Abstract
The risk of radiological and nuclear accidents, which possess serious threats to human health increases significantly due to the over demand and utilization of radiation technologies and radioisotopes. The main target of ionizing radiation is the DNA present within cellular structures. They can induce harm to DNA through direct and indirect methods. Ionizing radiation can induce different types of lesions in DNA such as single strand breaks, double strand breaks, base damage, non - double strand breaks clustered damage and DNA cross links. Therefore, exposure to ionizing radiation, whether intentional or accidental, carries negative health consequences that require minimization. The growing health concern highlights the need for developing optimal and efficacious radiation countermeasures, comprising radioprotectors, radiomitigators and therapeutics. A better understanding of the mechanism of action by which these countermeasures operate is of paramount importance for investigating their possible applications in the medical contexts. This review elucidates the different mechanisms of radiation induced DNA damage, the diverse type of radiation countermeasures available and the role of radioprotectors. Additionally, it explains the challenges and future prospects associated with radiation protectors.
Keywords
Ionizing radiation, DNA damage, Single strand breaks, Double strand breaks, Non-double strand break clustered damage, radiation countermeasures, radioprotectors, radiomitigators.
Introduction
Radiation has been a part of our environment since the birth of the planet. Radioactivity is a natural characteristic of our planet and has always existed. The Earth’s crust contains naturally occurring radioactive materials (NORM), and various naturally occurring radioactive elements are also present in our bodies, muscles, bones, tissues and the food we eat. Consequently, life revolved around an environment with substantial levels of ionizing radiation (Zakariya & Khan, 2014). The bulk of the exposure to these radiations comes from different natural sources such as highly penetrating cosmic rays stemming from outer space, as well as alpha, beta and gamma radiations originating from terrestrial sources (Eisenbud, 1984). A continuous spectrum is formed that comprises diverse electromagnetic waves ranging from the lowest frequency known as radio waves to the highest frequency waves such as X-rays and gamma rays (Alpen, 1997). Ionizing radiation is a form of radiation possessing sufficient energy to remove tightly bound electrons when it interacts with atoms, thereby causing the atom to become charged or ionized (Bakar et al., 2019). These radiations were discovered by Wilhelm Conrad Roentgen in the year 1985, following which their application in medical science for therapeutics and diagnostics was recognized (Samet, 2011). The application of radiation in industry, medical diagnostics and cancer therapy emphasizes its valuable role in everyday life (Han & Yu, 2009). Different techniques such as X- ray and CT scans employed in medical imaging have become essential components of contemporary medical diagnosis, resulting in increased exposure of patients to ionizing radiation (Smith et al., 2017). Detrimental health effects of radiations were recognized soon after the invention of x-rays in the year 1895. Epilation was first reported in 1896, followed by the explanation of skin burns soon after. Exposure to radiation is also linked to long- term health effects. A significant portion of our understanding of this statement arises from the atomic bomb incidents in Hiroshima and Nagasaki and the famous Chernobyl nuclear power plant incident, which occurred in 1986 (Kamiya et al., 2015). Subjection to ionizing radiation also leads to genomic instability, which is passed down through many generations post irradiation, affecting the progeny of surviving cells. Induced genomic instability leads to various delayed consequences including, lethal mutation, chromosomal instability and mutagenesis (Suzuki et al., 2003). The effect of radiation varies widely, including short-term and long-term health impacts as well as physical, social and psychological effects (Hasegawa et al., 2015). The health effects of radiation can be categorized into two main types. The first type is early deterministic effects, which occur at high doses that is dose more than 1 Sv (Sievert), inducing cell death in exposed tissues. These effects are frequently seen in cancer patients going through radiotherapy and include symptoms such as vomiting, diarrhoea and hair loss. The second type is the longer term effects, also known as stochastic effects. The most alarming stochastic effect is the development of radiation induced malignancies (Thomas & Symonds, 2016). The first case of radiation induced cancer was reported in 1902. By 1911, there were more instances of leukaemia induced by radiation in occupational workers exposed to radiation (Shah, Sachs, & Wilson, 2014).
REVIEW OF LITERATURE
TARGETS OF RADIATION – NUCLEUS AND DNA
The belief that a cell’s nucleus, specifically its genomic DNA, is the vital radiation sensitive structure accountable for the effects leading to cell death and cancer is supported by numerous indirect and inferential aspects. One primary factor is the size of DNA, which is the largest macromolecule in cells. Moreover, the nucleus has long been viewed as the brain or the control center of the cell, further supporting the concept that DNA is the primary target for the effects of ionizing radiations. Researchers have utilized radioactive substances that tend to build up in cell’s DNA, plasma membrane and cytoplasm. They have observed and concluded that less harm is caused when exposing the extranuclear regions of the cell to radiation compared to exposing the DNA within the nuclei (Iyer & Lehnert, 2000).
IONIZING RADIATION INDUCED DNA DAMAGE
The absorption of radiation energy by living materials can result in excitation or ionization. Sufficient energy possessed by the ionizing radiation is responsible for ejection of orbital electrons from an atom or molecule. Factors like the dose rate, total amount of exposure, the type of linear energy transfer (LET) and how the doses are divided over time determine the biological consequences caused by radiation (Obrador et al., 2020). Comprehending the mechanism of DNA damage induced by irradiation remains challenging due to the complexity of DNA molecule (Sakata et al., 2019). Tsoulou and colleagues inferred that damage caused to DNA by radiation is not a sudden effect rather instead a series of damaging events that happen at different times (Ahmed, 2005). Radiation can damage cells or tissues through direct or indirect action. The direct mode of action involves interacting with cellular components such as DNA, causing permanent injury. The radiation directly hits the DNA, causing disruptions in its structure, which can lead to cell injury or cell death. The damaged cells that survive may later induce malignancies or other abnormalities (Citrin et al., 2010; Desouky et al., 2015). The direct damage by ionizing radiation also involves the direct excitation or ionization of DNA components. The synthesis of enzymes required for DNA replication is also hindered by irradiation, thereby stopping mitotic divisions (Ahmed, 2005). Indirect effects occur when radiation interacts with adjacent molecules in the cells that are non-essential targets but are close enough to transfer this damage, in the form of free radicals. Free radicals are characterized by high reactivity due to presence of unpaired electrons; therefore, they react with DNA, causing structural damage (Citrin et al., 2010; Desouky et al., 2015).
MECHANISM OF DIRECT DAMAGE CAUSED BY IONIZING RADIATION
A. DOUBLE STRAND BREAKS (DSBs)
Double strand breaks (DSBs) in DNA induced by IR are most lethal form of damage (Santivasi & Xia, 2014). Ionizing radiation causes breaks in the phosphodiester backbone of both DNA strands, typically around 10 base pairs of each other (Lomax, Folkes, & O.Neill, 2013). If left unrepaired or repaired improperly, these DSBs can lead to chromosomal aberrations which in turn can cause human disorders, including cancer. Mammalian cells have two primary methods for fixing DSBs in DNA. The first approach is non – homologous end joining (NHEJ) and the second approach is homologous recombination (HR), which is primarily used to repair secondary DSBs that arises post exposure to ionizing radiation (IR) during the s-phase of the cell cycle. NHEJ involves several steps, including the recognition of DSBs, processing of the damaged DNA ends to remove non-ligatable groups and finally the ligation of the broken ends (Vignard, Mirey, & Salles, 2013).
B. BASE DAMAGE
Radiation causes certain structural changes in DNA, including base damage, such as unstacking of the bases and disordering of the B-form of the backbone (Sailer, 1996). It is anticipated that radiation induces damage to nitrogen bases within DNA. Irradiation induces the formation of sites that are susceptible to cleavage by endonucleases. Utilizing endonucleases with well defined specificities shows promise approach for quantifying base damage in irradiated DNA. The hydroxyl radicals formed due to ionizing radiation attacks the deoxyribose sugar in DNA, as well as 78% of the nucleotide bases (Hutchinson, 1985).
C. NON-DSB CLUSTERED DAMAGE
Ionizing radiation (IR) can cause both isolated DNA lesions and clustered damage. Non-DSB clustered damages consist of multiple closely spaced lesions, such as oxidized purines, oxidized pyrimidines and the formation of abasic sites. These clusters are challenging to repair and are more lethal or mutagenic. 80% of complex DNA damage is caused by clusters of oxidized bases and abasic sites, while the remaining 20% is caused by double strand breaks (Sutherland et al., 2000). These clustered DNA lesions can be situated on a single strand (tandem lesions) or on both strands (bistranded lesions). It is significant to record that higher linear energy transfer (LET) results in denser ionization tracks, enhancing the probability of producing complex lesions (Mavragani et al., 2019).
MECHANISM OF INDIRECT DAMAGE CAUSED BY IONIZING RAIDATION
- WATER RADIOLYSIS AND FORMATION OF REACTIVE OXYGEN SPECIES (ROS)
Nearly 80% of the biological system is made up of water. The primary pathway through which ionizing radiation damages cells is by generating reactive oxygen species (ROS), which are produced when high-energy ionizing radiation is absorbed by small molecules, primarily water, which surrounds cellular biomolecules. This interaction causes the radiolysis of water, leading to the production of hydrogen peroxide, hydrogen and free radicals such as hydroxyl and singlet hydrogen. The latter quickly changes into superoxide. Free radicals generated can lead to oxidative stress and cause damage to various cellular components, including DNA, proteins and lipids (Islam, 2017; Smith et al., 2017). Reactive oxygen species (ROS), when formed, damage DNA by causing loss of purine and pyrimidine base, single-strand breaks (SSBs), double-strand breaks (DSBs), destruction of sugar, DNA protein crosslinks and telomere malfunction (Mishra, Moftah, & Alsbeih, 2018). Figure -1 illustrates the mechanism of radiation induced DNA damage through the radiolysis of water.
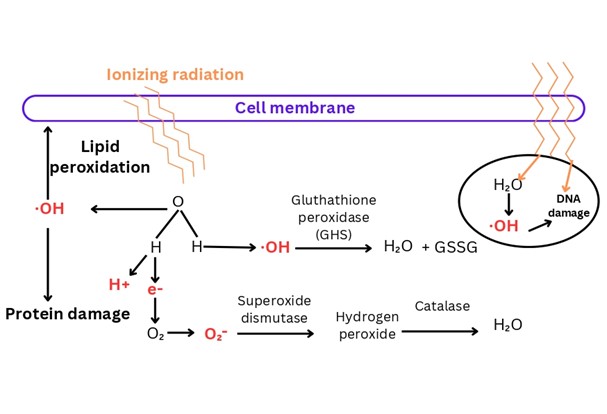
Figure-1 Illustrates the generation of reactive oxygen species (ROS) by ionizing radiation through the radiolysis of cellular water. The absorption of high energy ionizing radiation by water molecules leads to the production of hydroxide radicals and free electrons, which can cause protein damage or lipid peroxidation.
In malignant cells reactive oxygen species (hydroxyl radicals, superoxide radicals, hydrogen peroxide and singlet oxygen) directly influence the nuclear transcription factor and change the expression of Bcl-2, Bax and p53. These molecules are responsible for controlling the apoptosis, the rate of telomere shortening and they inhibit the growth and apoptosis of cancerous cells. In normal cells, ROS stimulate oncogenes and deactivate tumour suppressor genes which may start tumorigenesis and advancement of tumours (Sowa et al., 2012).
RADIATION COUNTERMEASURES
Radiation countermeasures involve substances that safeguard organisms from the adverse effects of ionizing radiations and minimize damage resulting from planned or unplanned exposure (Mishra, Moftah, & Alsbeih, 2018). Radiation countermeasures can be studied under three broad categories - radioprotectors, radiomitigators and therapeutics. Radioprotectors are administered prior to exposure to prevent damage. Radiomitigators are given shortly after radiation exposure, but before symptoms appear, to accelerate recovery or repair. Radiation therapeutics or treatments are administered after symptoms manifest to promote repair or regeneration (Singh et al., 2014). Figure - 2 depicts the ideal characteristics of the radioprotective substance.
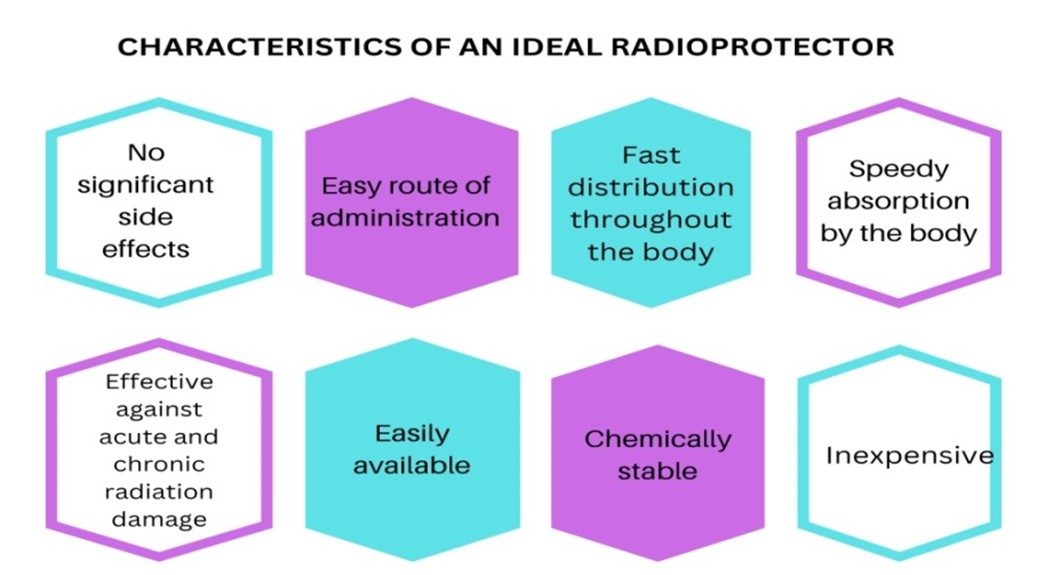
Figure -2:- An ideal radioprotector should have minimal or no side effects, be easily and quickly distributed throughout the body, be chemically stable, highly effective and inexpensive
RADIOPROTECTORS
The development of radioprotector is a highly promising strategy for both accidental and therapeutic exposure (Mun, Kim, Choi, Kim, & Lee, 2018). Radioprotectors, including synthetic compounds, natural plant extracts or phytochemical derivatives are effective in reducing radiation induced damage or toxicities when administered before radiation exposure (Montoro et al., 2023).
MECHANISM OF ACTION OF RADIOPROTECTORS
Radioprotectors exert their effects through several mechanisms including – scavenging free radicals by exhibiting antioxidant properties, enhancing DNA repair by triggering DNA damage repair pathways, donating a hydrogen atom to the radical formed during the interaction of IR with cellular molecules predominantly water, hypoxia induction in tissue, modulating redox sensitive genes, regulating the activity of growth factors and cytokines and by inhibiting apoptosis and delaying cellular division, which provides additional time for cells to repair DNA damage or undergo cell death (Mishra et al., 2018; Montoro et al., 2023; Soni et al., 2021). Radioprotective compounds inhibit free radical formation, eliminate formed free radicals or induce the production of natural radioprotectors such as superoxide dismutase, glutathione peroxidase and catalase. The enzyme glutathione peroxidase reduces hydroxide ions generated from the radiolysis of water due to ionizing radiation. Superoxide dismutase catalyzes the reduction of superoxide ions to hydrogen peroxide. The hydrogen peroxide produced by superoxide dismutase is subsequently used by catalase to generate water, as shown in figure-1 (Smith, et al., 2017). The induction of hypoxia and oxygen consumption by radioprotective agents, such as thiols and aminothiols, can effectively lower the concentrations of reactive oxygen species and hydrogen peroxide (Hosseinimehr, 2007). The following discussion elucidates the modes of action of a few radioprotectors, representing a subset of the wider array of radioprotective agents.
- MELATONIN
Melatonin chemically known as N-acetyl-5-methoxytryptamine is a hormone produced by pineal gland. It has been documented as a direct scavenger of free radicals and an indirect antioxidant because of its ability to stimulate antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione reductase and catalase. Previous studies have shown that melatonin decreased the formation of 8-hydroxy-2-deoxyguanosine, a DNA damage product resulting from free radicals, 60 to 70 times more efficiently than certain traditional antioxidants (Shirazi et al., 2012). Koc and colleagues found that administrating melatonin to rats prior to exposure to gamma radiation protected their liver tissue from oxidative harm (Kamran et al., 2016).
- VITAMINS
In recent years, there has been increasing interest in the effect of vitamins (such as vitamin A, vitamin E, vitamin C) on cancer, driven by the hypothesis that free radicals contribute to carcinogenesis. Vitamin E (also known as tocopherol), is a micromembrane stabilizer. It safeguards cell membranes from lipid peroxidation – induced damage by scavenging free radicals (Upadhyay et al., 2005). Studies have demonstrated that vitamin A and ?-carotenes, including lutein, lycopene, phytoene, reduced mortality and morbidity in mice subjected to partial or total body irradiation (TBI). Dietary vitamin A safeguards mice from radiation targeting the intestine (13 Gy, TBI) and the oesophagus (29 Gy). It was seen that a single dose of vitamin A administered intraperitoneally 2 hours prior to exposure to 2 Gy of ?-radiation, significantly reduced the number of micronuclei in the bone marrow and genetic damage. This reduction is due to its ability to scavenge free radicals (Montoro et al., 2023).
- OLIGOELEMENTS
The main oligoelements that have protective effects for radiation induced DNA damage are zinc, copper, selenium and manganese. These oligoelements are part of many defensive endogenous enzymes. Selenium serves as a co-factor for many enzymes including glutathione peroxidase, thioredoxin reductase and its derivative have proven to be a radioprotector in mice. An intraperitoneal administration of sodium selenite and selenomethionine, 24 hour and 1 hour before irradiation improved the survival rate in mice. Another derivative of selenium 3, 3-di-selenopropionic acid administered intraperitoneally at a dose rate of 2mg/kg for 5 days before whole body gamma radiation also exhibits radioprotection in mice by minimizing DNA damage and apoptosis. Past clinical data indicated that supplementation of oligoelements may act as an effective radiation protector for patients undergoing radiotherapy (Obrador et al., 2020).
D. FLAVONOIDS
Flavonoids are polyphenolic compounds that exhibit strong antioxidant activities. They act as radioprotective agents by scavenging free hydroxyl radicals. A study conducted by Devi and co-workers demonstrated that a flavonoid isolated from ocimum protects mice from exposure to 2 Gy of whole body gamma radiation. Narginin, a bioflavonoid found in grapefruit, guards against chromosomal damage induced by radiation in living organisms. Various flavonoids such as genistein, rutin, orientin and quercetin exhibit radioprotective properties (Kamran et al., 2016).
- SESAMOL
Sesamol, a natural phenolic antioxidant found in sesame seeds and sesame oil, possesses strong reactive oxygen (ROS) absorption and antioxidant properties. These properties can protect lymphocytes against ionizing radiation induced damage. The radioprotective effect of sesamol is influenced by the removal of reactive oxygen species (ROS) and the enhancement of DNA repair mechanism (Shivappa & Bernhardt, 2022).
- METFORMIN (MTF)
MTF has exhibited antioxidant, radioprotective and anticarcinogenic properties. MTF being a hydrogen rich compound is able to counteract free radicals and enhance the activity of certain enzymes such as SOD and CAT enzymes which promotes the antioxidant defenses of healthy cells. Additionally, metformin is also reported to reduce the generation of reactive oxygen species and activate DNA repair pathway by non-homologous end joining (NHEJ), homologous recombination and nucleotide excision repair (Montoro et al., 2023).
- AMINOTHIOLS
Aminothiols have been widely studied as radioprotectors. The underlying mechanism of action of aminothiols includes – scavenging free radicals, hydrogen atom or electron transfer to restore the damaged molecules, hypoxia induction, metal ion chelation. Treatment with aminothiols before irradiation provides protection against chromosomal aberrations. Aminothiols can produce significant alternations in cell metabolism, including modifications in cell cycle progression, DNA or protein synthesis, spindle formation and glycolysis (Murray, 2021).
Amifostine – a clinically used radioprotector
Amifostine, previously known as WR-2721, is a white crystalline powder that dissolves in water. WR-2721 being a prodrug requires activation by membrane bound alkaline phosphatase (AP) to form the active metabolite WR-1065. The active thiol, WR-1065 exhibits cytoprotective properties by scavenging free radicals and providing hydrogen atoms for DNA repair (Figure-3) (Müller et al., 2004; Santini, 2001). Amifostine can boost the synthesis of protein involved in repairing DNA damage and preventing cell death by affecting the function of Bcl-2 and hypoxia inducible factor-1?. (Andreassen, Grau, & Lindegaard, 2003) The American society of clinical oncology (ASCO) has recommended amifostine for prevention of xerostomia in patients undergoing radiotherapy for head and neck cancer (Singh & Seed, 2019). Continuous attempts have been made to improve the efficacy of amifostine by minimizing its toxicity and side effects, such as hypocalcaemia, fever, rash and malaise (Andreassen, Grau, & Lindegaard, 2003).
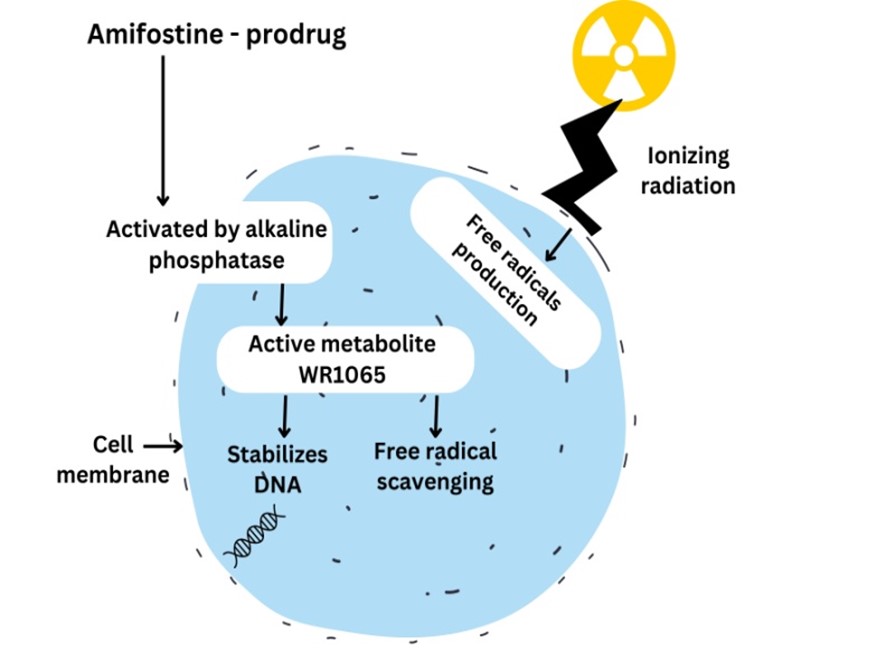
Figure -3:- Mechanism of action of amifostine
APPLICATIONS OF RADIOPROTECTORS
- Nuclear medicine –
Radiopharmaceutical agents are used in nuclear medicine for both imaging and treatment purpose. The ionizing radiation they emit can damage DNA, leading to side effects or the formation of secondary tumors. Radioprotective agents are employed to protect both the patients and staff from the effects of both internal (radiopharmaceuticals) and external sources of radiation (Hosseinimehr, 2009).
- Clinical uses –
Radioprotective compounds play a vital role in clinical radiation therapy by shielding surrounding normal and healthy tissues from the adverse effect of radiation and reduce the risk of secondary cancer formation (Abdollahi et al., 2015). Additional instances of ionizing radiation used in medical applications include positron beam utilized during positron emission tomography (PET) scans, x –rays employed for diagnostics, Iodine-131 isotopes for detecting thyroid cancer (Kumar & Singh, 2017).
- Military applications –
Radiation countermeasures are highly valuable for military personnel during a nuclear emergency or radiation exposure in the battlefield (Kumar & Singh, 2017).
- Space travel –
In addition to electromagnetic radiation, space radiation environment is also characterized by particle radiation, which consists of high energy particles possessing mass and charge or without charge. The space radiation poses significant risk for human space exploration and requires countermeasure strategies. When entering an environment or an area where exposure to ionizing radiation is a known risk, individuals were given radiation protectants (Montesinos et al., 2021).
- Research and study –
Phosphorous-32 and Tritium-99, used in research also emit ionizing radiation (Kumar & Singh, 2017). Radioprotectors are employed to prevent radiation exposure and to facilitate studies on its effects on living organisms, aiding in the development of new drugs for radiation protection.
CHALLENGES AND FUTURE DIRECTIONS
There is an urgent need for safe and effective measures to protect against the health hazards of ionizing radiation. An ideal agent, with minimal or no toxicity and favorable routes of administration, has yet to be developed. Several radioprotective compounds have shown promising results in preclinical studies and are in advanced stage of development. This progress suggests an increasing likelihood of readiness in the field of radiation countermeasures, which will be crucial in addressing threats from nuclear and radiological accidents and managing post radiotherapy complications. However, the process of developing any drug is challenging and complex, with low success rates despite advancements in knowledge, approaches and technology. One of the major reasons for the slow development of drugs to combat radiation- induced illness is the inability to conduct human efficacy studies due to ethical factors. Considering all these obstacles, it is clear that developing a radiation countermeasure is exceptionally difficult. Hence continuous and comprehensive efforts are needed. Efforts are underway to discover a radioprotective agent that is both safe and efficacious for human use, devoid of any toxicity.
CONCLUSION
Researchers have investigated the radiation chemistry of DNA to comprehend the specific changes caused by ionizing radiation in the DNA molecule. Damage to DNA is regarded as the main event induced by irradiation. Single-strand breaks (SSBs) and double-strand breaks (DSBs) are among the primary mechanisms through which ionizing radiation (IR) impacts DNA. Additionally, radiation induces clustered damage and the production of reactive oxygen species (ROS), further contributing to DNA damage. Radiotherapy is currently employed for both curative and palliative care in cancer patients. Irradiation of tumour tissue with ionizing radiation exposes surrounding normal and healthy tissue, which can result in acute and late radiation-induced normal tissue toxicity. Utilizing compounds to shield normal tissues and to mitigate toxicity post-damage could potentially lower the adverse effects of radiotherapy in patients and offer strategies for managing individuals exposed to radiation in different scenarios. Despite considerable research and efforts, the ideal radioprotector has yet to be developed. Various challenges, including issues related to drug toxicity and side effects, less radioprotection window, reduced effectiveness of agents against various types of radiation still need to be addressed for the development of an efficient and safe radioprotector. This review primarily examines the mechanisms underlying DNA damage induced by radiation, as well as the role of radioprotectors as countermeasures against radiation exposure.
REFERENCES
- Abdollahi, H., Shiri, I., Atashzar, M., Sarebani, M., Moloudi, K., &Samadian, H. (2015). Radiation protection and secondary cancer prevention using biological radioprotectors in radiotherapy. DNA repair, 29, 30.
- Ahmed, R. G. (2005). Damage pattern as function of various types of radiations. Medical Journal of Islamic World Academy of Sciences, 15(4), 135-147.
- Alpen, E. L. (1997). Radiation biophysics. Academic press.
- Andreassen, C. N., Grau, C., &Lindegaard, J. C. (2003, January). Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. In Seminars in radiation oncology (Vol. 13, No. 1, pp. 62-72). WB Saunders.
- Andreassen, C. N., Grau, C., &Lindegaard, J. C. (2003, January). Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. In Seminars in radiation oncology (Vol. 13, No. 1, pp. 62-72). WB Saunders.
- Bakar, N. F. A., Othman, S. A., Azman, N. F. A. N., &Jasrin, N. S. (2019, June). Effect of ionizing radiation towards human health: A review. In IOP Conference Series: Earth and Environmental Science (Vol. 268, No. 1, p. 012005). IOP Publishing.
- Citrin, D., Cotrim, A. P., Hyodo, F., Baum, B. J., Krishna, M. C., & Mitchell, J. B. (2010). Radioprotectors and mitigators of radiation-induced normal tissue injury. The oncologist, 15(4), 360-371.
- Desouky, O., Ding, N., & Zhou, G. (2015). Targeted and non-targeted effects of ionizing radiation. Journal of Radiation Research and Applied Sciences, 8(2), 247-254.
- Eisenbud, M. (1984). Sources of ionizing radiation exposure. Environment: Science and Policy for Sustainable Development, 26(10), 6-33.
- Han, W., & Yu, K. N. (2009). Response of cells to ionizing radiation. Adv. Biomed. Sci. Eng, 59, 204-262.
- Hasegawa, A., Tanigawa, K., Ohtsuru, A., Yabe, H., Maeda, M., Shigemura, J., ... &Chhem, R. K. (2015). Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. The Lancet, 386(9992), 479-488.
- Hosseinimehr, S. J. (2007). Trends in the development of radioprotective agents. Drug discovery today, 12(19-20), 794-805.
- Hosseinimehr, S. J. (2009). Potential utility of radioprotective agents in the practice of nuclear medicine. Cancer Biotherapy and Radiopharmaceuticals, 24(6), 723-731.
- Hutchinson, F. (1985). Chemical changes induced in DNA by ionizing radiation. Progress in nucleic acid research and molecular biology, 32, 115-154.
- Islam, M. T. (2017). Radiation interactions with biological systems. International journal of radiation biology, 93(5), 487-493.
- Iyer, R., & Lehnert, B. E. (2000). Effects of ionizing radiation in targeted and nontargeted cells. Archives of biochemistry and biophysics, 376(1), 14-25.
- Kamiya, K., Ozasa, K., Akiba, S., Niwa, O., Kodama, K., Takamura, N., ... & Wakeford, R. (2015). Long-term effects of radiation exposure on health. The lancet, 386(9992), 469-478.
- Kamran, M. Z., Ranjan, A., Kaur, N., Sur, S., & Tandon, V. (2016). Radioprotective agents: strategies and translational advances. Medicinal research reviews, 36(3), 461-493.
- Kumar, R., & Singh, A. K. (2017). Excellence is the real Enemy of Practicality! relevance to radiation countermeasure development.
- Lomax, M. E., Folkes, L. K., &O'neill, P. (2013). Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clinical oncology, 25(10), 578-585.
- Mavragani, I. V., Nikitaki, Z., Kalospyros, S. A., &Georgakilas, A. G. (2019). Ionizing radiation and complex DNA damage: from prediction to detection challenges and biological significance. Cancers, 11(11), 1789.
- Mishra, K. N., Moftah, B. A., &Alsbeih, G. A. (2018). Appraisal of mechanisms of radioprotection and therapeutic approaches of radiation countermeasures. Biomedicine & Pharmacotherapy, 106, 610-617.
- Montesinos, C. A., Khalid, R., Cristea, O., Greenberger, J. S., Epperly, M. W., Lemon, J. A., ... & Jones, J. A. (2021). Space radiation protection countermeasures in microgravity and planetary exploration. Life, 11(8), 829.
- Montoro, A., Obrador, E., Mistry, D., Forte, G. I., Bravatà, V., Minafra, L., ... & Mishra, K. P. (2023). Radioprotectors, Radiomitigators, and Radiosensitizers. In Radiobiology Textbook (pp. 571-628). Cham: Springer International Publishing.
- Müller, A. C., Pigorsch, S., Beyer, C., Lautenschläger, C., & Dunst, J. (2004). Radioprotective effects of amifostine in vitro and in vivo measured with the comet assay. Strahlentherapie und Onkologie, 180(8), 517.
- Mun, G. I., Kim, S., Choi, E., Kim, C. S., & Lee, Y. S. (2018). Pharmacology of natural radioprotectors. Archives of pharmacal research, 41, 1033-1050.
- Murray, D. (2021). Aminothiols. In Radioprotectors (pp. 53-107). CRC Press.
- Obrador, E., Salvador, R., Villaescusa, J. I., Soriano, J. M., Estrela, J. M., & Montoro, A. (2020). Radioprotection and radiomitigation: from the bench to clinical practice. Biomedicines, 8(11), 461.
- Sailer, K. (1996). Radiation-induced structural modifications in dsDNA analysed by FT-Raman spectroscopy. International journal of radiation biology, 69(5), 601-613.
- Sakata, D., Lampe, N., Karamitros, M., Kyriakou, I., Belov, O., Bernal, M. A., ... &Incerti, S. (2019). Evaluation of early radiation DNA damage in a fractal cell nucleus model using Geant4-DNA. Physica Medica, 62, 152-157.
- Samet, J. M. (2011). Radiation and cancer risk: a continuing challenge for epidemiologists. Environmental Health, 10(Suppl 1), S4.
- Santini, V. (2001). Amifostine: chemotherapeutic and radiotherapeutic protective effects. Expert opinion on pharmacotherapy, 2(3), 479-489.
- Santivasi, W. L., & Xia, F. (2014). Ionizing radiation-induced DNA damage, response, and repair. Antioxidants & redox signaling, 21(2), 251-259.
- Shah, D. J., Sachs, R. K., & Wilson, D. J. (2012). Radiation-induced cancer: a modern view. The British journal of radiology, 85(1020), e1166-e1173.
- Shirazi, A., Mihandoost, E., Mahdavi, S. R., &Mohseni, M. (2012). Radio-protective role of antioxidant agents. Oncology Reviews, 6(2).
- Shivappa, P., & Bernhardt, G. V. (2022). Natural radioprotectors on current and future perspectives: A mini-review. Journal of Pharmacy and Bioallied Sciences, 14(2), 57-71.
- Singh, V. K., & Seed, T. M. (2019). The efficacy and safety of amifostine for the acute radiation syndrome. Expert opinion on drug safety, 18(11), 1077-1090.
- Singh, V. K., Newman, V. L., Romaine, P. L., Wise, S. Y., & Seed, T. M. (2014). Radiation countermeasure agents: an update (2011–2014). Expert opinion on therapeutic patents, 24(11), 1229-1255.
- Smith, T. A., Kirkpatrick, D. R., Smith, S., Smith, T. K., Pearson, T., Kailasam, A., ... & Agrawal, D. K. (2017). Radioprotective agents to prevent cellular damage due to ionizing radiation. Journal of translational medicine, 15, 1-18.
- Soni, A. K., Soni, A., Upmanyu, N., Kannan, G. M., &Dongre, N. (2021). Radiation Injury: Mechanism of Toxicity and Countermeasures. Free Radicals and Antioxidants, 11(2), 29-34.
- Sowa, P., Rutkowska-Talipska, J., Sulkowska, U., Rutkowski, K., & Rutkowski, R. (2012). Ionizing and non-ionizing electromagnetic radiation in modern medicine. Polish Annals of Medicine, 19(2), 134-138.
- Sutherland, B. M., Bennett, P. V., Sidorkina, O., & Laval, J. (2000). Clustered damages and total lesions induced in DNA by ionizing radiation: oxidized bases and strand breaks. Biochemistry, 39(27), 8026-8031.
- Suzuki, K., Ojima, M., Kodama, S., & Watanabe, M. (2003). Radiation-induced DNA damage and delayed induced genomic instability. Oncogene, 22(45), 6988-6993.
- Thomas, G. A., & Symonds, P. (2016). Radiation exposure and health effects–is it time to reassess the real consequences?. Clinical Oncology, 28(4), 231-236.
- Upadhyay, S. N., Dwarakanath, B. S., Ravindranath, T., & Mathew, T. L. (2005). Chemical radioprotectors. Defence Science Journal, 55(4), 403.
- Vignard, J., Mirey, G., & Salles, B. (2013). Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiotherapy and Oncology, 108(3), 362-369.
- Zakariya, N. I., & Kahn, M. T. E. (2014). Benefits and biological effects of ionizing radiation. Sch. Acad. J. Biosci, 2(9), 583-591


 Pooja Belwal* 1
Pooja Belwal* 1



 10.5281/zenodo.11655004
10.5281/zenodo.11655004