Abstract
The present study was aimed to formulate, evaluate, and optimize a tablet that disintegrates and dissolved rapidly toshow a rapid onset action. Diclofenac sodium, non-steroidal- inflammatory, analgesic, and pyretic properties were selected asa model drug. Diclofenac sodium is among the most extensivelyused Non-steroidal anti-inflammatory drugs, employed inmucosal skeletal complaints, especially arthritis. In the present study, an attempt has been made to prepare fast dissolvingtablets of diclofenac sodium using super disintegrants like sodium starch Glycolate, Croscarmellose sodium, Crospovidone and starch by direct compression technique using 3 different concentrations of super disintegrants. The pre- compression parameters of the tablet-like hardness, friability, weight variation, disintegration time, in-vitro dissolution releaserate, and drug content. It was concluded that the batch which was prepared by using a combination of crospovidone and sodium starch Glycolate as a superdisintegrants shows excellentdisintegration time, enhance dissolution rate, taste-masking and hence lead to improve efficacy and bioavailability of a drug.
Keywords
Diclofenac sodium, Sodium starch Glycolate, Fast dissolving tablets
Introduction
Introduction conventional dosage form is very popular because of ease of self- administration, compact in nature, easy to manufacture and it can be delivered inaccurate dose. One important drawback of the conventional dosage form (tablet and capsule) is that it possesses higher disintegration time and pharmacological action is achieved after 30-45 min. of dosage of administration. To overcome this problem tablets that can rapidly disintegrate or dissolve within one minute in the oral cavity have attracted a great deal of attention. A fast-dissolving drug delivery system can be defined as a dosage form for oral administration, which when placed in the mouth, rapidly disintegrates or dissolves and can be swallowed in the form liquid. The conventional dosage form is very popular in pharmaceutical industries because of its low manufacturing cost. FDT disintegrate and/ or dissolve rapidly in the saliva without theneed for water. Some tablets are designed to dissolve in saliva remarkably fast, within a few seconds, and are true fast-dissolving tablets. The target populations for these new fast-dissolving/disintegrating dosage forms have generally been pediatric, geriatric, and bedridden or developmentally disabled patients. Patients with persistent nausea, who are traveling, or who have little or no access to water are also good candidates for FDT. The ease of administration of fast-dissolving/ disintegrating tablet, along with its pleasant taste, may encourage a patient to adhere to a daily medication regimen. Although FDT may not solve all compliance issues, it may be enough of an advance to be of therapeutic significance.1 Diclofenac sodium is a synthetic, non-steroidal anti-inflammatory & analgesic compound. The mechanism responsible for its anti-inflammatory, antipyretic, analgesic action is inhibition of prostaglandin synthesis by inhibition of cyclooxygenase (COX). Diclofenac mayalso be a unique member of the NSAIDs. There is some evidence that diclofenac inhibits the lipoxygenase pathways, thus reducing the formation of the leukotriene's (also pro- inflammatory autacoids). It is well absorbed orally and shows 100% bioavailability, morethan 99% protein- bound, metabolized and excreted both in urine and biles, and plasma t1/2 1.2-2hr.2 Diclofenac is used for musculoskeletal complaints, especially arthritis (rheumatoid arthritis, osteoarthritis, gout attacks, pain management in case of kidney stones and gallstones.2
AIM :
Formulation and Evaluation of Fast Dissolving Tablet of Diclofenac Sodium .
OBJECT:
To decrease dose.
Targeting of drug to better release.
DRUG PROFILE
- Diclofenac sodium:
Structure
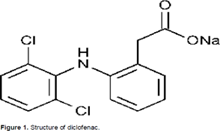
Figure No. 1: Structure of Diclofenac
“Diclofenac sodium” derives from its chemical name 2-(2,6dichloranilino) phenylacetic acid. Chemical Names: Diclofenac sodium salt; Voltaren;
Sodium diclofenac
Formula:
C14H11Cl2NNaO
Molecular Mass:
296.148g/mol Melting point: 284°C (543.2°F)
Dose:
The dose of diclofenac sodium is 100 mg given twice daily as a tablet. Category: Non-steroidal anti-inflammatory drug.
Storage:
Storage in a well-closed container, protected from light
Routes of administration:
Orel, rectal, intramuscular, intravenous (renal and gallstones), topical
Pharmacology
Diclofenac Sodium is the type of sodium salt derived form of Diclofenac, derived and non- steroidal anti-inflammatory drug (NSAID) with an analgesic, antipyretic and selective reversible and competitive inhibitor of cyclooxygenase (COX), subsequently blocking the conversion of arachidonic acid into prostaglandin precursors. This leads to an inhibition of the formation of prostaglandins that are involved in pain, in inflammation and fever.4, 5
EXCIPIENT PROFILE
- Mannitol:
Trade name:
Osmitrol
Empirical Formula:
C6H14O6
Mo1ecular Weight:
182.17g/mol
Structural Formula:
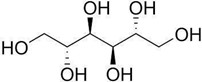
Figure No. 2: Structure of Mannitol
Solubility:
It is soluble in alkalis, methanol, and water and practically insoluble in ether. Melting Point: 166-168ºC
Usage:
It used as an excipient in the manufacture of chewable tablets, therapeutically used asan osmotic diuretic.9
2. Lactose
Structure:
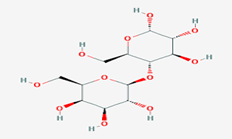
Figure No. 3: Structure of Lactose
Description:
A disaccharide of glucose and galactose in human and cow milk. It used intablets, as a medicine, and as a nutrient.
Chemical formula:
C12H22O11
Categories:
Sweetening Agents
Uses:
Lactose is milk sugar. It is a disaccharide composed of one galactose in the formulationof tablets lactose is used to help form tablets because it has excellent compressibility.7
3.Citric Acid
Structure:
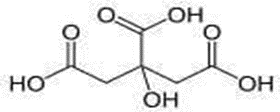
Figure No. 4: Structure of Citric acid
Citric acid is a week of organic tri-carboxylic acid. Citric acid is also a type of food. Chemical formula: C6H8O7
Molecular mass:
192.12g/mol Appurtenance: Crystalline white soli Odor: Odorless
Melting point:
156ºC Boiling point: 310ºC
Solubility:
Solubility in water 117.43g/100ml (10ºC).Soluble in alcohol, ether, ethyl acetate insoluble in toluene
Uses:
Citric acid is naturally used as a preservative. As a weak organic acid, it is commonly purchased in powder form but is found in all citric fruits in lemons, limes, oranges, and tangerines.9
4.Menthol
Menthol is an organic compound which is obtained from peppermint. It is in the form of crystalline substance, or white, which is solid at room temperature and melts slightly above.10
Structure :
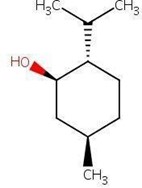
Figure No. 5: Structure of Menthol
Synonyms:
3-p-Menthol, Menthamenthol, Peppermint Chemical formula:
C10H20O
Molar mass:
156.27g/mol
Appearance:
White or colorless crystalline solid Density: 0.890g/cm
Melting point:
36 to 380C Boiling point: 2120C
Solubility:
Menthol is slightly soluble in water
Uses:
Menthol is used for: minor pain caused by a condition such as arthritis, bursitis, tendonitis, muscle strains or sprains, backache, bruising. It may also be used for other conditions as detracted by your doctor. Menthol also is a topical analgesic.
5.Magnesium Stearate
Structure:
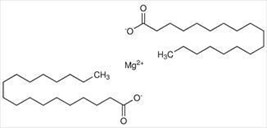
Figure No. 6: Structure of Magnesium Stearate
Magnesium Stearate is the chemical compound it is white in color and insoluble in water. IUPAC Name:
Magnesium octadecanoic
Chemical formula:
Mg (C18H35O2)2
Molar mass:
591.27g/mole
Appearance:
Light white powder
Odor:
Slight
Density:
1.026g/cm Melting point: 2000C
Uses:
It is also useful because it has lubricating properties, magnesium stearate is commonlyused lubricant for tablets.
Talc
Category:
Silicate mineral
Chemical Formula:
Mg3Si4O10 (OH)2
Color:
Light to dark green, brown, white, grey
Uses:
Talc is used as an ingredient in cosmetics, talcum, and baby powders.
6.Polyvinylpyrrolidone
(PVP) Chemical structure
Polyvinylpyrrolidone, also commonly called Polyvidone or Povidone is a water-soluble polymer made from the monomer N-vinylpyrrolidone.
Figure No. 7: Structure of PVP
Chemical name:
PVP, Povidone, Crospovidone, Polyvidone Chemical formula:
(C6H9NO) n
Molar mass:
2500 - 2500000 g.mol-1
Appearance:
White to light yellow hygroscopic, amorphous powder Density: 1.2 g/cm3
Melting point: 150-1800C
Solubility:
PVP is soluble in water and alcohol.
Uses:
It is used as a binder in many pharmaceutical tablets.
7.Hydroxyethylcellulose (HPMC)
Chemical structure

Figure No. 8: Structure of HPMC
Hypromellose in an aqueous solution, unlike methylcellulose, exhibits a thermal gelation property.
Chemical formula:
CH2CH (OH) CH3
Appearance
Hypromellose is a solid, and is a slightly off white to being powder and may be formed into granules.
Uses:
Hypromellose, short for hydroxypropyl methylcellulose (HPMC), is used as an ophthalmic lubricant and excipient.
METHOD AND MATERIAS
MATERIALS
All the materials used in this project work were commercial samples. Diclofenac sodium, Menthol, Citric acid, and PVP was received from Fine chemicals, Chennai, Mannitol received from Qualigens Fine Chemicals, Mumbai, Magnesium Stearate received from Hi media Laboratory, HPMC received from Loba Chemical PVT, LTD, Microcrystalline cellulose from Ignite Chemicals, Mumbai, Talc received from SD. Fine Chemical, LTD, Mumbai. All the reagents are used in analytical grade. Purified distilled water was used in thepreparation of diclofenac sodium.
METHOD
FDTs of Diclofenac Sodium using direct compression method:
Direct compression is used to define the process by which tablets are compressed directly from the powder blends of active ingredients and suitable excipients. Different batches of tablets prepared by direct compression methods. Six different batches of tablets prepared by taking super disintegrate (Sodium starch Glycolate, Croscarmellose sodium, Crospovidone) are used different concentrations of each with HPMC and compare with Six different batches of tablet prepared by taking another polymer (Starch) same as the concentration of HPMC. Thus all batches were prepared (all batch different combination shown in table). Different content was taken accordingly to the need for 20 tablets. Weight all the ingredients accurately and pass through sieve # 36 and Mix all the ingredients geometrically except Magnesium Stearate. Then lubricate the blend with Magnesium stearate.Tablets were compressed using tooling of 9.0 mm; circular punches with break line on one side and plain on the other side and dies were fixed to the 16 station single rotary tablets compression machine (Cadmach, Ahmadabad, India).
Table No. 2: Formulation design of fast dissolving tablet of Diclofenac Sodium
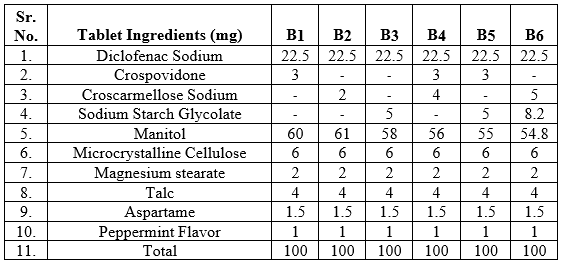
EVALUTION
Pre- Compression Parameter
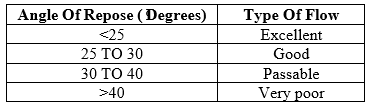
Angle of Repose:
Angle of repose was determined by using funnel methods. The powder was poured through the funnel that can be determined vertically until a maximum cone height (h) was obtained.A funnel was fixed on a stand at a high on the graph paper and powder was placed into the funnel and powder was allowed to flow down on the graph paper power made angle on the graph paper. The angle of repose was calculated with the help of the formula given below.30
Tan ? =2h/D
Table No. 3: Properties of Angle of repose
Bulk density
The bulk density of a powder is defined as the ratio of the mass of the powder to its bulk volume. It is used to describe the packing of particles. For bulk determination, a weighed volume of powder material was introduced into a graduated measuring cylinder and the amount of powder was determined.12
Bulk Density= Mass of the powder/ bulk volume
Tapped density
For the determination of tapped density, a weighed volume of powder was introduced into a graduated cylinder and mechanically either tapped or using a tape device until a constant volume was obtained.18
Tapped Density= Mass of the powder/ tapped volume
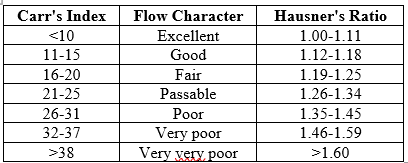
Carr’s compressibility index
The simplest method of measurement of the free flow of powder is compressive, an indication of the ease with which a material can be induced to flow. The compressive index is determined by Carr's index, which is calculated using the following formula,35
C= 100(1-B/T)
Where B is bulk density, T is tapped density
Hausner’s Ratio
Hausner’s ratio is an index of ease of powder flow. It is calculated by the following formula,
Hausner’s Ratio= Tapped density/ Bulk density
Lower Hausner’s ratio (< 1>1.25)
Table No. 4: Flow Characteristics
Post Compression Parameters
All formulated tablets were subjected to various physical characteristics such as crushing, friability, thickness, diameter, disintegration time, wetting time, weight variation, and drug content.28
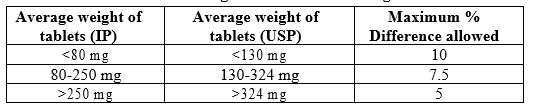
Weight Variation
A weight variation test was performed by weighing 20 tablets individually, using an electronic balance. Calculating average weight and comparing the weight of individual tabletsto the average weight.
Table No. 5: Weight Variation According to IP
Tablet thickness
Thickness was measured by placing the tablet between the two arms of the Vernier calipers. 5tablets were taken and their thickness was measured.2
Tablet hardness
The stiffness of a tablet, which is the force required to break a tablet into a diameter. The hardness tester used in the study was the Monsanto hardness tester, which applies force to thepellet with the help of an inbuilt spring31.
Tablet friability
The stability of the tablets was measured in a Roach Friabilator (Elite Scientific and Instruments). Tablets of known weight (Wo) or a sample of 20 tablets are cut into drums for a fixed time (100 revolutions) and weighed again (W). Percent stability was calculated from theloss in the weight given in the equation below. Weight loss should not exceed 1%. The determination was made in triplicate.7
% Friability = 100 (Wo -W) / Wo
In vitro characterization of prepared tablets
In the wetting time study
In the wetting time study, a piece of folded tissue paper was placed in a Petri plates (with internal diameter 6.5 cm) with 5 ml of distilled water. The tablet was placed on paper and the time for the tablet to become completely wet was measured in seconds.19
Disintegration test
The disintegration time of the fast-dissolving tablets was determined using the Disintegration test apparatus. Disintegration medium for 0.1N HCL for 2h followed by phosphate buffer[ ph 6.8] The operation was performed on 6 tablets.11
The in-vitro dissolution rate study
In-vitro dissolution rate study was done by using USP Type II apparatus which was rotated at 75 rpm. Phosphate buffer pH 6.8 (900 ml) was taken as a dissolution medium. The temperature of the dissolution medium was maintained at 37±0.5°C. Aliquots of dissolution medium were withdrawn at a specific time interval and it was filtered. The absorbance of filtered solution was determined by Spectrophotometer (SYSTRONICS-UV Double beam spectrophotometer-2101) at 283 nm and drug concentration was determined from the standard calibration curve.4
Determination of drug content
20 tablets were taken and powdered accurately. Powdered containing about 50mg of Diclofenac sodium was taken and shake it with 60ml methanol in 200ml volumetric flask anddilute to volume with methanol. 5ml of this solution was taken and diluted up to 100ml with methanol and absorbance was noted at 285 nm1.
Stability Studies
The best formulation was charged for stability studies at temperature and relative humidity of40ºC / 75%RH for one month. The parameter used to assess the effect of stress condition on tablets include Weight variation, Avg. Thickness, Friability, Disintegration Time, Avg. Hardness, Wetting time, Drug content and % Drug released.1
Table No. 6. Physical characteristics of tablets
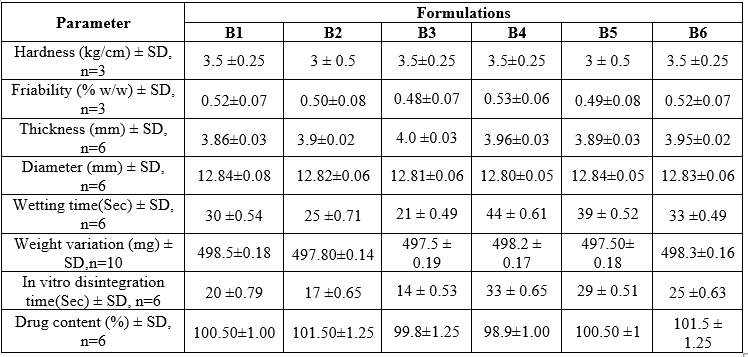
Table no. 7 Physical characteristics of tablets
RESULT AND DISCSUSSION
Diclofenac Sodium FDTs were prepared by the direct compression method. Sodium starch Glycolate and starch were used as super disintegrate which help in rapid and drug dissolution.
Weight Variation:
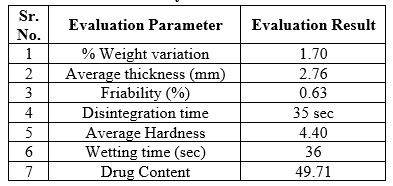
All tablets from each formulation passed weight variation test, as the % weight variation was within the Pharmacopeia limits
Friability:
The friability of the formulations was found to be between 0.24-0.66 percent and was within the official requirement (i.e. less than 1%)
Hardness:
The hardness was maintained to be within 4.40-4.91 kp, no variation in the hardness wasfound which indicates that the blending was uniform.
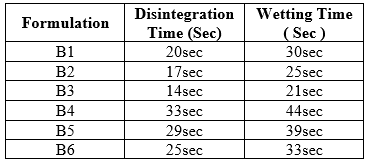
Disintegration time:
In-vitro disintegration time for all the formulations varied from 35 to 78 seconds. The formulation B4 shows a better disintegration time of 35 seconds
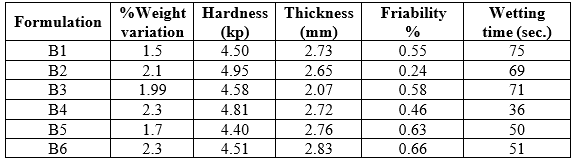
Table No. 8 Evaluation of Pre-formulation studies

Table No. 9: Evaluation of Post-formulation studies
In-vitro Dissolution study
The result of the in-vitro dissolution study indicates that process used to prepare the FDTs to enhance the rate and extent of dissolution data it was found that as the super Disintegrants increased, the drug release also increased. Among the different batches of formulation, B4 shows the highest dissolution rate were around 49.71.
Table No. 10: Comparative study of % drug release of FDT of diclofenac sodium of different batches
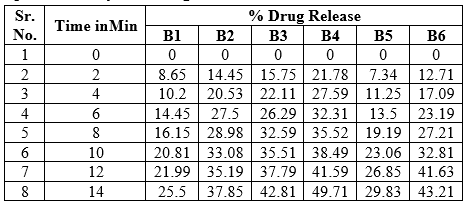
Figure No. 14: In-vitro dissolution studies
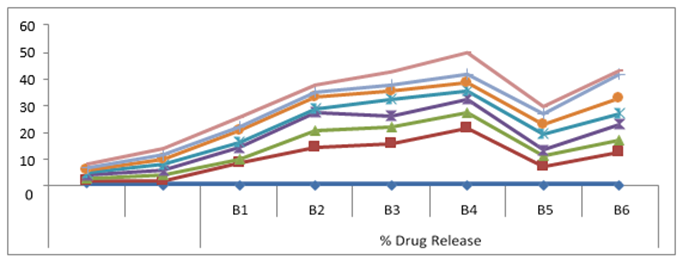
Table No. 11 Drug content study of FDTs of Diclofenac Sodium
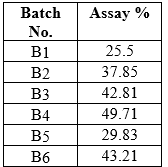
Table No. 12: Study of Different Parameter
CONCLUSION
Diclofenac Sodium is widely used NSAIDs for rheumatoid arthritis, inflammation, and pain relief. Fast dissolving tablets of Diclofenac sodium are a useful approach for pain management and a feasible alternative to the available conventional immediate release dosage form. Form the results, optimized B4 formulation showed improves drug release characteristics.
REFERENCE
- Prakash V, Maan S, Deepika, Yadav SK, Hemalatha et al. (2011) Fast disintegrating tablets: Opportunity in drug delivery system. J Adv Pharm Technol Res 2: 223-235.
- Ishikawa T, Watanabe Y, Utoguchi N, Matsumoto M (1999) Preparation and evaluation of tablets rapidly disintegrating in saliva containing bitter-tastemasked granules by the compression method. Chem Pharm Bull 47: 1451-4.
- Sammour OA, Mohammed A. Hammad, Nagia A Megrab, Ahmed S Zidan (2006) Formulation and optimization of mouth dissolve tablets containing rofecoxib solid dispersion. AAPS Pharm Sci Tech 7: E167-E175.
- Bhowmik D, Chiranjib B, Krishnakanth, Pankaj, Margret Chandira R et al. (2009) Fast Dissolving Tablet: An Overview. Journal of Chemical and Pharmaceutical Research 1: 163-177.
- NehalSiddiqui, Garima Garg, Pramod Kumar Sharma (2010) Fast Dissolving Tablets: Preparation, Characterization and Evaluation: An Overview. International Journal of Pharmaceutical Sciences Review and Research 4: 015.
- Schiermeier S, Schmidt PC (2002) Fast dispersible ibuprofen tablets. Eur J Pharm Sci. 15:295-305.
- MohitShrivastava, Deepak Chourasiya, Sanjay Soni, Deepak Patidar, Rajesh Jatav et al. (2012) Formulation and In-Vitro Evaluation of Mouth Dissolving Tablets of Phenytoin Sodium Using Different Disintegrating Agents. Int J Nov Drug Deliv Tech 2: 249-255.
- Sehgal Prateek, Gupta Ramdayal, Singh Umesh Kumar, ChaturvediAshwani, Gulati Ashwini, et al. (2012) Fast Dissolving Tablets: A New Venture in Drug Delivery. Am J Pharm Tech Res 2: 4.
- Deepak Sharma (2013) Formulation Development and Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate for Respiratory Disorders. ISRN Pharmaceutics 2013: 1-8.
- Sharma D, Reetika Chopra, NeenaBedi (2012) Development and Evaluation of Paracetamol Taste Masked Orally Disintegrating Tablets Using Polymer Coating Technique. International Journal of Pharmacy and Pharmaceutical Sciences 4: 3.
- Shukla, Dali, Chakraborty, Subhashism, Sanjay S, Mishra B (2009) Mouth Dissolving Tablets I: An Overview of Formulation Technology. ScientiaPharmaceutica 77: 309-326.
- Siddiqui, Md.Nehal, Garima Garg, Pramod Kumar Sharma (2010) Fast Dissolving Tablets: Preparation, Characterization And Evaluation: An Overview. International Journal of Pharmaceutical Sciences Review & Research 4: 87-96.
- Bhardwaj V, Bansal M, Sharma PK (2010) Formulation and evaluation of fast dissolving tablets of amlodipine besylate using different super disintegrants and camphor as sublimating agent. American-Eurasian Journal of Scientific Research 5: 264-269.
- Babji Movva, Laxman Kumar D, Mohan Ravi Kumar K (2013) Formulation and Evaluation of Fast Dissolving Tablets of Ranitidine Hydrochloride by Hole Technology. Asian J Pharm Clin Res 6: 143- 147.
- Kulkarni Upendra, Rao, Raghavendra NG (2011) Design and Development of Aceclofenac Fast Dissolving Tablets by Novel Hole Technology: A Novel Approach to Decrease the Disintegration Time and Increase the patient compliance. Drug Invention Today 3: 91-94.
- Peddi MG (2013) Novel Drug Delivery System: Liquid Solid Compacts. J Mol Pharm Org Process Res 1:108.
- Madhavi BR, Murthy VSN, Rani AP, Kumar GD (2013) Buccal Film Drug Delivery System-An Innovative and Emerging Technology. J Mol Pharm Org Process Res 1:107.
- Babu B, Meyyanathan SN, Gowramma B, Muralidharan S, Elango K, et al. (2012) Pharmacokinetic Evaluation of Newly Developed Oral Immediate Release and Sustained Release Dosage Forms of Losartan Potassium. J BioequivAvailab 4: 121-127.
- Shakeel F, Mohammed SF, Shafiq S (2009) Comparative Pharmacokinetic Profile of Aceclofenac from Oral and Transdermal Application. J BioequivAvailab 1: 013-017.
- Zheng C, Wang Y (2013) Prediction of Oral Bioavailability: Challenges and Strategies. J BioequivAvailab 6: 10000e47
- Dutta AK, Ikiki E (2013) Novel Drug Delivery Systems to Improve Bioavailability of Curcumin. J BioequivAvailab 6:1000172
- Balderas-Acata JI, Ríos-Rogríguez BuenoEP, del Castillo-García S, EspinosaMartínez C , Burke- Fraga V et al. (2011) Bioavailability of Two Coated-Tablet Formulations of a Single Dose of Pantoprazole 40 mg: An Open-Label, Randomized, Two-Period Crossover, Comparison in Healthy Mexican Adult Volunteers. J BioequivAvailab 3: 1000063
- Valenzuela F, Davila G, Ibañez Y, Garcia L, Crownover P, et al. (2011) Relative Bioavailability of Chewable and Conventional Film-Coated Tablet Formulations of Sildenafil 100 mg in Healthy Volunteers Under Fasting Conditions. J BioequivAvailab 3:1000087
- Bragatto MS, dos Santos MB, Pugens Pinto AM, Gomes E, Angonese NT, et al. (2011) Comparison between Pharmacokinetic and Pharmacodynamic of Single-Doses of Furosemide 40 mg Tablets. J BioequivAvailab 3: 191-197.
- Feleder EC, Yerino GA, Halabe EK, Carla S, Soledad G, et al. (2011) SingleDose Bioequivalence of a New Fixed-Dose Combination Tablet Containing Tenofovir Disoproxil Fumarate and Lamivudine. J BioequivAvailab 3: 236-243.
- Pechenkin MA, Balabushevich NG, Zorov IN, Staroseltseva LK, Mikhalchik EV, et al. (2011) Design, In Vitro and In Vivo Characterization of Chitosan-Dextran Sulfate Microparticles for Oral Delivery of Insulin. J BioequivAvailab 3: 244-250.
- Palma-Aguirre JA, Mireya LG, de Jesus CST, Mariel PG, Elisa ZB, et al. (2010) Bioavailability of Two Oral Tablet Formulations of citalopram 20 mg: Single-Dose, Open-Label, Randomized, Two- Period Crossover Comparison in Healthy Mexican Adult Subjects. J BioequivAvailab 2: 018-022
- Bapuji AT, VenkataRavikiran HL, Nagesh M, Syedba S, Ramaraju D, et al. (2010) Bioequivalence Testing - Industry Perspective. J BioequivAvailab 2: 098-101.
- Kanfer I (2010) Strategies for the Bioequivalence Assessment of Topical Dermatological Dosage Forms. J BioequivAvailab 2: 102-110.
- Junior EA, Duarte LF, PirasolVanunci ML, Teixeira ML (2010) Bioequivalence of Two Oral Contraceptive Drugs Containing Ethinylestradiol and Gestodene in Healthy Female Volunteers. J BioequivAvailab 2: 125-130.
- Khattak S, Malik F, Hameed A, Ahmad S, Rizwan M, et al. (2010) Comparative Bioavailability Assessment of Newly Developed Flurbiprofen Matrix Tablets and Froben SR® Tablets in Healthy Pakistani Volunteers. J BioequivAvailab 2: 139-144.
- Shakeel F, Ramadan W, Shafiq S (2009) Solubility and Dissolution Improvement of Aceclofenac using Different Nanocarriers. J BioequivAvailab 1: 0 39-043.
- Roda A, Simoni P, Roda G, Caponi A, Pastorini E, et al. (2009) Pharmacokinetics and Safety of a New 1200 mg Single-Dose Delayed Release MesalazineMicrogranule Formulation. J Bioequiv Availab 1: 044-051.
- Lima R, Vasconcelos T, Cerdeira R, Lefebvre M, Sicard E, et al. (2009) Bioequivalence of Final Tablet Formulation and Research Tablet Formulation of Eslicarbazepine Acetate in Healthy Volunteers. J Bioequiv Availab 1: 093098.
- Moreira RF, Rigato HM, Borges BC, Sverdloff CE, Oliveira RA,et al. (2009) Effect of Hyperlipemic Food on the ComparativeBioavailability of Two Bupropion Formulations after Administration of a Single Oral Dose of 150 mg in Healthy Human Volunteers. J Bioequiv Availab 1: 103-111.
- Teksin ZS, Agabeyoglu I, Yamac K (2009) Bioavailability of PentoxifyllineChitosan Oral Matrix Tablet in Healthy Subjects. J BioequivAvailab 1: 115-120.
- Dewan B, Sahu N (2010) Bioequivalence Study of Troxipide Tablet Formulations. J Bioequiv Availab 2: 050-054.
- Zhang CL, Jiao JJ, Wu YN, Song JQ, Gao WZ, et al. (2011) Study on Pharmacokinetics and Bioequivalence of Cefdinir Dispersible Tablet in Healthy Chinese Volunteers. J Bioequiv Availab 3: 114-117.
- Feng Y, Wang N (2013) The New Generation of Drug Discovery and its Analytical Technologies. J Bioequiv Availab 5: e42.
- Al-Ghananaeem A (2013) Sublingual and Nasal Trans mucosal Drug Delivery for Breakthrough Pain: A Frontier in Cancer Therapy. J Bioequiv Availab 5: e29.
- Harahap Y, Budi Prasaja MM, Lusthom W, Hardiyanti, Azmi F, et al. (2013) Bioequivalence of Trimetazidine Modified Release Tablet Formulations Assessed in Indonesian Subjects. J Bioequiv Availab 5: 117-120.
- Nichols AI, Richards LS, Behrle JA, Posener JA, Fruncillo R, et al. (2013) Effects of Age and Sex on the Pharmacokinetics, Safety, and Tolerability of Oral Desvenlafaxine in Healthy Adults. J Bioequiv Availab 5: 088-094.
- Mascoli V, Kuruganti U, Bapuji AT, Wang R, Damle B (2013) Pharmacokinetics of a Novel Orodispersible Tablet of Amlodipine in Healthy Subjects. J Bioequiv Availab 5: 076-079.
- Nishant T, Sathish Kumar D, Arun Kumar, Phaneendra M (2011) Role of Pharmacokinetic Studies in Drug Discovery. J Bioequiv Availab 3: 263-267.
- Ruiz A, Cuesta F, Parra S, Montoya B, Restrepo M, Archbold R, et al. (2012) Bioequivalence Evaluation of Two Formulations of Lamotrigine Tablets in Healthy Volunteers. J Bioequiv Availab 4: 030-034.
- Parthasarathi D, Gajendra C, Dattatreya A, Sree Venkatesh Y (2011) Analysis of Pharmacokinetic &Pharmacodynamic Models in Oral and Transdermal Dosage Forms. J Bioequiv Availab 3: 268-276.
- Wagh N, Gayakwad NJ, Christina AJM, Bhople A, Thakre A (2013) A Bioequivalence Study of Two Finofibrate Tablet Formulations in Indian Healthy
- Bhorepooja et al Int. Res. J. of Science & Engineering 2018, Special Issue A3: 43-48, ISSN:2322- 0015
- Amit Modi et al, PHARMACIA Volume 1, Issue 3, June 2012
- Damodar et al., J Mol Pharm Org Process Res 2014, 2:2 DOI: 10.4172/2329-9053.1000116
- Subramanyam CVS. Textbook of Physical Pharmaceutics, Vallabh Prakashan, 2nd edn; 2001; 235-237 “In vitro Dissolution” The United States Pharmacopoeia, United States Pharmacy Convention, Inc., Asian edition, 2000; 1941-1943.
- Habib W, Khankari R, Hontz J., "Fast-dissolving Drug Delivery Systems", Critical Reviews TM Therapeutic Drug Carrier Systems, 2000, 17(1), 61-72.
- Brown, D., Drug Delivery Tech., 2004.Parakh, S. R., and Gothoskar, A. V., Pharma. Tech., November 2003, 92-100.
- Kuchekar, B. S., Badhan, A. C., Mahajan, H. S., Pharma Times, June 2003, 35, 7-9.
- Lalla, J. K., and Sharma, A. H., Indian Drugs, 1994, 31(11), 503-508.
- Corveleyn, S. and Remon, J.P., “Formulation and Production of Rapid Disintegrating Tablets
- byLyophilization using Hydrochlorthiazide as a Model Drug", Int. J. Pharm., 1997, 152, 215-225.
- Corveleyn, S. and Remon, J.P., "Freeze-Dried Disintegrating Tablets", US patent No., US6 010719. 2000.
- 12.Masaki, K., “Intrabuccally Disintegrating Preparation and Production Thereof, US patent No.,US5466464


 Sujit S. Shinde *
Sujit S. Shinde *
 Sachitanand B. biradar
Sachitanand B. biradar
 Avinash A Bhagat
Avinash A Bhagat
 Madan K. Shinde
Madan K. Shinde





















 10.5281/zenodo.11400826
10.5281/zenodo.11400826