Abstract
The systemic delivery of nanomedicines represents a major step forward for nanotechnology. But for many medicinal applications, the capacity to achieve spatiotemporal control may be crucial. Due to its ability to succeed at the atomic scale, nanotechnology has extended itself to even molecular levels, opening up new horizons in both diagnostic and therapeutic domains. In recent years, a number of delivery methods have been put forward around the world. Dendrimers, magnetic nanoparticles, liposomes, quantum dots, and nanoburrs used for applications such tissue imaging, targeted cancer cell targeting, cancer treatment, virus detection, noninvasive vaccine delivery, etc. are examples of nanosystems. Here, we continue with sophisticated applications like nanoburrs, which are nanoparticles coated with a sticky protein that allows them to adhere to artery walls while gradually releasing medication. This study concludes that nanotechnology is widely applicable both now and, in the future, and it indicates that it has significant implications for the advancement of veterinary care as well as human medicine. The operation and uses of nanoburrs are covered in this article.
Keywords
Nanotechnology, Nanoburrs, Nanoparticles, Cardiovascular Diseases, Atherosclerosis, CREKA Targeting micelles
Introduction
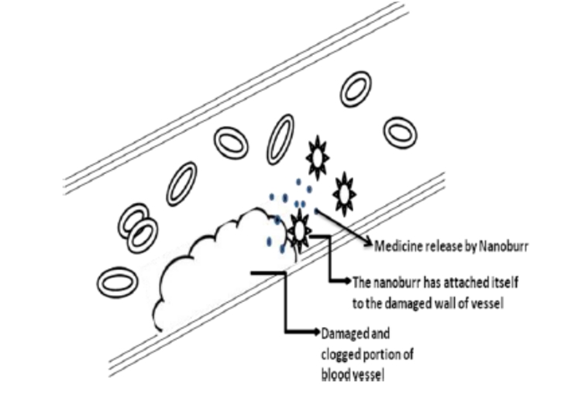
Small particles called nanoburrs travel through the bloodstream and adhere to afflicted arteries, where they administer medication straight to the injured tissue. Tiny protein bits cover nanoburrs, enabling them to adhere to injured arterial walls. Once tucked, they may release medications like paclitaxel, which blocks cell proliferation and aids in preventing the development of scar tissue that blocks arteries. The basement membrane, a particular structure that lines the arterial walls and is only accessible when those walls are broken, is the target of the nanoburrs. [1] Cardiovascular illness is responsible for about one-third of all male fatalities in our society, making it one of the leading causes of mortality. The percentage of cardiovascular disease-related male deaths has fallen since 1961, when it accounted for about half of all male deaths. Despite this, cardiovascular illness is the second-biggest cause of death for males, second only to cancer. The high mortality rate from cardiovascular illness can be explained by the fact that, on average, a man's blood cholesterol level is 5.5 millimoles per liter, which is 0.5 millimoles/liter higher than what the government advises. High cholesterol levels play a particularly important role in the development of atherosclerosis, which is brought about by the accumulation of cholesterol, fibers, and dead muscle cells into plaques (atheromas) on the lining of artery walls. Atherosclerosis results in the narrowing of arteries, which can then cause additional cardiovascular conditions like thrombosis and myocardial infarction. As a result, patients with constricted arteries or other inflammatory cardiovascular illnesses, as well as atherosclerosis, may be treated with drugs delivered via nanoburrs. The nanoburrs structure may facilitate production since the targeted peptides are connected to an outer shell rather than the drug-carrying center, which would necessitate a more complex chemical interaction. This design also lessens the possibility of nanoparticle rupture and promotes the safe release of medications. [1,4] The field of nanotechnology is expanding at a breakneck pace, with seemingly limitless potential in practically every area. everything that we do. For example, the food we eat, the drugs we use, the chemicals we use, the vehicle we drive, and many other things.
2. Multifunctional Micelles
Engineers at the University of California Santa Barbara have designed and tested a nanoparticle which they consider will target atherosclerotic plaques, specifically the “shoulder” region – the place where the plaque is most likely to rupture and causes in heart disease complication. This nanoparticle was a multifunctional micelle – a lipid based collection of molecules that form a sphere shape [3] ( Fig 1). The micelle has a peptide, a piece of protein [pentapeptide (cysteine-arginine-glutamic acid- lysine-alanine)], on its surface, and that peptide binds to the surface of the plaque. Fibrin has already been used widely as a target for drug delivery to specific sites and it is widely predictable as being deposited in atherosclerotic plaques.[2]
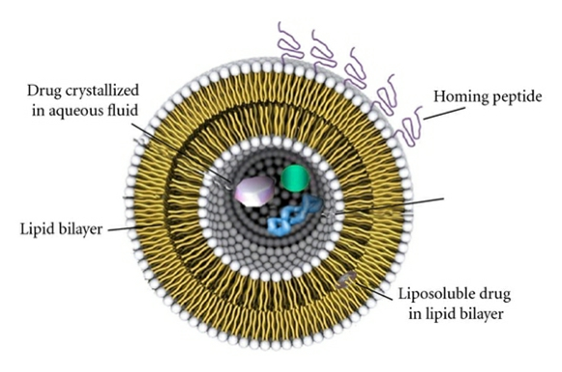
Fig 1: Multifunctional Micelles
They used these findings to test if their micelles could be used to deliver thrombin inhibiting drugs to the site of atherosclerotic plaques. Thrombin has been found to both induce clotting and increase the progression of atherosclerosis by causing smooth muscle cells to bind to Low Density Lipoproteins (LDL). If thrombin can be inhibited, then the progression of atherosclerosis can be halted and its adverse effects avoided. The production of thrombin 6 is induced when an atherosclerotic plaque ruptures – the specific place where the CREKA-targeting micelles were found to concentrate. It is with this CREKA-targeting micelle that a treatment for atherosclerosis can be developed. By testing it on humans and approving it, we can advance the treatment for both atherosclerosis and thrombosis (and therefore myocardial infarction) through the use of the inhibitor drug Hirulog. The many benefits of micelles, outlined above, mean that they are excellent for progressing our medical treatment of atherosclerosis and, although not discussed here, tumours. Research shows that there is great potential for CREKA-targeting micelles in the treatment of atherosclerosis and the prevention of its adverse effects. The problem faced now is that these findings are only reported in mice at present and therefore might not translate into the same positive effects in humans. However, if the same conclusions can be drawn for humans as for mice, then CREKA-targeting micelles would be an excellent solution to the current problem. [2,5]
Advantages of multifunctional micelles
- Long circulation time.
- Designed to target the plaques that are most vulnerable to rupture
- Self-assembly and easy to be constructed.
3. Nanoburrs
A polymer core is surrounded by a lipid shell interface in the unique spherical structure of nanoburrs, or burrs, which are 60 billionths of a meter (60 nanometers) in diameter. On the period at the end of this statement, one might put thousands of them. The drug in each is connected to a polymer that breaks down gradually in the inner core. Burrs contain minute, hook-like structures that enable them to adhere to bare areas of injured arteries. They can also deliver the drug to the areas around the stent struts, serving as a backup for drug-eluting stents. [2,3] These A lipid shell interface surrounds a polymer core to form nanoburrs (Fig. 2). In the beginning, the only purpose of nanoburrs will be to deliver medication to lower the swelling of the injured tissue or to disintegrate the atheroma. Additional nanoparticles may be used to administer further medication following this treatment in order to repair the established damage to the intima, which would reinforce the coronary artery's lining. The identical method of drug delivery utilizing nanoburrs can be utilized to prevent ischemic stroke, since the nanoburrs would function similarly in delivering a dose of a drug that could break down an atheroma in one of the arteries.
4. Structure
It comprises three layers:
• Inner core: This is where the medication payload and a polymer chain, such as PLA, are located. (Polylactide).
• Middle core: It is composed of fatty material, such as soybean lecithin/lipid.
• Outer covering (antibiofouling layer, PEG – Polyethylene glycol)
A polymer PEG coats the outside of these burrs, shielding them as they move through the bloodstream. PEG is hydrophilic in nature and avoids RES scavenging, allowing burrs to remain in the bloodstream for an extended period of time. Burrs can be coated with a sticky protein that makes them adhere to artery walls while releasing drugs loaded simultaneously. The inner core regulates the release of the drug. The length of the polymer chain that is there determines it. A reaction—ester hydrolysis—moderates the activity for a longer period as the chain gets longer. The drug releases from the polymer during this reaction to exert its therapeutic effect. Nanoburrs with 7-amino peptides on the surface, which are used to target the damaged vasculature of the basement membrane, are discussed in Pam Baker's report, as illustrated in Figure 3 below [2,7].
5. Working:
Due to the peptides taken from bacterial phages, viruses that attack bacteria, that cover the nanoburrs, they adhere to damaged arterial walls. Studies have revealed that one peptide binds to the collagen that makes up the basement membrane of arteries, making it helpful in selectively attaching to the basement membrane of the artery wall that is exposed whenever the artery is damaged, such as during an angioplasty, when the inflated angio balloon presses against the artery wall, removing the top layer of cells.Because of the nanoburr's stickiness, these small hybrid-polymeric particles are far more likely to reach the treatment target than nanoparticles that don't have the protein hooks. The burred nanoparticles were two to four times more likely to attach to damaged arterial tissue than non-burred variants, according to the present study, which was conducted on live rats' carotid arteries and on arterial cell cultures in a dish [1,9,10]. The nanoburrs' "sticky" exterior covering (peptide coating) adheres to the tissue basement membrane. Only in damaged sections of the arterial wall is the tissue membrane exposed. The burrs, when given parenterally, Use the basement membrane to pinpoint arteries that require treatment. They adhere to this basement membrane, and the gradual release of medication helps to lessen the harm caused to arteries (e.g. paclitaxel) and prevent cell division. However, it is only revealed when the walls of the arteries are harmed. Additionally, it aids in preventing the development of scar tissue that can block arteries. This is a novel technology that may be combined with a number of additional therapies, particularly stents. These burrs have the benefit of being able to be used even in situations when stents cannot be used. By injecting these into the rat tails, Justin Borad demonstrated that they were able to migrate upstream by adhering themselves to the injured left carotid artery of the rat. The use of animal testing and additional improvements are being made to this [2,11].
6. Example:
The nanoburrs are designed to target the structure, particularly the basement membrane, which borders the arterial walls and is only visible when those walls are broken. As a result, they may be employed to administer medications for the treatment of atherosclerosis and other inflammatory cardiovascular illnesses. The present research employs Paclitaxel, a medication that blocks cell division and prevents the development of scar tissue that might block arteries. The particles are made up of spheres that are only 60 nanometers in diameter, which is over a hundred times smaller than a red blood cell. Each one is covered with microscopic protein fragments that enable it to adhere to the fractured surface of injured artery walls. The particle is primarily composed of a drug intended to treat blood vessel constriction linked to a chain-like polymeric molecule. The medicine gradually releases over several days and begins to treat the artery. Changing the polymer length allows for precise regulation of the drug's release time. According to the researchers [12], nanoburrs may be used in conjunction with vascular stents, the conventional treatment implants that physically open blocked arteries.
Rats as Subjects for Testing:
The team is conducting a two-week trial of the nanoburrs in rats to identify the optimal dosage for treating injured vascular tissue. Additionally, the particles might be helpful in delivering medications to tumors. The rate at which the burrs attached to the fractured walls was double that of nontargeted nanoparticles. Since the particles can be injected intravenously and are capable of releasing medication over a longer period of time. "This technology could have broad applications over other important diseases, including cancer and inflammatory diseases where vascular permeability or vascular damage is commonly observed" [12].
7. Planning:
The basement membrane's surface molecules are bound by short peptide sequences known as C-11, which are composed of seven amino acids. The nanoparticles' outer layer was later coated with these. The medication is carried by the inner core of the 60-nanometer-diameter particles, which are linked to a polymeric chain called Poly Lactic Acid (PLA). Between the core and the outer shell, which is made of a polymer called Polyethylene Glycol (PEG) that shields the particles as they circulate in the bloodstream, is a middle layer of soybean lecithin, a fatty substance. Esther hydrolysis, a slow reaction, causes the medication to detach from the PLA polymer chain, at which point it is released. By adjusting the length of the polymer chain, it is possible to modify the timing of the drug's release because this process takes longer the longer the polymer chain [1,4,6,9,10]. By self-assembly and nano precipitation, nanocombs are created. To create nanoparticles, a hot lipid solution is gradually mixed with acetone, vortexed thoroughly, and allowed to self-assemble for 2 hours. Maleimide-thiol was used to peptide functionalize the nanoparticles [2,13]. These burrs are protected by a polymer PEG coating when moving through the bloodstream. Burrs stay in the bloodstream for a long period because PEG is naturally hydrophilic and prevents scavenging. Burrs can be made to adhere to artery walls by coating them with a sticky protein, which also allows for the gradual release of the medicines they carry. The drug release is regulated by the inner core. The length of the polymer chain determines this. The duration of action regulated by a reaction, such as ester hydrolysis, increases with the length of the chain. The drug separates in this reaction.
8. Benefits of nanoburrs:
I. Nanoburrs are capable of attaching to damaged artery walls and delivering medications (such aspaclitaxel) to teat damage area[2].
II. The rate of medication release is regulated by the PLA polymer chain, and the length is determined by the depending on the length of the chain. Drug release was found to occur for more than 12 days during testing [2].
III. As the body fluids are made up of, the release brought about by hydrolysis is ideal.primarily water.
IV. Drug attachment usually necessitates a chemical reaction, making it more complex. This issue is made less complicated by the fact that burrs attach the targeted moieties to the outside shell rather than the core[2].
V. The frequent drawback of conventional vesicular drug delivery methods is thatearly explosion that releases the medicine in the wrong area and dosage. This disadvantage can be mitigated in burrs where the construction is designed to prevent the structures from breaking.
VI. The route of administration, where the medication is administered, is the most important advantage.Delivery may originate from a location far away from the afflicted region[11]. Justin Borad's research, which showed that burrs adhered to injured arteries at over twice the rate of healthy ones, supports this. nanoparticles that have been targeted [2,13].
VII. The use of burrs can be expanded with greater accuracy for tumors related to the changes in arterial permeability brought about by vascular injury [2,7].
VIII. They may be given intravenously at a location far away from the afflicted tissue. Research conducted in rats showed that nanoburrs injected close to the tail were able to target the walls of damaged carotid arteries at twice the rate of stents, but not healthy carotid arteries [1,9].
IX. Due to the particles' capacity to provide medications for a longer amount of time and to Patients would not have to receive multiple, surgically invasive injections directly into the site that needs therapy if the treatment were administered intravenously [1,14].
10. Uses of Nanoburrs:
It has potential applications in a variety of cardiovascular illnesses [1]. Tumors and inflammatory diseases are just a few of the significant illnesses that can be diagnosed with nanoburr, which detects specific vascular rupture or permeability [1]. According to Chan, "the nanoburrs may be useful for targeting solid tumors and for diseases where vascular damage and permeability is observed," such as inflammatory illnesses like inflammatory bowel disease.
11. Adaptability of Nanoburrs in Body:
Process to keep the nanoburrs sticking and circulating in the blood for a longtime is necessary for cardiovascular disease patient because as soon as it enters the patient body then body's natural defences will quickly muster attacks against it, treating as a foreign particle for the body [1,6]. To prevent this, nanoburrs are to be sheathed in soy lecithin, a fatty substance and then later should be coated with polyethylene glycol (PEG) because PEG is an inert hydrophilic substance and is able to evade much of the body's defences [10].
12. Nanoparticles with Medical uses
Nanoburrs: Targeting solid tumors and for diseases where vascular damage and permeability is observed.
- Dendrimers: Targeting of cancer cells, drug delivery, imaging.
- Magnetic nanoparticles: Specific targeting of cancer cells, tissue imaging
- Liposome: Specific targeting of cancer cells, gene therapy, drug delivery
- Goldnanoshells: Tissue imaging, thermal ablative cancer therapy
- Quantumdots: Tissue imaging
- Transfersomes: Noninvasive vaccine delivery, drug delivery
13. Nanoburrs Vs. Stents:
Tiny particles called nanoburrs travel through the bloodstream, attach to diseased arteries, and deliver medication straight to the damaged tissue. Initially, they seem to function like medicated stents, the typical therapy for blocked arteries. On closer inspection, though, it becomes evident that nanoburrs are a unique response to a significantly more complicated query [1]. One of the current common methods for treating blocked and damaged arteries involves the use of a vascular stent, which supports the artery open and delivers medications like paclitaxel. The researchers hope that this novel nanoburr may be employed in conjunction with stents or as a substitute for them in the treatment of injuries that occur in regions that are not ideal for stents Stents, like those close to an artery's bifurcation [1,6,14]. Stents are excellent at keeping arteries open and preventing them from collapsing suddenly. On the other hand, a stent is fixed in place, resembling a miniature Atlas carrying the burden of a collapsing artery wall [6]. Nanoburrs, in contrast, can target just damaged tissue and spread across isolated locations. Target proteins, which are identified with specific forms of tissue injury, bind to them like Velcro [1,7]. Comparing existing technology to novel technology such as burrs is common. A very frequent comparison is made to stents. Stents are widely used and undoubtedly beneficial because they prevent the arteries from suddenly collapsing by keeping them open. They are also able to provide medications to the injured artery walls. However, the drawback is that stents are immobile and have a fixed nature. They are confined to one location and must endure the burden caused by the collapsing artery, which will worsen over time. Burrs are, in contrast, more mobile. They can disperse themselves over vast areas while also having the ability to target injured tissues. Since it is harmful to people with diabetes, renal failure, and hypertension, stents may not be an option for everyone. In addition to this, stents may not be able to be placed in all the areas/locations, such as forks of the artery (bifurcation lesions), diffuse lesions, large arteries, already stented arteries having more than one lesion, etc. [3,7]. Stent insertion may not be appropriate for people whose arteries have constricted. Burrs are helpful in all these situations since they allow for greater flexibility and adaptability in the course of treatment.
14. CONCLUSION:
This approach was first used to target blood vessel walls in cardiovascular illness. The nanoburr mechanism has a wider range of applications. Using nanotechnology, we may develop a viable therapy and diagnosis for atherosclerosis, a potentially fatal and significant risk to our society. As previously mentioned, atherosclerosis and the vascular tissue injury that comes before it can be reduced by employing both nanoburrs and micelles. Moreover, the use of nanoburrs is especially cost-effective because it reduces the number of surgical procedures. The investigation conducted to propose these is comparable to the majority of studies. The ideas have brought up a lot of moral questions, particularly that of animal research. The issues are far outweighed by the potential benefits that this study may yield. The non-invasive treatment for atherosclerosis that we seek will likely be provided by nanotechnology in the form of micelles and nanoburrs in the future.
REFERENCES
- International Research Journal of Pharmacy, Jaihlia Rajiv et al. IRJP 2(5) 2011.
- Gajanan Shinde, novel approach: of nanotechnology, international journal of pharmacy & teaching & practices 2013, vol.4, issue4,
- Brendon Nafziger. Nanoburrs Stick to Injured Arteries. January 22, 2010.
- Chan J. Zhang L.; Tong R.; Ghosh D., Gao W. spantiotemporal controlled delivery of nanoparticles to injured vasculature. Proceedings the national academy of sciences. http/web.mit.edu/news office/2010/nanoburrs.html.
- http:/ww.pnas.org/content/106/24/9815
- Heart Statistics http://www.heartstats.org/latestadditions.asp?id=83
- Pam Baker Tiny Nanoburrs Stick to Damaged Arteries and Repair Tissue, Tech News World, 01/21/10, 10:54, PT, am. Available from: http://www.technewsworld.com/story/69154.html.
- Nanoburrs seek heal injury in artery, Harv. heart. lett.2011 Jan 21(5):6
- Trafton A. Nanoburrs. MIT news office article 2010. Available from: URL:
- http://web.mit.edu/newsoffice/2010/nanoburrs.html. http://www.dotmed.com/news/story/11380
- Justin Barad. Nanoburrs’ Stick to and Deliver Drugs to Damaged Arteries. Jan 19, 2010,11:09 am.
- www.irandaily.com/1388/11/1/MainPaper/3600/Page/8/MainPaper_3600_8. pd Nanoburrs 'Help Treat Heart Patients
- Scientists Enlist Nanoburrs to Unclog Arteries, Published: January 21, 2010. Available from: http://www.qmed.com/mpmn/blog/17161/scientists-enlist- nanoburrs-unclog-arteries
- Trafton A, MIT News Office, Massachusetts Institute of Technology. Editorial Adaptations by Science Daily Staff. Available From: URL: http.//web.mit.edu/news office/2010/nanoburrs.html
- Juliana M. Chan, Liangfang Zhang, Rong Tong, Omid C. Farokhzad. Spatiotemporal controlled delivery of nanoparticles to injured vasculature
- Hamish Mcclatchey, Sam Thomas ,Catherine Ward, Applications Of Nanotechnology In Coronary Heart Disease,Pass With Merit ,Research Paper Based On Pathology Lectures atwww.medlink-uk.net/wp-content/uploads
- http://www.understandingnano.com/nanoparticle-targeted-drug-delivery-arterial-plaque.html
- Information on drug-eluting stents http://en.wikipedia.org/wiki/Drug-eluting stent.


 V. M. Barethiya*
V. M. Barethiya*


 10.5281/zenodo.15645820
10.5281/zenodo.15645820