Abstract
The aim of this review on gastroretentive drug delivery systems is to gather and present recent literature, particularly highlighting the various gastroretentive methods that have recently emerged as prominent techniques in the realm of site-specific, orally administered, controlled release drug delivery. Floating drug delivery systems represent advanced technology that allows these systems to float on gastric fluids, potentially enhancing the bioavailability and intestinal absorption of encapsulated drugs. The effectiveness of these systems depends on both physiological and formulation factors that influence gastric retention, essential for the successful development of floating drug formulations. These systems can be categorized into single-unit or multiple-unit floating designs. By extending and managing the rate of gastric emptying, these formulations support prolonged drug release. Gastro-retentive systems extend the residence time in the stomach, improving drug bioavailability and solubility in higher pH conditions, and ensuring maximum drug concentration at the target site. This review explores different gastro-retentive drug delivery systems, including those made from natural and synthetic polymers, and their applications. It also highlights the factors impacting these systems, as well as the challenges in the process and future prospects for their commercialization.
Keywords
Gastro-retentive, floating, gastric, bioavailability.
Introduction
Gastroretentive drug delivery systems represent an innovative approach in drug delivery. The primary goal of these systems is to extend the duration a drug remains in the stomach, thereby ensuring site-specific release in the upper gastrointestinal tract for either local or systemic effects. Drug formulations designed for gastroretentive delivery stay in the stomach for extended periods, significantly increasing their gastric retention time (GRT). Numerous methods for gastroretentive drug delivery have been developed over recent decades.Oral drug administration is the most convenient and preferred method for delivering medication to the systemic circulation. Recently, there has been growing interest in oral controlled release drug delivery systems within the pharmaceutical field due to their potential for enhanced therapeutic benefits. These benefits include ease of dosing, improved patient compliance, and flexibility in formulation. Medications that are rapidly absorbed from the gastrointestinal tract (GIT) and have short half-lives are quickly cleared from the system, necessitating frequent dosing to maintain therapeutic levels. To address this issue, oral sustained-release formulations have been developed to release drugs gradually into the GIT, thereby maintaining effective drug concentrations in the systemic circulation over an extended period. After oral administration, these delivery systems are designed to remain in the stomach, releasing the drug in a controlled manner, which allows for continuous drug supply to absorption sites in the GIT. However, these systems face challenges such as short gastric retention time (GRT) and unpredictable gastric emptying time (GET), which can lead to incomplete drug release in the absorption zone (stomach or upper part of the small intestine) and reduced efficacy of the dose. To enhance site-specific controlled release dosage forms, achieving prolonged gastric residence time is desirable. Extended gastric retention can improve bioavailability, prolong the duration of drug release, reduce drug waste, and enhance the solubility of drugs that are less soluble in high pH environments. Additionally, longer gastric retention time can be beneficial for local action in the upper part of the small intestine, such as in the treatment of peptic ulcers.[1-4]
For instance, various systems are employed to enhance gastric retention of drugs:
- High-Density (Sinking) System: This approach keeps the drug settled at the bottom of the stomach.
- Low-Density (Floating) System: This method enables the drug to float in the gastric fluid.
- Mucoadhesive System: This system allows the drug to adhere to the stomach lining.
- Unfoldable, Extendible, or Swellable System: These systems prevent the dosage form from passing through the pyloric sphincter.
- Superporous Hydrogel and Magnetic Systems: These represent additional examples of gastric retention drug delivery systems (GRDDS).[5]
ADVANTAGES
The Gastroretentive Drug Delivery System (GRDDS) offers several benefits:
- Enhances the bioavailability and therapeutic effectiveness of medications.
- Optimizes the economic use of dosages.
- Minimizes the risk of antibiotic resistance by maintaining consistent therapeutic levels, thus eliminating fluctuations.
- Improves drug release efficiency, especially for drugs with short half-lives.
- Influences pharmacokinetic properties.
- Promotes patient adherence by reducing the frequency of doses.
- Overcomes issues related to Gastric Retention Time (GRT) and Gastric Emptying Time (GET) by staying buoyant in gastric fluid due to its lower bulk density.[5-8]
DISADVANTAGES
GRDDS come with several disadvantages:
- They necessitate an increased volume of stomach fluids.
- GRDDS are unsuitable for drugs with low solubility in gastric fluids, those that cause gastrointestinal irritation, those that are ineffective in acidic environments, and those designed for selective release in the colon.
- The continuous renewal of the stomach's mucus layer makes it difficult to predict the adherence of drugs.
- The effectiveness of GRDDS can vary depending on whether they are taken before or after a meal, as the drug’s residence time in the stomach is influenced by the subject’s digestive state. [5-8]
FACTORS INFLUENCING GASTRIC RETENTION TIME OF DOSAGE FORMS
When developing gastroretentive dosage forms, it is crucial to consider the anatomical and physiological features of the stomach. For particles to pass through the pyloric valve into the small intestine, their size should generally be between 1 and 2 mm. Key factors affecting the gastric retention time (GRT) of oral dosage forms include the dosage form’s density, size, and shape, as well as factors related to food intake such as its nature, caloric content, and frequency. Other influencing factors are body posture, gender, age, sleep patterns, body mass index, physical activity, and existing health conditions like chronic diseases or diabetes. Additionally, medications that affect gastrointestinal transit, such as anticholinergics (e.g., atropine, propantheline), opiates (e.g., codeine), and prokinetic agents (e.g., metoclopramide, cisapride), can play a role. The drug's molecular weight and lipophilicity, influenced by its ionization state, are also important factors to consider.
1. Impact of Dosage Form Density
The density of a dosage form significantly influences its gastric emptying rate and its positioning within the stomach. Dosage forms with a density lower than that of the gastric contents tend to float to the upper part of the stomach, while those with a higher density settle at the bottom. This positional difference can affect the system's proximity to the pylorus. For a dosage form to demonstrate floating properties, its density needs to be less than 1.0 gm/cm?3;.
2. Influence of Shape and Size
The shape and size of dosage forms play a crucial role in the design of indigestible single-unit solid dosage forms. The gastric residence time of non-floating dosage forms varies widely and is significantly influenced by their size, which can range from large to small units. Generally, larger dosage forms tend to have longer gastric residence times. The retention time of dosage forms, referred to as Gastric Retention Time (GRT), can be influenced by the size of the dosage form. Larger dosage forms may take longer to pass through the pyloric antrum into the intestines. Dosage forms with diameters greater than 7.5 mm tend to have improved gastric residence time compared to those with diameters around 9.9 mm. Shapes such as rings and tetrahedrons generally offer better gastric retention compared to other configurations.
3. Food intake and its nature
The presence and nature of food intake significantly impact the GRT of dosage forms. Factors like food viscosity, volume, caloric content, and feeding frequency can affect how long a dosage form remains in the stomach. Typically, having food in the gastrointestinal tract enhances the GRT of dosage forms, which can lead to improved drug absorption by extending the time the drug spends at the absorption site. Additionally, higher acidity and caloric value of food can slow gastric emptying time, further enhancing the retention of dosage forms.
4. Effect of gender, posture and ageDifferences in gastric emptying rates are also observed between genders and age groups. Generally, females experience slower gastric emptying compared to males. Posture does not significantly impact GRT when comparing upright, ambulatory, and supine states. However, elderly individuals tend to have slower gastric emptying rates. [9-13]
APPLICATIONS
The following are applications of Gastroretentive Drug Delivery Systems (GRDDS):
1. Enhanced Bioavailability
Gastroretentive dosage forms (GRDF) such as those containing riboflavin show improved bioavailability compared to non-GRDF polymeric formulations. Various processes related to drug absorption and gastrointestinal transit act together to affect the drug absorption rate.
2. Sustained Drug Delivery/Reduced Frequency of Dosing
For drugs with a short biological half-life, sustained and slow release from GRDF improves pharmacokinetic profiles and reduces dosing frequency. This enhances patient compliance and improves overall therapy.
3. Targeted Therapy for Local Ailments in the Upper GIT
Prolonged and sustained drug administration from GRDF can provide targeted therapy to the stomach and small intestine. This ensures therapeutic drug concentrations locally while maintaining minimal systemic concentrations post-absorption and distribution.
4. Reduced Fluctuations of Drug Concentration
Continuous drug intake following GRDF administration maintains blood-drug concentrations within a narrower range compared to immediate-release forms. This reduces fluctuations in drug effects and minimizes adverse effects caused by higher concentrations.
5. Site-Specific Drug Delivery
For drugs with limited absorption sites in the upper small intestine, a floating dosage form can be used. Controlled and slow drug release to the stomach achieves local therapeutic levels and limits systemic exposure. This approach reduces side effects in the bloodstream and decreases dosing frequency due to prolonged gastric retention.[14-16]
Table 1. Commonly used drug in formulation of gastro retentive dosages forms
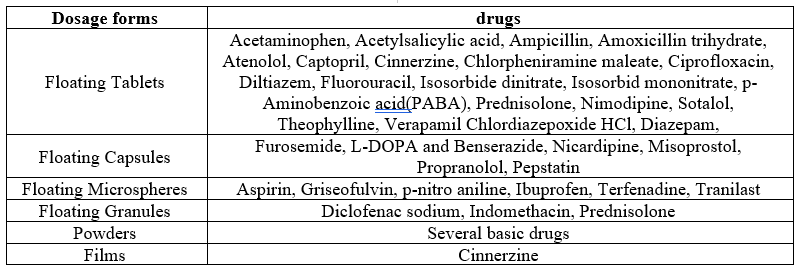
Table 2. Gastroretentive products available in the market

APPROACHES TO ACHIEVE GASTRIC RETENTION
The following strategies are employed to extend the duration drugs spend in the gastrointestinal tract:
- Pharmacological Strategy
This method involves using drugs or integrating them into the dosage form to slow down gastrointestinal emptying. For example, antimuscarinic agents like propantheline are used to achieve this effect.
- Physiological Strategy
This approach utilizes natural substances or fat derivatives, such as triethanolamine myristate, to activate receptors in the duodenum or jejunum, thereby slowing gastric emptying.
- Pharmaceutical Strategy
Given the toxicity concerns associated with the first two methods, various pharmaceutical techniques are employed, including:
- Low Density or Floating Drug Delivery System
This system has a bulk density lower than that of gastric fluids, allowing it to stay buoyant in the stomach. These systems, also known as Hydrodynamically Balanced Systems (HBS), can be further classified into:
- Effervescent Systems: These include gas-generating and volatile liquid-containing systems.
- Non-Effervescent Systems: These consist of expandable or swellable systems and inherently low-density systems.
2. High Density or Sinking Drug Delivery System
This system ensures that the drug remains at the bottom of the stomach.
- Modified Shape or Unfolding System
- This design allows the drug to expand to a larger size, preventing it from passing through the pyloric sphincter too quickly.
- Bioadhesive or Mucoadhesive Drug Delivery System:
- This type of system enables the drug to stick to the gastric mucosa.
- Super Porous Hydrogel System
- Magnetic System[17-25]
LOW DENSITY OR FLOATING DRUG DELIVERY SYSTEM
To enhance drug bioavailability, a floating drug delivery system is employed to ensure optimal gastric retention. This approach is suitable for drugs that are absorbed in the stomach or the upper part of the small intestine. Unlike other methods, it does not affect the rate at which the stomach empties over time. Due to its lower density compared to gastric fluids, the system remains afloat in the stomach, allowing for a gradual release of the drug. The release of the drug follows the emptying of the residual system from the stomach, which prolongs gastric retention and stabilizes plasma drug levels. Key requirements for an effective floating drug delivery system include:
- A reservoir for controlled release of the contents.
- A specific gravity lower than that of gastric fluids (1.004-1.01 gm/cm?3;).
- The formation of a cohesive gel barrier.
MECHANISM OF FLOATING DRUG DELIVERY SYSTEM
The floating drug delivery system releases the drug at a controlled rate while navigating through the gastric contents. Once the drug is released, the system is subsequently removed from the
stomach. To ensure effective buoyancy and
maintain the dosage form's floatation over the meal surface, there must be a minimum level of gastric contents and an appropriate floating force (F). The floating force's kinetics can be assessed using a device that measures the force equivalent to F over time while the object remains submerged. A higher positive force indicates better object flow (see Figure 1). [18]

Figure 1: Mechanism of Floating Drug Delivery Systems, GF– Gastric Fluid, CO2 – Carbon Dioxide
This device helps in optimizing floating drug delivery systems (FDDS) and mitigating issues related to unexpected variations in intragastric buoyancy, stability, and durability.
The floating force (F) can be calculated using the equation:
???? = ???? buoyancy ? ???? gravity
???? = ( ???? ? ???? ???? ) ? ???? ? ???? F=(D f ?D s )?v?g
Where:
= Total vertical force,
???? ???? = Fluid density,
???? ???? = Object density,
= Volume,
= Acceleration due to gravity.
CLASSIFICATION OF FLOATING DRUG DELIVERY SYSTEM
Floating drug delivery system is classified as shown in figure 2, based on the buoyancy mechanism:
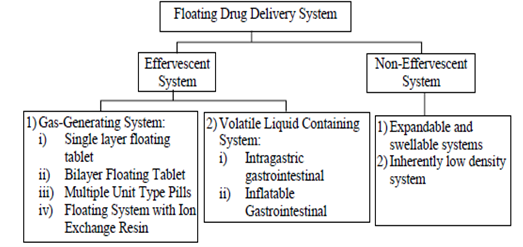
Figure 2: Classification of Floating System
EFFERVESCENT SYSTEMS
Effervescent systems utilize gas-producing agents, such as carbonates (e.g., sodium bicarbonate) and organic acids (e.g., citric acid, tartaric acid), to generate carbon dioxide (CO2) gas. This process reduces the system's density, enabling it to float on gastric fluids. Alternatively, some systems incorporate a liquid-containing matrix that releases a gas upon reaching body temperature.[26]
Effervescent systems can be divided into two main categories:
1) Effervescent/Gas-Generating Systems
These systems create gas bubbles to achieve buoyancy. They often use matrices composed of swellable polymers, like polysaccharides (e.g., chitosan), alongside effervescent agents (e.g., sodium bicarbonate, citric acid, tartaric acid). An ideal ratio of citric acid to sodium bicarbonate for optimal gas production is 0.76:1. In these systems, CO2 is released, causing the formulation to float in the stomach. Various materials and methods are also employed, including mixtures of sodium alginate and sodium bicarbonate, multiple-unit floating dosage forms that generate CO2 upon ingestion, and floating mini capsules containing sodium bicarbonate, lactose, and PVP, coated with HPMC. Additionally, floating systems based on ion exchange resins and bilayer or multilayer systems—where drugs and excipients are independently formulated and gas-generating agents are included in one of the layers—are used. Some systems are further modified with a polymer coating that is water-permeable but carbon dioxide-impermeable.
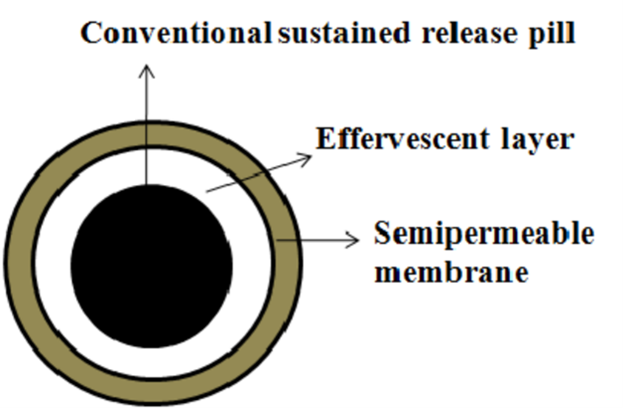
Figure 3: Effervescent (Gas-Generating) System
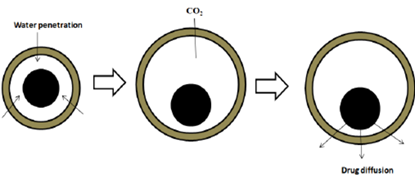
Figure 4: Drug Release from Effervescent (Gas-Generating) System
One limitation of gas-generating systems is the challenge of differentiating between the elasticity, plasticity, and permeability of various polymers. These systems are often categorized as follows:
i) Single Layer Floating Tablet or Hydrodynamically Balanced System (HBS)
This system, illustrated in Figure 5, involves incorporating CO2-generating agents into the matrix tablet alongside the drug. Due to the lower bulk density of this system compared to gastric fluids, the tablet remains buoyant in the stomach for extended periods, independent of the gastric emptying rate. The drug is gradually released from the floating tablet at a controlled rate. Once the drug is fully released, the remaining tablet is expelled from the stomach. This mechanism enhances the gastric residence time (GRT) and stabilizes plasma drug concentration levels.[27-29]

Figure 5: Single Layer Floating Tablet
ii) Bilayer Floating Tablet
This formulation consists of a tablet with two distinct layers: one for immediate release and the other for sustained release (see Figure 6).
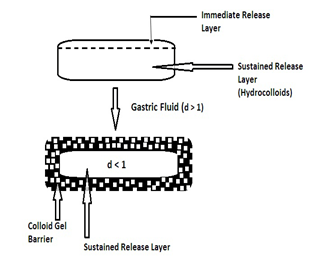
Figure 6: Bilayer Floating Table
iii) Multiple Unit Floating Pill
This type of system (see Figure 7) features sustained-release pills encased in two layers. The inner layer has effervescent agents, while the outer layer is made of a swellable membrane. Upon contact with the dissolution medium at body temperature, the system sinks and forms swollen pills that float due to their reduced density. This low density results from CO2 generation and entrapment within the system. [29]
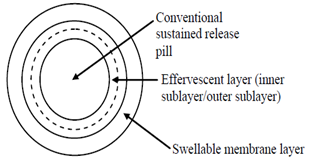
Figure 7: Multiple Unit Floating Tablet
iv) Ion Exchange Resin System
This multiple-unit oral dosage system extends the gastric residence time of the medication. It includes drug-resin complex beads that are charged with bicarbonate ions and covered with a hydrophobic polymer. When the beads enter the stomach, chloride ions are swapped with bicarbonate and drug ions, producing CO2 gas that is captured by the polymer coating, causing the beads to float.[30]
2) Volatile Liquid-Containing System:
To enhance the gastric retention time, this system contains an inflatable chamber filled with a liquid (e.g., ether, cyclopentane) that vaporizes at body temperature, causing the chamber to expand in the stomach. It may also feature a bioerodible plug made of materials like polyvinyl alcohol or polyethylene, which dissolves over time to release gas from the chamber. This results in the chamber collapsing after a set period, allowing the system to exit the stomach.
- Intragastric Floating Gastrointestinal Drug Delivery System:
This system (see Figure 8) includes a flotation chamber designed to keep the system buoyant in the stomach. The chamber is either vacuum-sealed or filled with air or a safe gas, and the drug is contained within a microporous compartment. [31-32]
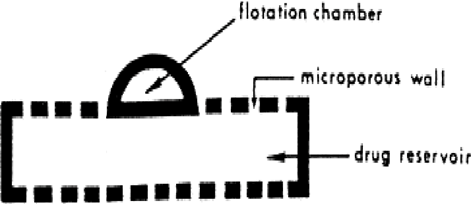
Figure 8: Intragastric Floating Gastrointestinal Drug Delivery System
- Inflatable Gastrointestinal Delivery System
This system incorporates an inflatable chamber filled with liquid ether that vaporizes at body temperature, leading to chamber inflation in the stomach (see Figure 9). It is constructed by combining
an inflatable chamber with a drug
reservoir (which may be a drug-loaded polymeric matrix) and encasing it in a gelatin capsule. Upon oral administration, the capsule dissolves, releasing both the drug reservoir and the inflatable chamber, which then inflates and retains the drug in the gastric fluid.
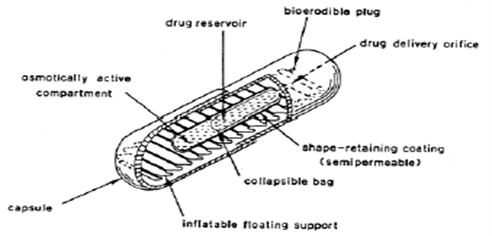
Figure 9: Inflatable Gastrointestinal Delivery Syste
Non-Effervescent System
The non-effervescent floating drug delivery system operates based on either polymer swelling or bioadhesion to the gastrointestinal mucosa. It commonly uses gel-forming cellulose derivatives, hydrophilic gums, polysaccharides, matrix-forming materials like polycarbonate and polystyrene, and bioadhesive polymers such as chitosan.
There are several types of non-effervescent systems:
Expandable or Swellable System
These systems are designed to be larger than the pyloric sphincter to ensure they stay in the stomach longer. They must be small enough to be swallowed and should not cause blockage, either by themselves or through accumulation. This type of system typically features:
i) A compact size for oral ingestion,
ii) An expanded form to enhance gastric retention,
iii) A final compact state for elimination after the drug is released.
Improving gastroretentivity involves ensuring the system’s size and rigidity can withstand stomach movements. Recent research has focused on developing effective gastroretentive drug delivery systems (GRDDS) with swellable components. These systems expand through osmotic water absorption, allowing them to remain in the gastrointestinal tract while being initially small enough to be ingested. [33-34]
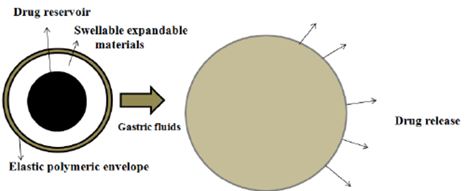
Figure 10: Drug Release from Swellable System
System with Naturally Low Density
This system addresses the issue of premature gastric emptying by initially settling and then floating. There are two main approaches to achieve a naturally low-density system:
- Air Entrapment (Hollow Microspheres/Microballoons)
These can be created using the following techniques:
a) Emulsion-Solvent Diffusion Technique: This involves mixing a polymer and drug solution (dissolved in ethanol or methylene chloride) with an agitated aqueous polyvinyl alcohol solution. Ethanol quickly disperses into the aqueous phase, causing the polymer to precipitate around methylene chloride droplets. As methylene chloride evaporates, it forms internal cavities within the microparticles (refer to Figure 11a).
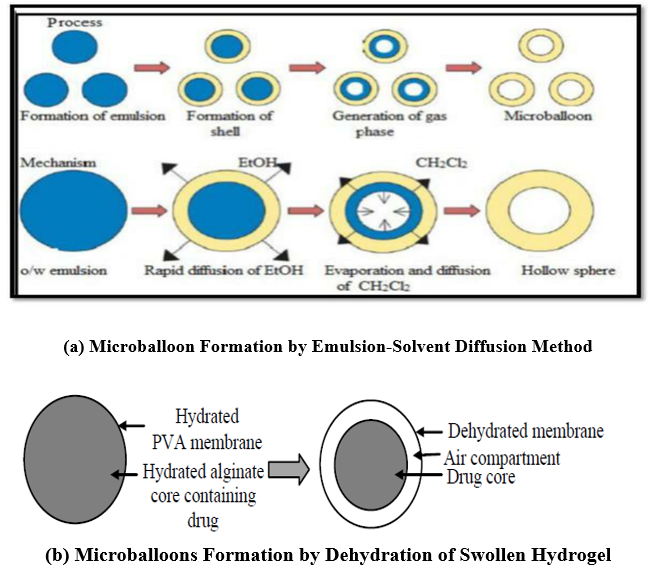
Figure 11: Microballoons for GRDD
b) Modified Solvent Evaporation Technique
In this approach, drug powder is mixed with a 1:1 solution of cellulose acetate butyrate and Eudragit RL 100 in acetone. This mixture is then subjected to carbon dioxide pressure, which causes the formation of bubbles that help in reducing the pressure. As bubbles are created, the mixture is quickly emulsified into an external oil phase. Some of the CO2 bubbles get trapped within the drug-polymer droplets, resulting in internal cavities within the solidified microspheres.
c) Dehydration of Swollen Hydrogel
This technique involves a drug-loaded calcium alginate core that is hydrated and then covered with a polyvinyl alcohol layer. As the hydrogel dries, it creates an air pocket due to the shrinkage of the hydrated core (see figure 11 b).
d) Hollow Chamber System
This method involves applying a coating to drugs on hollow cores such as pop rice, empty gelatin capsules, or polystyrene beads. The drug is then covered with a membrane that controls the release rate.
ii) Use of Low-Density Materials
This technique employs inherently low-density materials like fats or lightweight polymers to create floating drug matrices. For example, a floating system might consist of polypropylene foam powder, matrix-forming polymers, the drug, and a filler (see figure 12a). The foam powder, known for its high porosity, ensures a low density, enhancing the in vitro floating characteristics of the system. Microporous foam particles are saturated with an organic drug-polymer solution (e.g., Eudragit RS 100) and then dried to form floating drug microparticles based on polypropylene foam powder (see figure 12 b). [26-29]
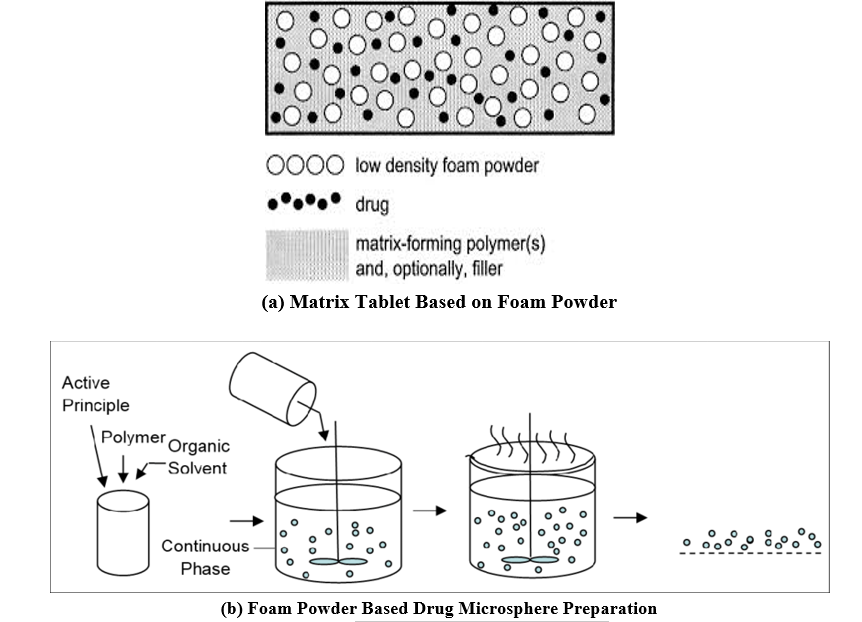
Figure 12: GRDDS Based on Foam Powder
HIGH-DENSITY (SINKING) OR NON-FLOATING DRUG DELIVERY SYSTEM
High-density or non-floating drug delivery systems are designed with dosage forms that have a density greater than the typical stomach content (~1.004 gm/cm?3;). These formulations are created by either coating the drug onto a heavy core or by combining the drug with inert materials such as iron powder, barium sulfate, zinc oxide, or titanium dioxide. These materials can increase the density to between 1.5 and 2.4 gm/cm?3;. A density of approximately 2.5 gm/cm?3; is generally required to significantly extend the gastric residence time. However, this system is not effective in humans, and such dosage forms are not commercially available. [20-26]
Modified Shape or Unfolding System
The modified shape or unfolding system is designed to expand to a larger size to prevent passage through the pyloric sphincter. Drugs in this system are incorporated into various geometric shapes (such as tetrahedrons, rings, cloverleaves, discs, strings, spirals, and pellets/spheres) which are then tightly packed into a gelatin capsule. Once the capsule dissolves in the stomach, the shapes unfold. This system includes at least one erodible polymer, one non-erodible polymer, and a drug dispersed within the polymer matrix. A key disadvantage of this system is that the expanded material may remain in the stomach for an extended period.
Bioadhesive or Mucoadhesive Drug Delivery Systems
In gastroadhesive drug delivery systems, mucoadhesive polymers are employed to stick to the gastric mucosal surface, thereby extending the time the drug remains in the gastrointestinal tract. The ability of these polymers to adhere to the mucus layer makes them highly effective as excipients in gastroretentive drug delivery systems (GRRDS). Mucoadhesive polymers can be derived from natural sources (such as sodium alginate, gelatin, and guar gum) or be semi-synthetic (including HPMC, carbopol, and sodium carboxymethyl cellulose). These polymers can exhibit cationic, anionic, or neutral properties. [15]
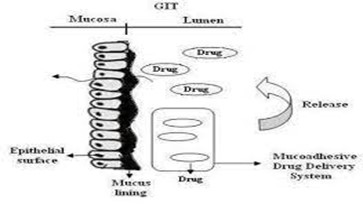
Figure 13: Mucoadhesive Drug Delivery System
The adhesion of polymers to mucus membranes can be facilitated through hydration, bonding, or receptor-specific interactions. Hydration-mediated adhesion occurs when hydrophilic polymers absorb water, becoming sticky and enhancing their mucoadhesive properties. Bonding-mediated adhesion involves physical or chemical bonding, including ionic, covalent, or Van der Waals forces, between the polymer and the mucus membrane. In receptor-mediated adhesion, polymers bind to specific receptors present on gastric cells.[34]
Hydration-Mediated Adhesion
Hydrophilic polymers gain bioadhesive characteristics by absorbing water, which makes them sticky. By adjusting the dissolution rate of these polymers, the duration of gastroretention in bio/muco-adhesive delivery systems can be managed.
Bonding-Mediated Adhesion:
Polymers adhere to mucus or epithelial cell surfaces through various bonding mechanisms. Adhesive materials can form physical or mechanical bonds by filling the mucosal crevices. Secondary chemical bonds, which contribute to bioadhesion, include dispersive interactions (such as Van der Waals forces) and stronger specific interactions with the cell surface. Receptor-mediated adhesion often involves hydrogen bonding between hydroxyl (OH) and carboxylic groups (COOH) on the polymers and the mucosal surfaces.
Receptor-Mediated Adhesion
Certain polymers specifically bind to receptor sites on gastric cells, improving the retention of dosage forms. Plant lectins, such as tomato lectins, can interact specifically with sugar groups found in mucus or on the glycocalyx. [33]
Super-Porous Hydrogel System
The super-porous hydrogel system represents a distinct category from traditional systems due to its unique characteristics. This system enhances gastric retention by utilizing super-porous hydrogels with average pore sizes exceeding 100 ?m. These hydrogels quickly reach their equilibrium size within 60 seconds as they absorb water rapidly through their numerous interconnected pores. They expand significantly, with a swelling ratio of 100 or more, and acquire enough mechanical strength to endure the pressure from gastric contractions. This system is particularly suited for formulating hydrophilic particulate substances.
Magnetic System
The magnetic system aims to extend gastric retention time by incorporating a small magnet within the dosage form and positioning an external magnet on the abdomen over the stomach. While this method has proven effective, the precise placement of the external magnet is crucial, which may affect patient compliance. [35-36]
CONCLUSION
Based on the literature surveyed, it may be concluded that gastroretentive drug delivery offers various potential advantages for drugs with poor bioavailability due to their absorption being restricted to the upper gastrointestinal tract (GIT). These drugs can be delivered efficiently, thereby maximizing their absorption and enhancing absolute bioavailability. Due to the complexity of pharmacokinetics and pharmacodynamics parameters, in vivo studies are required to establish the optimal dosage form for a specific drug. Another promising area of research for gastroretentive drug delivery systems is the eradication of Helicobacter pylori, which is now believed to be the causative bacterium of chronic gastritis and peptic ulcers. Although this microorganism is highly sensitive to many antibiotics, its complete eradication requires high concentrations of antibiotics to be maintained within the gastric mucosa for prolonged periods. An important feature to consider is the stomach's physiology, particularly the timing of drug administration (during or apart from a meal). Developing an efficient gastroretentive dosage form is a real challenge to pharmaceutical technology. The drug delivery system must remain in the stomach for a sufficient time, which is not compatible with the stomach's normal physiology. Various gastroretentive drug delivery systems (high density, floating, expandable or unfoldable, swelling, superporous, bioadhesive, magnetic systems, etc.) are being studied, each presenting its own advantages and disadvantages. Currently, a significant amount of work is being done to develop different types of gastroretentive delivery systems for various drugs. In the future, it is expected that these systems will become increasingly important, ultimately leading to improved efficiencies in various types of pharmacotherapies.
REFERENCE
- Streubel A, Siepmann J, Bodmeier R. Gastroretentive drug delivery system. Expert Opin Drug Deliv 2006; 3(2): 217- 33.
- Iannucelli V, Coppi G, Bernabei MT, Camerorni R. Air compertment multiple-unit system for prolonged gastric residence. Part-I. Formulation study. Int J Pharm 1998; 174: 47-54.
- Garg R, Gupta GD. Progress in controlled gastroretentive delivery systems. Trop. J Pharm Res 2008; 7(3): 1055-66.
- Rouge N, Allemann E, Gex-Fabry M, Balant L, Cole ET, Buri P, Doelker E. Comparative pharmacokinetic study of a floating multiple-unit capsule, a high density multipleunit capsule and an immediate-release tablet containing 25 mg atenolol. Pharm Acta Helbetiae 1998; 73: 81-7.
- Streubel A, Siepmann J, Bodmeier R. Multiple unit Gastroretentive drug delivery: a new preparation method for low density microparticles. J Microencapsul 2003; 20: 329-47.
- Goole J, Vanderbist F, Aruighi K. Development and evaluation of new multiple-unit levodopa sustained-release floating dosage forms. Int J Pharm 2007; 334: 35-41.
- Shrma S, Pawar A. Low density multiparticulate system for pulsatile release of meloxicam. Int J Pharm 2006; 313: 150-58.
- Santus G, Lazzarini G, Bottoni G, Sandefer EP, Page RC, Doll WJ, Ryo UY, Digenis GA. An in vitro- in vivo investigation of oral bioadhesive controlled release furosemide formulations. Eur J Pharm Biopharm 1997; 44: 39-52.
- Klausner EA, Lavy E, Friedman M, Hoffman A. Expandable gastroretentive dosage forms. J Control Release 2003; 90: 143-62.
- Deshpande AA, Shah N, Rhodes CT, Malik W. Development of a novel controlled-release system for gastric retention. Pharm Res 1997; 14: 815-19. 11. Park K. Enzyme-digestible swelling as platforms for longterm oral drug delivery: synthesis and characterization. Biomaterials 1988; 9: 435.
- Fujimori J, Machida Y, Nagai T. Preparation of a magnetically-responsive tablet and configuration of its gastric residence in beagle dogs. STP Pharma Sci 1994; 4: 425-30.
- Wilson CG, Washington N. The stomach: its role in oral drug delivery. In: Rubinstein, MH, editors. Physiological Pharmaceutical: Biological barriers to drug absorption. Chichester, U.K.: Ellis Horwood. 1989. p. 47-70.
- Streubel A, Siepmann J, Bodmeier R. Drug delivery to the upper small intestine window using Gastroretentive technologies. Curr Opin Pharmacol 2006; 6: 501-8.
- Larhed AW, Artursson P, Grasjo J, Bjork K. Diffusion of drugs in native and purified gastrointestinal mucus. J Pharm Sci 1997; 86(6): 660-65.
- Dubernet C. Syste`mes a` liberation gastrique prolonge`e. In: Falson-Rieg F, Faivre V, Pirot F, editors. Novelles formes me`dicamenteuses. Editions Me`dicales Internationales. Editions TEC and DOC. Cachan. 2004. p. 119-33.
- Arrora S, Ali J, Khar RK, Baboota S. Floatng drug delivery systems: A review. AAPS Pharm Sci Tech 2005; 6(3): 372-90.
- El-Kamel AH, Sokar MS, Al Gamal SS, Naggar VF. Preparation and evaluation of ketoprofen floating oral delivery system. Int J Parm 2001; 220: 13-21.
- Garg S, Sharma S. Gastroretentive drug delivery systems. Business Briefing: Pharmatech 2003: 160-66. 20. Khosla R, Feely LC, Davis SS. Gastrointestinal transit of non-disintegrating tablets in fed subjects. Int J Pharm 1989; 53: 107-17.
- Mojaverian P, Vlasses PH, Kellner PE, Rocci Jr ML. Effects of gender, posture and age on gastric residence time of an indigestible solid: Pharmaceutical considerations. Pharm Res 1988; 10: 639-44.
- Vyas SP, Khar RK. Gastroretentive systems. In: Controlled drug Delivery. Vallabh Prakashan, Delhi, India. 2006. p. 197-217.
- Clarke GM, Newton JM, Short MD. Gastrointestinal transit of pellets of differing size and density. Int J Pharm 1993; 100(13): 81-92.
- Moes AJ. Gastric retention systems for oral drug delivery. Business Briefing: Pharmatech 2003: 157-59.
- Sing BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Rel 2000; 63: 235-59.
- Sungthongjeen S, Paeratakul O, Limmatvapirat S, Puttipupathachorn S. Preparation and in-vitro evaluation of multiple-unit floating drug delivery system based on gas formation technique. Int J Pharm 2006; 324: 136-43.
- Krogel I, Bodmeier R. Development of a multifunctional matrix drug delivery system surrounded by an impermeable cylinder. J Control release 1999; 61: 43-50.
- Goole J, Vanderbist F, Aruighi K. Devlopment and evaluation of new multiple-unit levodopa sustained release floating dosage forms. Int J Pharm 2006; 313: 150-158.
- Santus G, Lazzarini G, Bottoni G, Sandefer EP, Page RC, Doll WJ, Ryo UY, Digenis GA. An in vitro-in vitro investigation of oral bioadhesive controlled release furosemide formulations. Eur J Pharm Biopharm 1997; 44: 39-52.
- Klausner EA, Lavy E, Friedman M, Hoffman A. Exapandable gastroretentive dosage forms. J Control Release 2003; 90: 143-162.
- Deshpande AA, Shah N, Rhodes CT, Malik W. Devlopment of a novel controlled-release system for gastric retention. Pharm Res 1997; 14: 815-819.
- Park K. Enzyme-digestible swelling as platforms for long term oral drug delivery: synthesis and characterization. Biomaterials 1988; 9: 435.
- Fujimori J, Machida Y, Nagaui T. Preparation of a magnetically- responsive tablet and configuration of its gastric residence in beagle dogs. STP Pharm Sci 1994; 4: 425-430.
- Clarke G.M., Newton J.M., Short M.D., Gastrointestinal transit of pellets of differing size and density, Int. J. Pharm. 100 (1-3), 1993, 81-92.
- Clarke G.M., Newton J.M., Short M.D., Comparative Gastrointestinal Transit of Pellet Systems of Varying Density, Int. J. Pharm. 114 (1), 1995, 1-11.
- Chen J, Blevins WE, Park H, Park K, Gastric retention of superporous hydrogel composites, J Control Release., 64, 2000, 39-51.


 Dr. Jeevan R. Rajguru* 6
Dr. Jeevan R. Rajguru* 6















 10.5281/zenodo.12818744
10.5281/zenodo.12818744