007. High?Performance Liquid Chromatographic Determination of Parecoxib in Human Plasma and Pharmaceutical Formulations. Analytical letters, 40(15), pp.2925-2934.
- Jin, X., Zhou, F., Liu, Y., Cheng, C., Yao, L., Jia, Y., Wang, G. and Zhang, J., 2018. Simultaneous determination of parecoxib and its main metabolites valdecoxib and hydroxylated valdecoxib in mouse plasma with a sensitive LC–MS/MS method to elucidate the decreased drug metabolism of tumor bearing mice. Journal of Pharmaceutical and Biomedical Analysis, 158, pp.1-7.
- Li, S.L., Zhu, Y.L., Zhu, C.Y., Li, S.B., Li, Z.H. and Qiu, X.J., 2020. Simultaneous determination of parecoxib and its metabolite valdecoxib concentrations in beagle plasma by UPLC-MS/MS and application for pharmacokinetics study. Drug Design, Development and Therapy, 14, p.1117.
- Liu, M., Yu, Q., Li, P., Zhu, M., Fang, M., Sun, B., Sun, M., Sun, Y., Zhang, P., He, Z. and Sun, J., 2016. Simultaneous determination of parecoxib sodium and its active metabolite valdecoxib in rat plasma by UPLC–MS/MS and its application to a pharmacokinetic study after intravenous and intramuscular administration. Journal of Chromatography B, 1022, pp.220-229.
- Amini, M., Hamedani, M.P., Vosooghi, M., Nabavi, M. and Shafiee, A., 2005. Pre-column derivatization of rofecoxib for determination in serum by HPLC. Analytical and bioanalytical chemistry, 382(5), pp.1265-1268.
- Dem?rtÜrk, E., Nemutlu, E., ?ah?n, S. and Öner, L., 2020. Development and validation of an HPLC method for determination of rofecoxib in bovine serum albumin smicrospheres. Turkish Journal of Chemistry, 44(3), pp.647-655.
- Sattari, S. and Jamali, F., 2000. High performance liquid chromatographic determination of cyclooxygenase II inhibitor rofecoxib in rat and human plasma. J Pharm Pharmaceut Sci, 3(3), pp.312-316.
- Sava?er, A., Özkan, Y., Özkan, C.K., Ta?, Ç. and Özkan, S.A., 2004. RP?HPLC Assay of Rofecoxib from Pharmaceutical Dosage Forms and Human Plasma and Its Drug Dissolution Studies. Analytical letters, 37(1), pp.81-97.
- Woolf, E., Fu, I. and Matuszewski, B., 1999. Determination of rofecoxib, a cyclooxygenase-2 specific inhibitor, in human plasma using high-performance liquid chromatography with post-column photochemical derivatization and fluorescence detection. Journal of Chromatography B: Biomedical Sciences and Applications, 730(2), pp.221-227.
- Hamama, A.K., Ray, J., Day, R.O. and Brien, J.A.E., 2005. Simultaneous determination of rofecoxib and celecoxib in human plasma by high-performance liquid chromatography. Journal of chromatographic science, 43(7), pp.351-354.
- Navas, N., Urena, R. and Capitan-Vallvey, L.F., 2008. Determination of celecoxib, rofecoxib, sodium diclofenac and niflumic acid in human serum samples by HPLC with DAD detection. Chromatographia, 67(1), pp.55-61.
- Werner, U., Werner, D., Mundkowski, R., Gillich, M. and Brune, K., 2001. Selective and rapid liquid chromatography–mass spectrometry method for the quantification of rofecoxib in pharmacokinetic studies with humans. Journal of Chromatography B: Biomedical Sciences and Applications, 760(1), pp.83-90.
- Rajput, S.J., Sankalia, M.G. and Patel, F.T., 2005. Spectrofluorophotometric determination of rofecoxib and mosapride citrate in their individual dosage form. Indian journal of pharmaceutical sciences, 67(5), p.582.
- Shehata, M.A., Ashour, A., Hassan, N.Y., Fayed, A.S. and El-Zeany, B.A., 2004. Liquid chromatography and chemometric methods for determination of rofecoxib in presence of its photodegradate and alkaline degradation products. Analytica chimica acta, 519(1), pp.23-30.
- Radhakrishna, T., Rao, D.S. and Reddy, G.O., 2001. LC determination of rofecoxib in bulk and pharmaceutical formulations. Journal of pharmaceutical and biomedical analysis, 26(4), pp.617-628.
- Gandhimathi, M., Ravi, T.K. and Varghese, S.J., 2005. Simultaneous LC determination of tizanidine and rofecoxib in tablets. Journal of pharmaceutical and biomedical analysis, 37(1), pp.183-185.
- Lalla, J.K., Hamrapurkar, P.D., Yadav, S.P. and Vyas, P.M., 2004. An improved HPLC method of analysis of rofecoxib. Indian journal of pharmaceutical sciences, 66(3), p.338.
- Tadikonda, Y.K. and Dhanalakshmi, M., RP-HPLC Method Development and Validation of Rofecoxib in Bulk and Dosage Form.
- Nagoji, K.V., Vijayasrinivas, S., Kumar, M.K., Mathivanan, N., Kumar, M.S. and Rao, M.B., 2004. A new reverse phase high performance liquid chromatographic method for analysis of rofecoxib in tablets. Indian journal of pharmaceutical sciences, 66(1), p.129.
- Subramanian, G. and Udupa, N., 2004. RP-HPLC estimation of rofecoxib and tizanidine in combination tablets. Indian journal of pharmaceutical sciences, 66(5), p.699.
- Pai, P.S. and Khan, H., 2005. HPLC method for Simultaneous estimation of Rofecoxib and Tizanidine hydrochloride in Tablets. Indian journal of pharmaceutical sciences, 67(4), p.504.
- Subramanian, G., Sheety, R., Agarwal, S. and Udupa, N., 2005. Simultaneous reverse phase HPLC estimation of paracetamol and rofecoxib in tablets. Indian journal of pharmaceutical sciences, 67(2), p.247.
- Kaul, N., Dhaneshwar, S.R., Agrawal, H., Kakad, A. and Patil, B., 2005. Application of HPLC and HPTLC for the simultaneous determination of tizanidine and rofecoxib in pharmaceutical dosage form. Journal of Pharmaceutical and Biomedical Analysis, 37(1), pp.27-38.
- Pawar, U.D., Sulebhavikar, A.V., Naik, A.V., Pingale, S.G. and Mangaonkar, K.V., 2009. Simultaneous determination of rofecoxib and tizanidine by HPTLC. E-Journal of Chemistry, 6(1), pp.295-302.
- Kaul, N., Dhaneshwar, S.R., Agrawal, H., Kakad, A. and Patil, B., 2005. Application of HPLC and HPTLC for the simultaneous determination of tizanidine and rofecoxib in pharmaceutical dosage form. Journal of Pharmaceutical and Biomedical Analysis, 37(1), pp.27-38.
- Ravi, T.K., Sireesha, K.R. and Jacob, S., 2006. HPTLC method for the simultaneous estimation of Tizanidine and Rofecoxib in tablets. Indian journal of pharmaceutical sciences, 68(2).
- Cheremina, O., Brune, K. and Hinz, B., 2006. A validated high?performance liquid chromatographic assay for determination of lumiracoxib in human plasma. Biomedical Chromatography, 20(10), pp.1033-1037.
- Sun, J., Zhang, L., Zhang, L. and Liu, Q., 2021. A validated UHPLC–MS/MS method for simultaneous determination of lumiracoxib and its hydroxylation and acyl glucuronidation metabolites in rat plasma: Application to a pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis, 201, p.114105.
- Moreira, T.S., Pierre, M.B.R., Fraga, C.A.M. and Sousa, V.P., 2008. Development and validation of HPLC and UV spectrophotometric methods for the determination of lumiracoxib in tablets. Revista de Ciências Farmacêuticas Básica e Aplicada, 29(3).
- Sangoi, M.S., Wrasse?Sangoi, M., Oliveira, P.R. and Bernardi, L.S., 2011. Determination of lumiracoxib by a validated stability?indicating MEKC method and identification of its degradation products by LC?ESI?MS studies. Journal of separation science, 34(15), pp.1867-1874.
- Oliveira, P.R., Bernardi, L.S., Mendes, C., Sangoi, M.S. and Silva, M.A., 2010. Liquid chromatographic determination of lumiracoxib in pharmaceutical formulations. Journal of pharmaceutical and biomedical analysis, 51(3), pp.728-732.
- https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadConsumer/banneddrugs.pdf


 Vinay V. Sarode* 1
Vinay V. Sarode* 1



































 10.5281/zenodo.11183353
10.5281/zenodo.11183353