Abstract
Herbal plants have been used worldwide for several years since ancient time up to till date for treatment of various ailments in the human beings. Because herbal has large amounts of active phyto compounds found it. In this study, chemicals seen in natural products derived from Holy basil (Ocimum sanctum Linn.) leaves, whether in the form of pure chemicals, offers countless possibilities for developing new therapeutic drugs. Global interest in edible plants has increased due to the growing need for chemical diversity in screening programs and the search for therapeutic medicines from natural sources. Holy basil (Ocimum sanctum Linn.) leaves preparations contain a variety of bioactive compounds. The extraction, isolation, and characterization of active ingredients such as Isothymusin, Oleanolic acid and Ursolic acid are analyzed in this paper. The present discourse delves into the examination of bioactive chemicals found in Holy basil (Ocimum sanctum Linn.) leaves through the use of standard qualitative and quantitative techniques like TLC and HPLC.
Keywords
Holy basil, Ingredients, Phenolic compounds, Therapeutic medicines.
Introduction
India is one of the 12 major biodiversity countries in the world and has around 8% of the globe’s estimated biodiversity with a lot of different 12600 medicinal species. Tulsi (Ocimum sanctum) is a widely popular and highly prestigious, wonder sacred medicinal plant in India commonly known as Holy basil or ‘’Tulsi’’ belonging to the family Lamiaceae. This plant can grow in tropical and semitropical regions of India. Most people they kept Tulsi as a household plant for home remedy purposes. Natural products will provide incomparable compounds from medicinal plants for the creating plant-based drugs. But the wild plant variety has a unique complex mixture of phyto-chemical constituents and these compounds are not easy to separate and purify. The whole Tulsi plant parts such as flower, seed, stem, root will be used for therapeutic purpose against various illnesses in humans. The plant also harboring potential bioactive compounds available which is known as Herbal Medicine [1]. Ocimum sanctum has been used in the cosmetic and pharmaceutical industries for the production of medicines, tonics, and cosmetics [2]. Currently, human beings as well as domestic animals are unexpectedly infected by various pathogens. The medicinal plant parts and their ingredients have medicinal properties for treating many diseases. Tulsi plant leaf ingredients contain a lot of secondary metabolites are accountable for creating phytodrugs against disease resistant pathogens [3]. Tulsi has widely potential bioactive chemicals that are antibacterial, antiviral, adaptogenic, and immune-enhancing properties and also a strong antioxidant, that promote general health and support the body’s natural defense against stress and diseases[4]. The phenolic compounds have antioxidant potential also its redox property and act as reducing agents, hydrogen donors, and singlet oxygen quenchers [5]. Tulsi plant extract possess three groups of bioactive compounds are phenolic di and tri terpenes, flavonoids, phenolic acids, sterols, along with other compounds like glycosides, saponins, tannins, carbohydrates, and proteins, [6, 7]. The unique phytochemical properties of the Tulsi is highly complex. There are numerous beneficial compounds known as phytochemicals. The phytocompounds of Tulsi plant has been used in the cosmetic and pharmaceutical sectors for the production of medicines, tonics and cosmetics from its secondary metabolites and essential oils. In this study Tulsi leaf has significant amount of phytoconstituents such as alkaloids, flavonoids, glycosides, Phenolic, saponins, triterpenoids, tannins, carbohydrates, and proteins which indicate the highly active against various illness. We focus on the separation and identification of bio-active compounds from methanolic extract of Tulsi leaf by using modern techniques likewise Thin Layer Chromatography and Reverse Phase High Performance Chromatography. This study highlights the potential pharmacological qualities of Tulsi plant leaf extract in methanol, which could be advantageous for the medical industry.
MATERIAL AND METHODS
- Collection of plant material and extraction
Fresh and healthy leaves of a wild variety of Tulsi (Ocimum sanctum L.) were collected from the garden of the Holy Redeemer convent church in Theni city, Tamil nadu, India and this plant was identified and authenticated at PG and Research Department of Botany, Thiagarajar College, Madurai, Tamil Nadu, India. The leaves were washed thoroughly with sterile water and dried in room temperature for five days and ground well. Then 1Kg powdered material was soaked in 2.5 L methanol and kept in a refrigerator for seven days. Then it was filtered with Whatmann No 1 filter paper to get methanolic extract using Buchi Rotavapor R-210 (Switzerland) at 40 °C [8,9,10,11]. Finally, the dried extract is obtained and stored at room temperature. The extract was used for investigation of TLC and HPLC.
TLC bio autographic analysis
Four spots of the concentrated methanolic Tulsi extract were loaded on the TLC plates just above 1.5 cm from the bottom using capillary tube. Each spot had a gap of 1.2 cm. Thin layer chromatography (TLC) for Tulsi leaf extract was performed on Merck-silica Gel 60 plates, with Hexane:Ethyl acetate (60:40 v/v) as mobile phase. The separated components were visualized under Ultraviolet and Visible light (254 and 360 nm) [12]. The separated compounds were shown in the TLC plate 1. Rf Values were calculated.
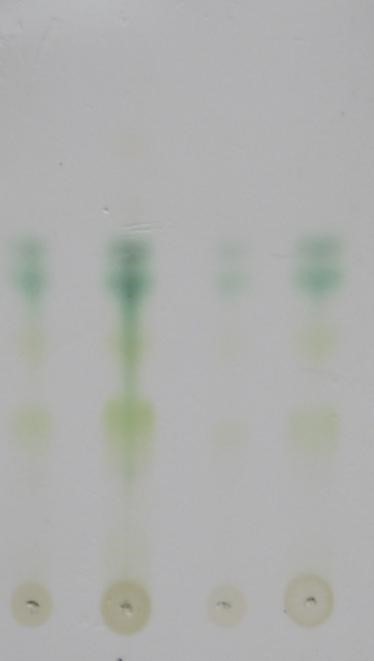
Plate: 1 TLC of Tulsi Leaf Extract
Qualitative analysis of secondary metabolites by RPHPLC system
The separation of compounds were performed by Reversed-phase HPLC (RP-HPLC) system. The injection volume was set at 20 µl. The dissolved compound was injected into the LC-8A Shimadzu C18 column (5 µm; 250 x 4.60 mm). A gradient (100%) of an HPLC grade acetonitrile-water system was setup over 45 min at a flow rate of 0.5 mL/min with detection at 290 nm. The peaks were identified by comparing the retention times of the reference standards with the extract. This study describes the chemical investigation of the methanolic leaf extracts of Ocimum sanctum contains most of the polar and non-polar compounds were effectively identified by RPHPLC method. The separation of the compounds were undertaken for a complete identification of the data assignment. The pure compound was isolated by drying and the precipitate and dissolved in HPLC grade of methanol.
RESULTS
Isothymusin
The RPHPLC profiles for the methanolic extract of Ocimum sanctum Leaf are shown for the detector wavelength 270 nm. The one phenolic component (peak) was
identified by this analysis as the following known compound: Isothymusin (C17H14O7). The peak identification of this compound is depicted in reference standard Fig1. This peak was previously reported at retention time of 4.2 mint for the isothymusin compound [13]. The results obtained through this peak showed the retention time at 3.709 mint Fig.2.
Fig No.1 Reference standard Isothymusin
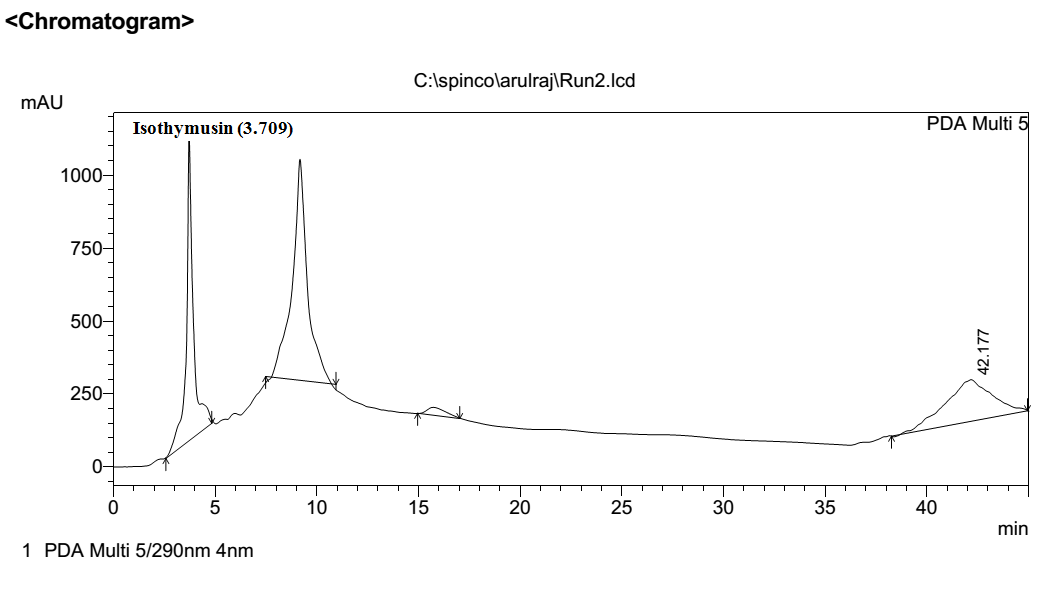
Fig No.2 RPHPLC chromatogram of Isothymusin extracted from holy basil leaf
The phenolic compounds of Isothymusin (C17H14O7) is a natural substance and extractive that appears as a yellow powder. It is also known as 6,7-dimethoxy-5,8,4'-trihydroxyflavone. (Figure 3).
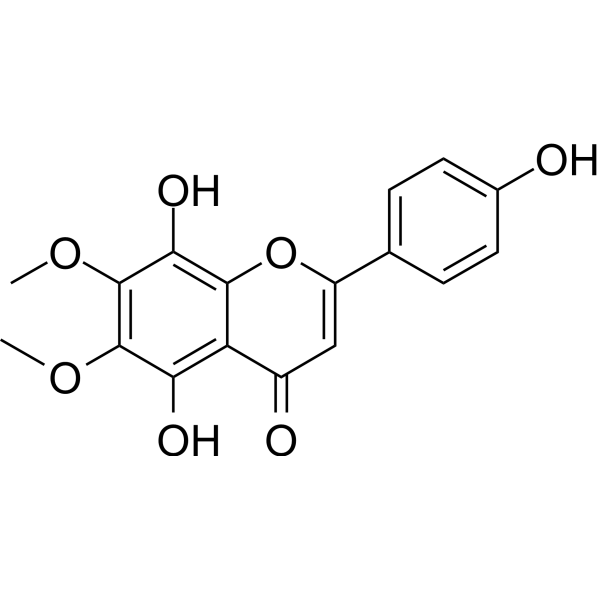
Fig No.3 Structure of Isothymusin
Oleanolic acid
This compound was previously [14] at retention time of 9.2 mint (Fig. 4). This Tulsi extract separated and its retention time 8.2 mint (Fig.5). The result peak was compared with reference standard.
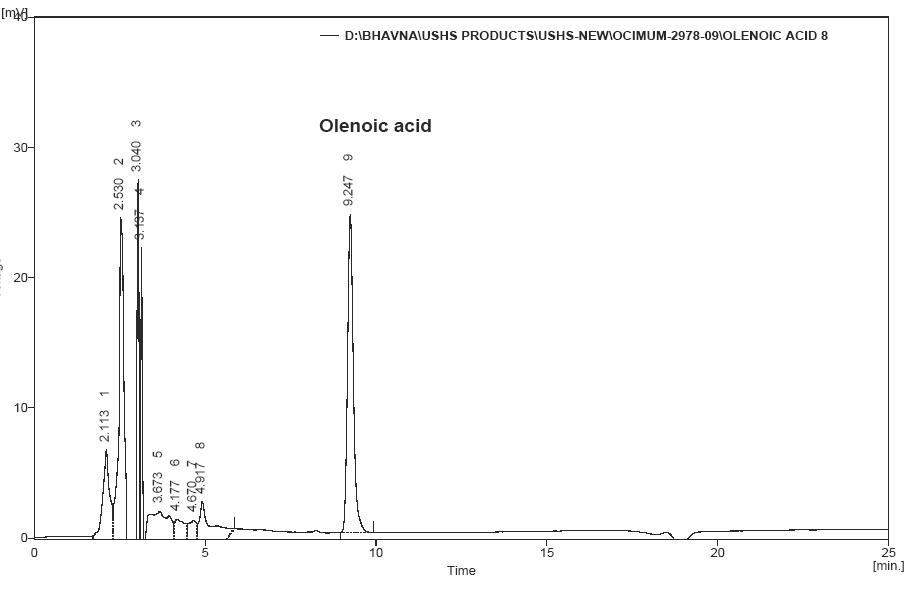
Fig No.4 Reference standared Oleanolic acid
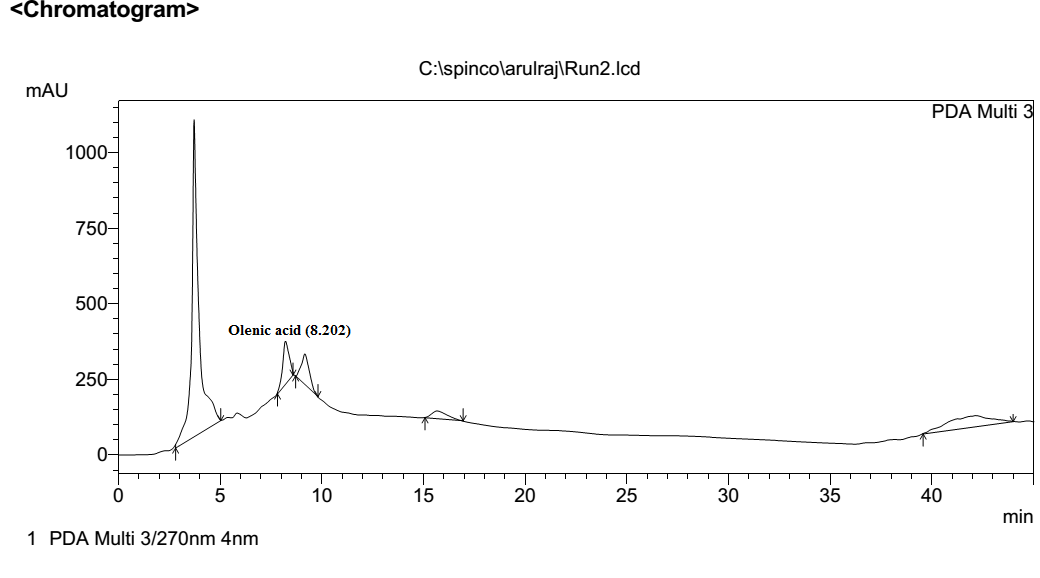
Fig No.5 RPHPLC chromatogram of Oleanolic Acid extracted from holy basil leaf
The oleanolic acid (C30H48O3), which is fundamentally a pentacyclic triterpenoid, exists as a free acid.
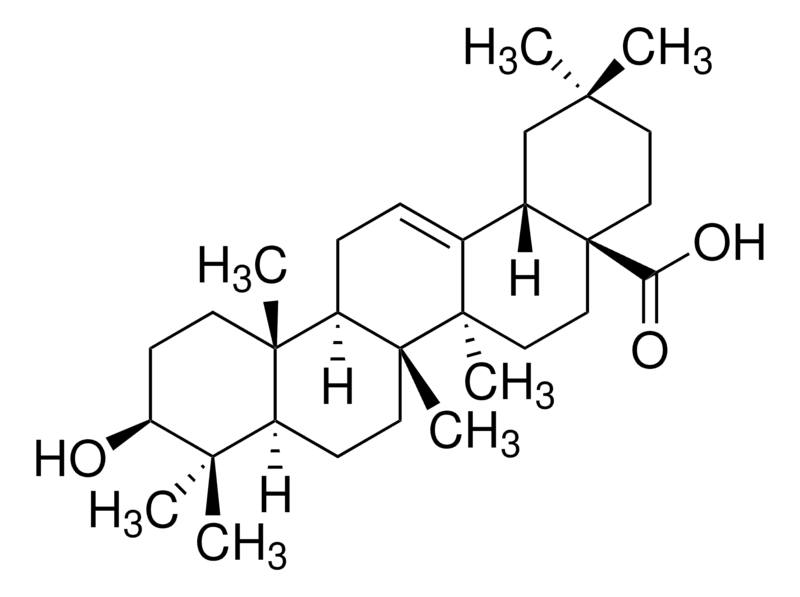
Fig No.6 Structure of Oleanolic acid
Ursolic acid
This peak was previously reported [14] at retention time of 9.47 mint (Fig.7). This compound was separated and its retention time 9.16 mint. (Fig.8). This result was compared with reference standard.
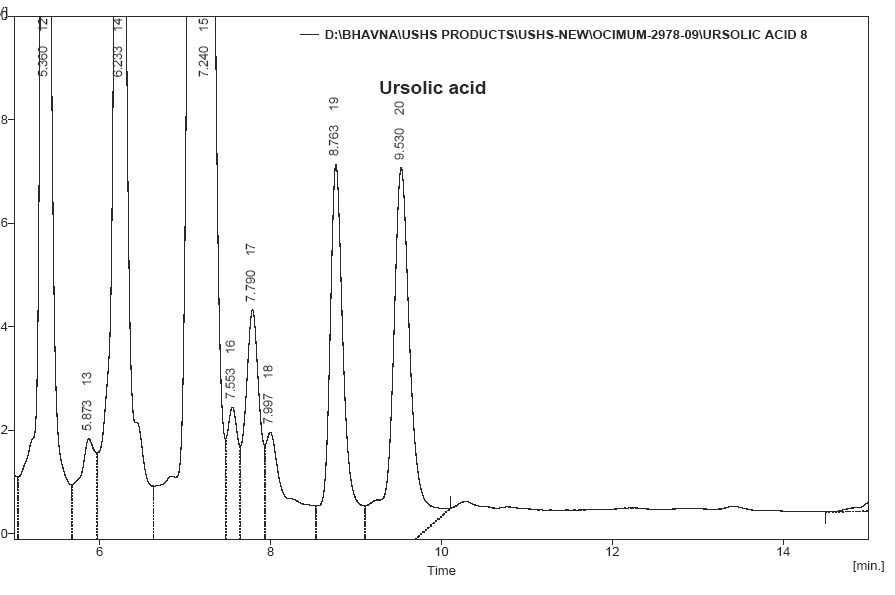
Fig No.7 Reference standard of Ursolic acid
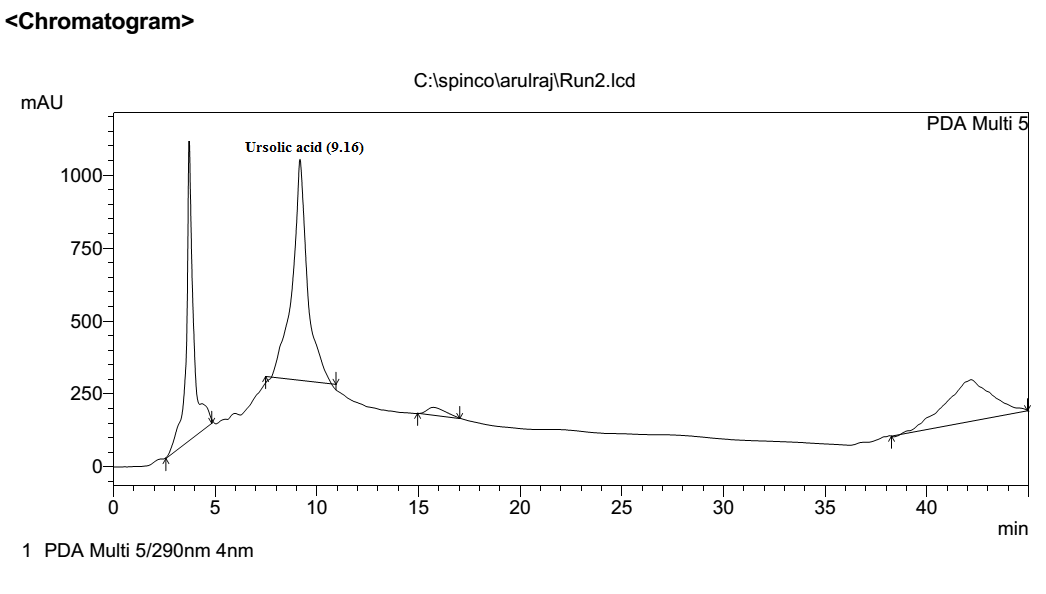
Fig No.8 RPHPLC chromatogram of Ursolic Acid extracted from holy basil leaf
Ursolic Acid (C30H48O3) is a pentacyclic triterpenoid found in various fruits, vegetables and medicinal herbs.
Fig No.9 structure of Ursolic acid
DISCUSSION
Isothymusin, Oleanolic acid and Ursolic acid
The presence of the phenolic compound Isothymusin (IT) in holy basil plant leaf has been documented previously. The phenolic compounds, such as cirsilineol, cirsimaritin, isothymusin, apigenin and rosmarinic acid, and appreciable quantities of eugenol (a major component of the volatile oil) from OS extract of fresh leaves and stems found great antioxidant activity [15]. Similar study was carried out on the methanolic extract of Ocimum basilicum var.basilicum, Ocimum basilicum varieties for anti-arthritic activity as well as quantification of three marker compounds such as ursolic acid, oleanolic acid and eugenol by HPTLC and HPLC and to check interspecies [16]. The demonstration of Ocimum sanctum plant and its bioactive compounds such as Vicenin, Caryophyllene, Cirsimaritin, Isothymusin and Isothymonin had significant binding activity to COVID-19 Main protease enzyme (Mpro) and led to the potential control viral multiplication and showed the immunomodulatory activity in the host cells [17]. Based on the literature survey phytochemical investigation of Ocimum sanctum showed the presence of peroxidase enzyme and a substantial quantity of phenolic compound especially isothymusin which had anti-oxidant property [18].
The air-dried leaves of Ocimum gratissimum (4.1 kg) were extracted with ethanol and the solvent was removed under reduced pressure given a solid which was submitted to a chromatographic silica gel column, sequentially eluted with hexane, dichloromethane, ethyl acetate and methanol, Ursolic acid is a very important compound due to its biological potential as an anti-inflammatory, trypanocidal, antirheumatic, antiviral, antioxidant and antitumoral agent. In that study presents the HPLC analysis of ursolic acid (UA) content in eight different Ocimum species: O. americanum L., O. basilicum L, O. basilicum var purpurascens Benth, O. basilicum var. minimum L, O. gratissimum L, O. micranthum Willd, O. selloi Benth. And, O. tenuiflorum L. grown in Northeastern Brazil [19]. Tandem electrospray ionization mass spectrometry (ESI-MS) has been performed on Tulsi leaf extracts in methanol to establish the identity of the compounds were Ursolic acid (UA) and oleanolic acid (OA) [20]. HPLC analysis of the Methanolic Tulis leaf extract was carried out for the presence of eugenol [21]. The result obtained from UV and HPLC analysis shows that the, Ocimum sanctum Linn. contains higher amount of eugenol and established Ultra-violet spectroscopy and high-performance liquid chromatographic method for quantification of the phenolic compound or Eugenol in the leaves of the plant has been sensitive and reliable [22]. The HPLC analysis of methanolic and aqueous leaf extracts of Tulsi confirmed the presence of Urosolic acid(UA) Oleanolic acid (OA), and Betulinic acid (BA) [23]. The solvent system contains Toluene-ethyl acetate-acetic acid 30:3:1 which was used for presence of bioactive compounds such as Urosolic acid as determined by HPTLC [24]. Its leaf contains volatile oil eugenol, euginal (also called eugenic acid), ursolic acid, carvacrol, linalool, limatrol, caryophyllene, methyl carvicol (also called Estragol), sitosterol, anthocyans are found in this plant [25,26,27].
The hepatoprotective effect of Oleanolic Acid (OA) and Ursolic Acid (UA) against liver damage induced by anti-TB drugs lengthens the list of the multiple biological activities possessed by these triterpenes, highlighting the already reported antimicrobial activity and antitubercular effect of the mixture [28]. The higher amount of ?-tocopherol and polyphenolic compounds probably contributes to greater antimicrobial activity of transgenic Codonopsis lanceolata (Bonnet bell flower against Salmonella typhimurium, Klebsiella pneumoniae, and Escherichia coli, [29]. With regard to the mixture of Triterpenes Ursolic acid the natural product-of-interest in the ongoing study, in vivo anti-TB activity was already demonstrated in an experimental mouse model of progressive pulmonary TB, and a significant reduction of bacterial loads and pneumonia with a higher expression of Interferon gamma (IFNg) and Tumor necrosis factor alpha (TNF-a) in the lungs was described [30]. A recent study demonstrated that Ursolic acid had a nephroprotective effect by attenuating renal damage induced by toxic compounds suggesting that this natural product has the potential to be considered as a prototype drug [31]. A recent report stated that the biochemical parameters associated with hepatic and renal functions were evaluated by Golden hamsters (Mesocricetus auratus), treated with UA isolated from the leaves of Baccharis uncinella (Asteraceae) did not present alterations in the levels of aspartate aminotransferase (AST), seric alanine transaminase (ALT), creatinine and urea. Taken together, these findings indicate that UA is an interesting natural compound that should be considered for the development of prototype drugs against visceral Leishmaniasis [32]. The mixture of Ursolic Acid (UA) and Oleanolic Acid (OA) was obtained from methanolic extract of Bouvardia ternifolia aerial parts. Mice treated with the mixture of triterpenic acids exhibited significantly decreased aspartate transaminase and alanine aminotransferase levels and amelioration of the histopathological alterations produced by the anti-TB drugs which induced liver toxicity [33]. The presence of Oleanolic Acid in the Tulsi plant exhibited anti-ageing and anti-oxidant property [34]. Evidences both in vitro and in vivo suggest that Ursolic Acid (UA) possesses multi-fold biological properties, including anti-inflammatory, anti-oxidative, hipolipidemic and a hepatic metallothionein inducer property, hepatoprotector effect, as well as other significant effects [35,36,37].
CONCLUSION
Plant phenolic compounds are mostly secondary metabolites possessing high antioxidant activity and are widespread in the family members of Lamiaceae. Methanolic Tulsi leaf extract subjected to the separation and quantification of phenolic and pentacyclic triterpenoid compounds by Thin Layer Chromatography (TLC) and Reversed-phase HPLC (RP-HPLC) were almost widely used. This current study reveals that Tulsi plant has rich bioactive compounds such as Isothymusin, Oleanolic acid and Ursolic acid. Therefore, further analysis is recommended to confirm and purify the biologically active phyto-compounds of this plant to optimize the production of these compounds in the medical and food industry.
ACKNOWLEDGMENT
Authors are sincere gratitude to the Thiagarajar College Principal and Management, The Chariperson, School of Biological Sciences, and UGC-Networking Resource Center for Biological Sciences, (UGC – NRCBS), Madurai Kamaraj University, Madurai, Tamil Nadu, India.
REFERENCES
- Nikhil, S.G., Chaitanya, D.G, Amol., G.J., & Prashant, A.P. (2021). Study of medicinal uses of Ocimum sanctum (Tulsi). J Pharmacogn Phytochem, 10(2), 1427-31.
- Rakesh, K.J., William, N.S., & Joyce Kelly, D.S. (2016). Phytoconstituents,Traditional medicinal uses and bioactivities of Tulsi (Ocimum sanctum Linn.): A review. American Journal of Essential Oils and Natural Products, 5, 18-21
- Anjana, S., Rani, V., & Padmini, R. (2008). Anti- bacterial activity of some medicinal plants used by tribals against uti causing pathogens. World Applied Sciences Journal, 7(3), 332-339.
- Kruti, P., Bhavna, S., Kunal, M., Nilesh, G., & Surendra, B. (2011). Natural Herbal Supplements-A Study on Their Nutritional Value and Their Phytochemical Constituents. International Journal of Pharmaceuetical Sciences and Research, 14;2(6), 1480-1494.
- Caragay, A.B. (1992). Cancer-preventive Foods and Ingredients. Food Technology, 46(4), 65-68.
- Aruoma, O.I., Halliwell, B., Aeschbach, R., & Loliger, J. (1992). Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica, 22, 257-68.
- Raj, K.J., Reecha, M., & Balbir, S. (2003). Anticonvulsant potential of holy basil, Ocimum sanctum Linn. and its cultures. Indian Journal of Experimental Biology, 41(11), 1329-1333.
- Cooper Gunn: Tutorial pharmacy, In: Carter S.J. (Ed.). New Delhi:CBS Publishers and Distributors, 2005
- Ghule, B.V., Murugananthan, G., Nakhat, P.D., & Yeole, P.G. (2006). Immunostimulant effects of Capparis zeylanica Linn. Leaves. Journal of Ethnopharmacology, 108, 311-315.
- Rajbir, S., Sukhpreet, S., Subodh, K., & Saroj A. (2007). Free radical-scavenging activity of acetone extract/fractions of Acacia auriculiformis A. Cunn. Food Chemistry, 103(4), 1403-1410.
- Venkatalakshmi, S., & Dinakaran Michael, R. (2001). Immunostimulation By leaf extract of Ocimum sanctum linn. in Oreochromis mossambicus (Peters). Journal of Aquaculture in the Tropics, 16(1), 1-10.
- Harish Kumar, K., Hullatti, K.K., Sharanappa, P., & Paras, S. (2010). Comparative antimicrobial activity and TLC-Bioautographic analysis of root and aerial parts of Andrographis serpyllifolia. International Journal of Pharmacy and Pharmaceutical Sciences, 2(1), 52-54.
- Lukmanul Hakkim, F., Gowri Shankar, C., & Girija, S. (2007). Chemical Composition and Antioxidant Property of Holy Basil (Ocimum sanctum L.) Leaves, Stems, and Inflorescence and their in Vitro Callus Cultures. Journal of Agricultural and Food Chemistry, 55(22), 9109-9117.?
- Kruti P, Bhavna S, Kunal M, Nilesh G, Surendra B. Natural herbal supplements – A study on their nutritional value and their phytochemical constituents, International Journal of Pharmaceutical Sciences and Research. 2011 June 01;2(6):1480-1494.
- Kelm, M.A., Nair, M.G., Strasburg, G.M., & DeWitt, D.L. (2000). Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine, (1), 7-13.
- Sapna, P., Rahul, P., Vaibhav, S., & Kakasaheb, M. (2013). Comparative Phytochemical And Pharmacological Evaluations of Two Varieties of Ocimum Basilicum for Antiarthritic Activity. Journal of Pharmacognosy and Phytochemistry, 2(2), 158-167.
- Nem Kumar, J., Anurag, A., Giriraj, T.K., & Mukul, T. (2021). Molecular Docking Study On Phytoconstituents Of Traditional Ayurvedic Drug Tulsi (Ocimum Sanctum Linn.) Against Covid-19 Mpro Enzyme: An In Silico Study. International Journal of Pharmacy and Pharmaceutical Sciences, 4(4), 44-50.
- Deepak, P., Prativa, B., Jitu, H., Priya, C., Debasmita, D., Vineet, K.R., Biswakanth, K., Durga, M.K., Goutam, R., & Goutam, G.A. (2022). Comprehensive review on phytochemistry, molecular pharmacology, clinical and translational outfit of Ocimum sanctum L. South African journal of Botany, (150), 342-360.
- Silva, V.G.M., Vieira, P.G.I., Mendes, P.N.F., Albuquerque, L.I., Dos Santos, N.R., Silva, O.F. (2008). Morais MS. (2008). Variation of Ursolic Acid Content in Eight Ocimum Species from Northeastern Brazil. Molecules, (13), 2482-2487.
- Depanjan, S., Amitava, S., & Pradeep, T. (2012). Rapid identification of molecular changes in tulsi (Ocimum sanctum Linn) upon ageing using leaf spray ionization mass spectrometry. This journal is ª The Royal Society of Chemistry, Analyst, (137), 4559–4563.
- Bhatt Mehul, K., Shankar, M.B., Saluja Ajay, K., Dholwani Kishor, K., & Captain A.D. (2012). Evaluation of anti-microbial activity of Ocimum sanctum methanolic extract. Journal of Pharmaceutical And Scientific Innovation, 1(4), 1-3.
- Sharma, V., Joshi, A., & Dubey, B.K. (2012). Development of an analytical method to determine eugenol concentrations in alcoholic extracts of different species of Ocimum’s. International Journal of Phytopharmacy, 1(2), 35-42.
- Ali, R., Chauhan, V., Farooq, S., Khan, A., & Farooq, U. (2014). In-vitro Analysis of Antibacterial Activity of Ocimum sanctum Against Pathogenic Bacteria and Quantification of Ursolic Acid and Oleanolic Acid. International Journal of Pharmaceutical Sciences Review and Research, 25(2), 13-17.
- Ravindra, P., Swarnlata, S., & Saraf, S. (2007). HPTLC: A prominent tool for standardization of herbals. Natural Products An Indian Journal Review, 3(3), 118-125.
- Priyabrata, P., Pritishova, B., Debajyoti, D., & Sangram, K.P. (2010). Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn Rev, 4(7), 95-105.
- Shishir, S., Sekhar, M., Sanjeev, B., & Bharat, B.A. (2003). Ursolic acid inhibits nuclear factor-kappa B activation induced by carcinogenic agents through suppression of I kappa B alpha kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9 and cyclin D1. Cancer Res, 63(15), 4375-4383.
- Shahedur, R., Rezuanul, I., Kamruzzaman, M., Khasrul, A., & Abu Hena, M.J. (2011). Ocimum sanctum L. A Review of Phytochemical and Pharmacological Profile. American Journal of Drug Discovery and Deveolpment, 1-15.
- Ghimire, B.K., Seong, E.S., Yu, C.Y., Kim, S-H., Chung, I-M. (2017). Evaluation of phenolic compounds and antimicrobial activities in transgenic Codonopsis lanceolata plants via overexpression of the ?-tocopherol methyltransferase (?-tmt) gene. South African Journal of Botany, 109, 25–33.
- Songtao, L., Xilu, L., Fanyu, M., Yemei, W., Zongxiang, S., Fuchuan, G., Xiaoxia, L., Man, M., Ying, L., & Changhao, S. (2014).Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS One, 9(1), 86724.
- Adelina, J.A., Julieta, L.H., Jorge, C.G., Sonia, L.G., María Eugenia, C.M., Mariana, M.F., Dulce, M.E., Brenda, M., Javier, T., & Rogelio, H.P. (2013). Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement Altern Med, 13, 258.
- Preethi, G.P., Savindika, C.N., Avdhooth, K., Umma, H., Sudarshan, R.C., Srinivas, T., & Jnaneshwara, P.S. (2012). Nephroprotective Effect of Ursolic Acid in a Murine Model of Gentamicin-Induced Renal Damage. ISRN Pharmacol, 2012, 1-6.
- Jessica, A.J., Thais, N.F., Eduardo, S.Y., Marcia, D.L., Marcelo, S.S., Aurea, F.F., Joao Henrique, G.L., Gabriela, S.G., & Luiz Felipe, D.P. (2017). Therapeutic Effect of Ursolic Acid in Experimental Visceral Leishmaniasis. International Journal for Parasitology: Drugs and Drug Resistance, 7(1), 1-11.
- Gabriel A Gutiérrez, R., Georgina Siordia, R.A., Mariana, M.F., & Adelina, J.A. (2016). Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver Damage. Asian Pacific Journal of Tropical Medicine, 9(7), 644–651.
- Shweta, S. K., Nilesh, R. B., Divyani, D. P., Pavan, P. K., Prashant, S. B. (2023). A Research on Futuristic Magical effect of Oleanolic Acid, Asian Journal of Pharmacy and Technology, 13(4), 1-2.
- Jorge, C.G., Germán, C., Leticia, G.S., Rogelio, H.P., (2012). María Adelina JA. Acute and subacute toxicity (28 days) of a mixture of ursolic acid and oleanolic acid obtained from Bouvardia ternifolia in mice. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, 11(1), 91-102.
- Yasutaka, I., Akira, M., & Hajime, O. (2008). Ursolic acid: an anti- and pro-inflammatory triterpenoid. Molecular Nutrition Food Research, 52(1), 26-42.
- Liu, J. (1995). Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol, 49(2), 57-68


 Arulraj Jeyaraj*
Arulraj Jeyaraj*










 10.5281/zenodo.14301560
10.5281/zenodo.14301560