Abstract
Nanotechnology has the potential to generate advancements and innovations in formulations and delivery systems. This fast-developing technology has been widely exploited for diagnostic and therapeutic purposes. The application of nanotechnology in cosmetics has been shown to overcome the drawbacks associated with traditional cosmetics and also to add more useful features to a formulation. Nano cosmetics and nano cosmeceuticals have been extensively explored for skin, hair, nails, lips, and teeth, and the inclusion of nanomaterials has been found to improve product efficacy and consumer satisfaction. This is leading to the replacement of many traditional cosmeceuticals with nanocosmeceuticals. Novel nanocarriers like liposomes, niosomes, nanoemulsions, microemulsion, solid lipid nanoparticles, nanostructured lipid carrier, and nanospheres have replaced the usage of conventional delivery system. These systems enhance the bioavailability, stability, and efficacy of active ingredients, offering controlled release and targeted delivery features that are crucial for optimizing cosmetic results. Nanotechnology facilitates improved penetration of actives into the skin, protects ingredients from degradation, and ensures sustained release, thereby enhancing the therapeutic effectiveness of cosmeceuticals. This review highlights the various novel carriers used for the delivery of cosmeceuticals, their positive and negative aspects, marketed formulations, and their toxicity.
Keywords
Nanocosmeceutical, advancements, liposomes, niosomes, nanoemulsions, microemulsion, solid lipid nanoparticles, nanostructured lipid carrier.
Introduction
According to the Food and Drugs Act [FDA] the cosmetics can be defined as any product/ article to be rubbed, poured, sprinkled or sprayed on, introduced to or otherwise applied to the human body or part of it, except soap, intended for cleansing, beautifying, promoting attractiveness, or altering the appearance[1].The variety of products which belongs to the class cosmetics are shampoos, hair colours, nail lacquers, lipsticks, preparations meant to be applied on eye and face to be beautify them, toothpastes and deodorants etc.[2]. Within last 2-4 cosmetics has gained tons of recognition in both in males and females and considerd as essential parts in life. They are not only attracting the people towards it but also imparts the physiological effect.[3]. Cosmetics are not medicines nor still governed and regulated by FDA [2]. however, some cosmetic products could contain ingredients that can impart a biological effect with mild therapeutic action or drug-like benefit on the skin, hence termed as “Cosmeceuticals” [1] which improves appearance of skin, prevent wrinkles, skin dryness, dark spots, uneven complexion, hyperpigmentation, photo ageing and hair damage [4]. It is midway between both cosmetics and drug as these contains medicinal agents to mitigate, prevent or change any biological function of skin.[2]. Nanotechnology have the potential to enhance the effectiveness and affect the cellular responses associated with various cosmetics with extensive applications such as enhancement in physiochemical properties and the visual appeal of the cosmetic, enhancement in stability of the actives, modulation in penetration of actives, alteration of the route of penetration (intracellular, transcellular or appendageal), cause site specific accumulation, modulation in systemic toxicity of actives and enhancement in the intrinsic photostability of the cosmetics[5]. Furthermore, they offer targeted delivery, painless remedies, customized therapies and simplified solutions over the conventional therapies . Nanoparticles are the sub- micron sized particles in the size range of 1100 nm [4]. which influences their dermal penetration and cellular internalization. Size dependant accumulation of the nanocarriers may be obtained in various layers of the skin where the effects are specific to each type of nanopartices and hence theris no common cellular effect of all nanoparticles [5]. Various non-scientific approaches actively involved in beauty enhancement with the better entrapment, dispersibility, performance, quality, protection, penetration and patient compliance are Liposomes, Niosomes, Transferosomes, Cubosomes, Ethosomes, Dendrimers, Solid lipid nanoparticles, Nanoemulsion, Gold nanoparticles, Fullerene, Nanocapsules and Nanospheres etc.
2. 0 Types of Nanocosmeceuticals
- Liposomes
- Niosomes
- Solid lipid nanoparticle
- Nanostructured Lipid Carrier
- Nanoemulsions
- Gold Nanoparticles
- Dendrimers
- Cubosomes
- Ethosomes
- Nano capsule
- Fullerence
- Transferosomes
2.1 Liposomes
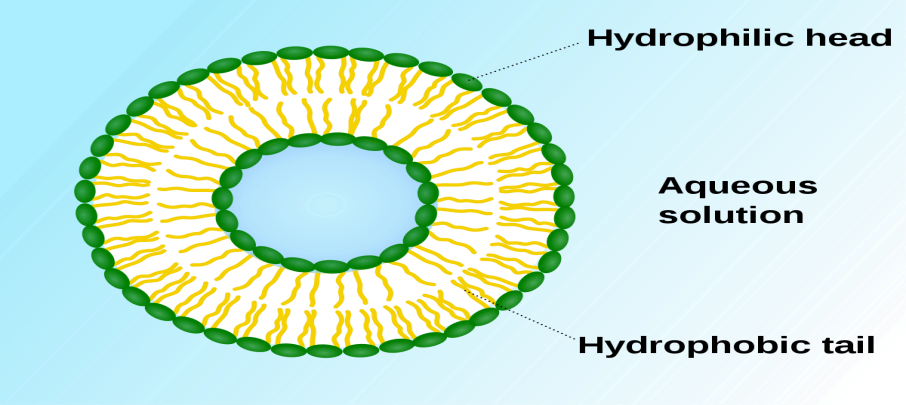
Figure 1: Liposomes
Liposomes are most widely used for the cosmeceutical preparations. They are the vesicular structures having an aqueous core which are enclosed by a hydrophobic lipid bilayer [6]. The main component of liposome lipid bilayer is phospholipids; these are GRAS (generally recognized as safe) ingredients, therefore minimizing the risk for adverse effects [7]. To protect the drug from metabolic degradation, liposome encapsulates the drug and releases active ingredients in a controlled manner [8]. Liposomes are suitable for delivery of both hydrophobic as well as hydrophilic compounds. Their size varies from 20 nm to several micrometers and can have either multilamellar or unilamellar structure [9]. Antioxidants such as carotenoids, CoQ10, and lycopene and active components like vitamins A, E, and K have been incorporated into liposomes, which are used to amplify their physical and chemical stability when dispersed in water[10]. Phosphatidylcholine is the key component of liposomes which has been used in various skin care formulations like moisturizer creams and so on and hair care products like shampoo and conditioner due to its softening and conditioning properties. Due to their biodegradable, nontoxic, and biocompatible nature, liposomes are used in variety of cosmeceuticals as they encapsulate active moiety[11]. Vegetable phospholipids are widely used for topical applications in cosmetics and dermatology because of their high content of esterified essential fatty acids. For topical applications in cosmetics and dermatology vegetable phospholipids are being widely used as they have high content of esterified essential fatty acids. After the application of linoleic acid, within a short period of time the barrier function of the skin is increased and water loss is decreased. The transport of linoleic acid into the skin is done by these phospholipids [12,13]. In a clinical study, it was proven that flexible liposomes help in wrinkle reduction and show effects like decreasing of efflorescence in the acne treatment and an increase in skin smoothness [14]. Liposomes are being developed for the delivery of fragrances, botanicals, and vitamins from anhydrous formulations, such as antiperspirants, body sprays, deodorants, and lipsticks. They are also being used in antiaging creams, deep moisturizing cream, sunscreen, beauty creams, and treatment of hair loss[15]. Several positive and negative aspects of liposomes are discussed[16].
Various marketed formulations of Liposomes are given in Table 1 . [17,18,19]
Table 1: Various Marketed Formulations of Liposomes
|
|
Product Name
|
Marketed By
|
Uses
|
|
Natural Progesterone Liposomal Skin Cream
|
NOW
Solutions
|
Maintenance of healthy feminine balance
|
|
C-Vit Liposomal Serum
|
Sesderma
|
Hydration, boosts collagen synthesis, enhances skin’s elasticity and firmness, and
brightens the complexion
|
|
Decorte Moisture
Liposome Face
Cream
|
Decorte
|
Moisturizer
|
|
Decorte Moisture
Liposome Eye Cream
|
Decorte
|
Moisturizes, firms, and brightens the delicate skin around the eyes
|
2.2 Niosomes
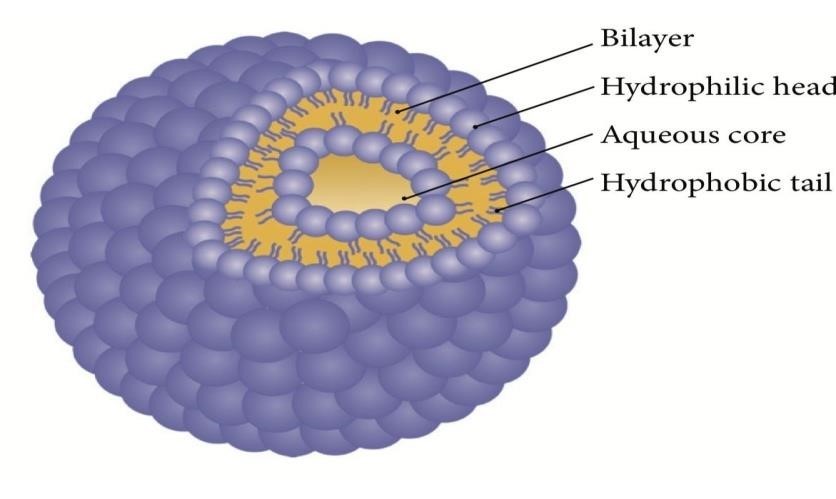
Figure 2: Structure of Niosomes
Niosomes are defined as vesicles having a bilayer structure that are made up by self-assembly of hydrated nonionic surfactants, with or without incorporation of cholesterol or their lipids[20]. Niosomes can be multilamellar or unilamellar vesicles in which an aqueous solution of solute and lipophilic components are entirely enclosed by a membrane which are formed when the surfactant macromolecules are organized as bilayer[21]. Size ranges from 100 nm to 2 m in diameter. Size of small unilamellar vesicles, multilamellar vesicles, and large unilamellar vesicles ranges from 0.025–0.05 μm, =>0.05 μm, and 0.10 μm, respectively[22]. Major niosomes components include cholesterol and nonionic surfactants like spans, tweens, brijs, alkyl amides, sorbitan ester, crown ester, polyoxyethylene alkyl ether, and steroid-linked surfactants which are used for its preparation[23] Niosomes are suitable for delivery of both hydrophobic as well as hydrophilic compounds. As a novel drug delivery system, niosomes can be used as vehicle for poorly absorbable drugs[24]. It provides encapsulation to the drug, due to which the drug in the systemic circulation is for prolonged period and penetration is enhanced into target tissue. Niosomes overcome the problems associated with liposomes, like stability problems, high price, and susceptibility to oxidation[25]. Niosomes are used in cosmetics and skin care applications since skin penetration of ingredients is enhanced because it possesses the property of reversibly reducing the barrier resistance of the horny layer, allowing the ingredient to reach the living tissues at greater rate. There is increased stability of entrapped ingredients and improvement in the bioavailability of poorly adsorbed ingredients. There are many factors affecting formation of niosomes, namely, nature and structure of surfactants, nature of encapsulated drug, membrane composition, and temperature of hydration which influences shape and size[26]. Specialized niosomes are called proniosomes; these are nonionic based surfactant vesicles, which are hydrated immediately before use to yield aqueous niosome dispersions. To enhance drug delivery in addition to conventional niosomes, proniosomes are also being used [27,28].
Various marketed formulations and uses of Niosomes are given in Table 2 . [29,30,31].
Table 2: Various Marketed Formulation and Uses of Niosomes
|
Product Name
|
Marketed By
|
Uses
|
|
Niosome+ Perfected Age Treatment
|
Lancome
|
Removes wrinkles
|
|
Niosome+
|
Lancome
|
Foundation cream, clear and white skin tone
|
|
MayuNiosome Base Cream
|
Laon Cosmetics
|
Whitening and moisturizing
|
|
Anti-Age Response Cream
|
Simply Man Match
|
Treatment of wrinkles
|
2.3 Solid Lipid Nanoparticles

Figure 3: Solid Lipid Nanoparticles
Solid lipid nanoparticles are among the nanotechnologies extensively used in both pharmaceuticals and cosmeceuticals. Commercially, many cosmeceutical companies produce cosmeceuticals using this technology to enhance beauty and therapy. 50 to 1000 nm is the size range of solid lipid nanoparticles [32] . They are composed of single layer of shells and the core is oily or lipoidal in nature. Solid lipids or mixtures of lipids are present in the matrix drug which is dispersed or dissolved in the solid core matrix. Phospholipids hydrophobic chains are embedded in the fat matrix. These are prepared from complex glycerides mixtures, purified triglycerides, and waxes; liquid lipid is replaced by solid lipid or blend of solid lipid which is solid at body and room temperature and is stabilized by surfactants or polymers[33]. Lipophilic, hydrophilic, and poorly water-soluble active ingredients can be incorporated into SLNs which consist of physiological and biocompatible lipids. With the use of biocompatible compounds for preparing SLN, toxicity problems are avoided[34]. Two principle methods for preparation of SLNs are high pressure homogenization method and precipitation method. Controlled release and sustained release of the active ingredients are possible; SLN which has drug enriched core leads to a sustained release and SLN having drug enriched shell shows burst release. Solid lipid nanoparticles have advantages in stability, higher production, and long-term storage compared to other conventional and nano delivery techniques.
Various marketed formulations and uses of Solid Lipid Nanoparticals are given in Table 3 . [35,36]
Table 3: Various Marketed Formulation and Uses of SLN
|
Product Name
|
Marketed By
|
Uses
|
|
Allure Body Cream
|
Chanel
|
Body moisturizer
|
|
Allure Parfum Bottle
|
Chanel
|
Perfume
|
|
Allure Eau Parfum Spray
|
Chanel
|
Perfume
|
|
Soosion Facial Lifting Cream SLN Technology
|
Soosion
|
Antiwrinkle cream
|
2.4 Nanostructured Lipid Carriers (NLC)

Figure 4: Nanostructured Lipid Carriers (NLC)
Nanostructured lipid carriers are considered as the second generation of the lipid nanoparticles. NLC have been developed so that the drawbacks associated with SLN can be overcome. NLC are prepared through blending by solid lipids along with spatially incompatible liquid lipid leading to amorphous solids in preferable ratio of 70 : 30 up to 99.9 : 0.1 being solid at body temperature [37,38]. NLC are mainly of three types on the basis of which the structure is developed according to the formulation composition and production parameters, namely, imperfect type, amorphous type and multiple type. The particle size ranges from 10 to 1000 nm[39] . In October 2005, the first products containing lipid nanoparticles were introduced in the cosmetic market, namely, NanoRepair Q10® cream and NanoRepair Q10® serum, Dr. Rimpler GmbH, Germany, offering increased skin penetration. Currently there are more than 30 cosmetic products available in the market containing NLC[40,41] .
List of marketed products, and uses of NLC is given in Table 4 . [42,43,44,45].
Table 4: Various Marketed Products and Uses of NLC
|
Product Name
|
Marketed By
|
Uses
|
|
Cutanova-Cream Nanorepair Q10
|
Dr. Rimpler
|
Smoothing of fine lines, promotes restructuring of skin & aging
|
|
Intensive Serum Nanorepair Q10
|
Dr. Rimpler
|
Antiwrinkle serum, fights sign of aging
|
|
Cutanova Cream Nanovital Q10
|
Dr. Rimpler
|
Antiaging treatment with UV-protection, protective,
|
|
Iope Supervital Extra Moist Softner
|
Amore
Pacific
|
Moisturizes dry and rough skin
|
2.5 Nanoemulsions

Figure 5: Structure of Nanoemulsion
Nanoemulsions are considered as the kinetically or thermodynamically stable dispersion of liquid in which an oil phase and water phase are in combination with a surfactant. Their structure can be manipulated on the basis of method of preparation, so as to give different types of products. Depending on the composition different types of nanoemulsions are oil in water, water in oil, and bicontinuousnanoemulsion. They exhibit various sizes ranging from 50 nm to 200 nm. These are dispersed phase which comprises small particles or droplets, having very low oil/water interfacial tension[46]. They have lipophilic core, which is surrounded by a monomolecular layer of phospholipids, making it more suitable for delivery of lipophilic compounds. Nanoemulsions are widely used as medium for the controlled delivery of various cosmeceuticals like deodorants, sunscreens, shampoos, lotions, nail enamels, conditioners, and hair serums[47] . In cosmetics formulation, nanoemulsions provide rapid penetration and active transport of active ingredients and hydration to the skin.
Various marketed products name and uses of Nanoemulsion are given in Table 5 . [48,49,50].
Table 5: Various Marketed Products Name and Uses of Nanoemulsion
|
Product Name
|
Marketed By
|
Uses
|
|
Korres Red Vine Hair Sun Protection
|
Korres
|
Prevents hair color from fading away
|
|
Nanocream
|
Sinerga
|
Wet wipes
|
|
Vital Nanoemulsion Α-VC
|
Marie Louise
|
Nutrition and miniaturization
|
|
Bepanthol-Protect Facial Cream Ultra
|
Bayer HealthCare
|
Moisturizing, antiaging, and antipollution
|
2.6 Gold Nanoparticles
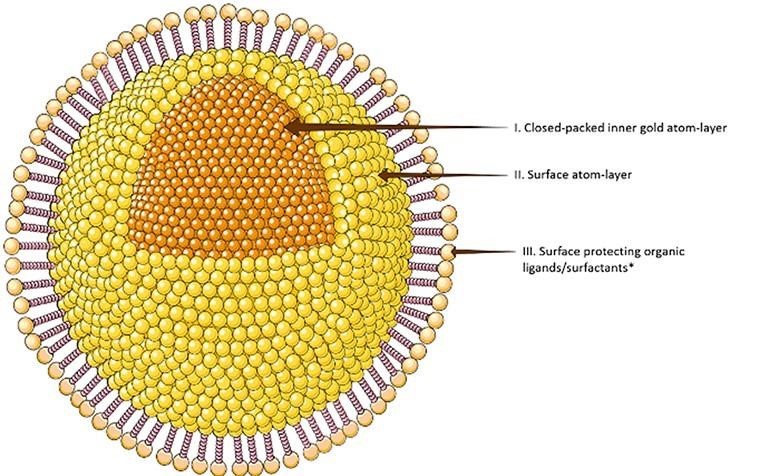
Figure 6 : Structure of Gold Nanoparticles
Nanogold or gold nanoparticles exhibit various sizes ranging from 5 nm to 400 nm. in determination of their properties[51]. They exhibit different shapes such as nanosphere, nanoshell, nanocluster, nanorod, nanostar, nanocube, branched, and nanotriangles. Shape, size, dielectric properties, and environmental conditions of gold nanoparticles strongly affect the resonance frequency. The color of nanogold ranges from red to purple, to blue and almost black due to aggregation[52]. Gold nanoparticles are inert in nature, highly stable, biocompatible, and noncytotoxic in nature. Nanogold is very stable in liquid or dried form and is nonbleaching after staining on membranes; they are also available in conjugated and unconjugated form[53]. They have high drug-loading capacity and can easily travel into the target cell due to their small size and large surface area, shape, and crystallinity[54]. Main properties of nanogold in beauty care consist of assets, namely, acceleration of blood circulation, anti-infammatory property, antiseptic properties, improvising frmness and elasticity of skin, delaying aging process, and vitalizing skin metabolism [55].
Various marketed products name and uses of Nanoemulsion are given in Table 5 .[56-60].
Table 6: Various Marketed Products Name and Uses of Nanoemulsion

2.7 Dendrimers

Figure7: Structure of Dendrimers
The term “dendrimer” arises from two Greek words: namely, “Dendron” that means tree and “Meros” meaning part. Dendrimers are highly branched, unimolecular, globular, micellar nanostructure, and multivalent nanoparticles whose synthesis theoretically affords monodisperse compounds. A dendrimer is typically built from a core on which one or a number of successive series of branches are engrafted in an arborescent way and often adopts a spherical three-dimensional morphology[61]. Generation of the dendrimer is determined by total number of series of branches: if it has one series of branches, then it is first-generation dendrimer; if it has two series, then it is second generation and so on. They are extremely small in size, having diameters in the range of 2–20 nm [62]. Its other properties like monodispersity, polyvalence, and stability make it an ideal carrier for drug delivery with precision and selectivity. To attach biologically active substances for targeting purpose terminal groups are modified. Dendrimers provide controlled release from the inner core and drugs are incorporated in interior as well as being attached on the surface[63] . Dendrimers are new class of macromolecular architecture and are also being used as nanotechnology based cosmeceuticals for various applications like in hair care, skin care, and nail care. Dendrimers have utility in various cosmetic products like shampoos, sunscreen, hair-styling gels, and antiacne products[64]. Companies like L’Oreal, The Dow Company, Wella, and Unilever have several patents for application of dendrimers in cosmeceuticals.
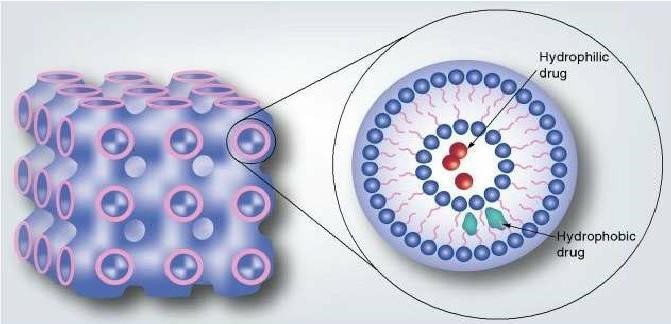
Figure 8: Structure of Cubosomes
Cubosomes are the advanced nanostructured particles which are discrete, submicron, and self-assembled liquid crystalline particles of surfactants with proper ratio of water that provides unique properties. Cubosomes are formed by self-assembled structures of aqueous lipid and surfactant systems when mixed with water and microstructure at a certain ratio[65]. Cubosomes are bicontinuous cubic liquid phase, which encloses two separate regions of water being divided by surfactant controlled bilayers and wrapped into a three dimension, periodic, and minimal surface, forming a strongly packed structure[66]. They consist of honeycombed (cavernous) structure and they appear like dots which are slightly spherical in structure. They exhibit size range from 10 to 500 nm in diameter. They have ability to encapsulate hydrophilic, hydrophobic, and amphiphilic substances. Cubosomes have relatively simple preparation methods; they render bioactive agents with controlled and targeted release, possess lipid biodegradability, and have high internal surface area with different drug-loading modalities[67,68]. Cubosomes are an attractive choice for cosmeceuticals, so for this reason a number of cosmetic giants are investigating cubosomes. Various patents have been filed regarding the cosmetic applications of cubosomes.
3.0 Advantages of nanocosmeceuticals.
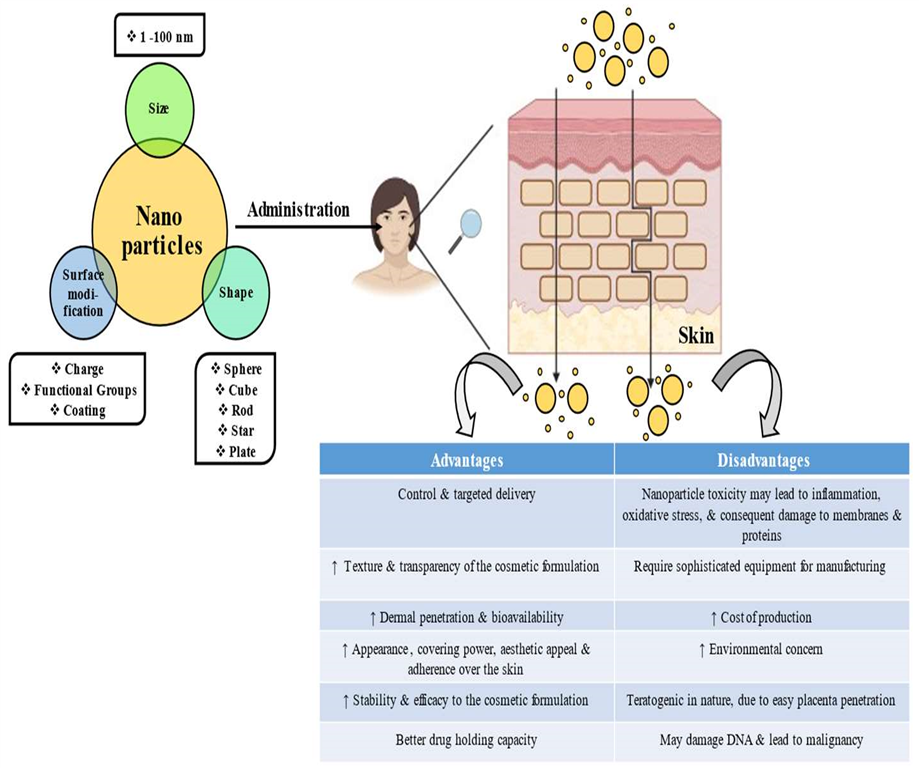
Figure no.9
4.0classes Of Nanocosmeceuticals
Cosmeceuticals are contemplated as the fastest growing segment of personal care industry. A plethora of nanocosmeceuticals are assimilated in nail, hair, lip, and skin care.
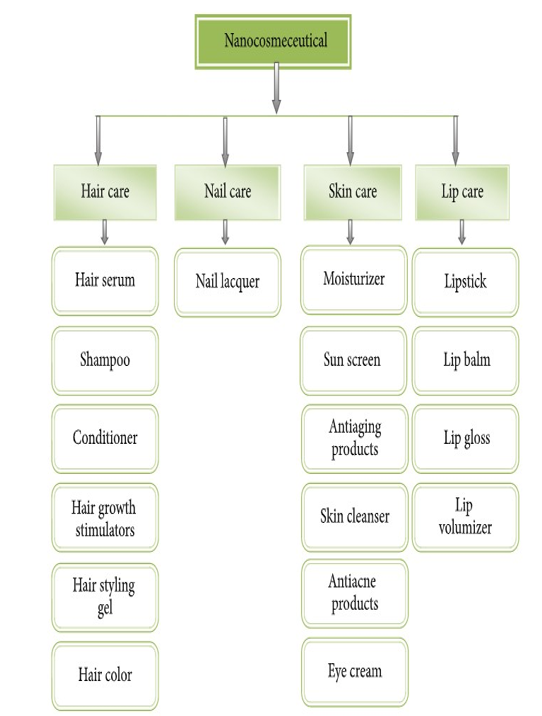
Figure 10: Classes of Nanocosmeceuticals [30].
4.1 Hair Care
Hair care is the most promising cosmeceutical sector. People of all ages are concerned about their hair. Hair loss may occur at any age. Hair loss conditions are associated with scalp inflammation and growth disorders, which vary with the individual [83-86]. Hair nanocosmeceutical products include shampoos, conditioning agents, hair growth stimulants, coloring, and styling products. Hair follicle, shaft targeting, and increased quantity of active ingredient are achieved by intrinsic properties and unique size of nanoparticles. Nanoparticles subsuming in shampoos seals moisture within the cuticles by optimizing resident contact time with scalp and hair follicles by forming protective film[69]. Conditioning nanocosmeceuticals agents have purposive function of imparting softness, shine, silkiness, and gloss and enhance disentangling of hair. Novel carriers like niosomes, microemulsion, nanoemulsion, nanospheres, and liposomes have major function of repairing damaged cuticles, restoring texture and gloss, and making hair nongreasy, shiny, and less brittle[70] .
Hair serum

Figure 11: Hair Serum
Hair serum is a styling product that coats the surface of your hair. It's made with a base of silicone, a rubber-like substance that sits on top of your strands. ... Depending on the product's formula, a hair serum might reduce frizz, add shine, or straighten your hair.
Shampoo

Figure 12: Shampoo
Shampoo is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in bar form, like a bar of soap. Shampoo is used by applying it to wet hair, massaging the product into the scalp, and then rinsing it out.
Hair conditioner

Figure 13: Hair Conditioner
Hair conditioner is a hair care product used to improve the feel, appearance and manageability of hair. Its main purpose is to reduce friction between strands of hair to allow smoother brushing or combing, which might otherwise cause damage to the scalp.
Hair Gel

Figure 15 : Hair Gel
Hair gel is a gelatinous preparation used in styling hair.
Hair growth stimulants
Scalp massage increases hair thickness by stretching the cells of hair follicles. This, in turn, stimulates the follicles to produce thicker hair. It's also thought that a scalp massage may help dilate blood vessels beneath the skin, thereby encouraging hair growth.

Figure 14: Hair growth Stimulant
Hair colour
Hair coloring, or hair dyeing, is the practice of changing the hair color. The main reasons for this are cosmetic: to cover gray or white hair, to change to a color regarded as more fashionable or desirable, or to restore the original hair color after it has been discolored by hairdressing processes or sun bleaching

Figure 16: Hair Colour
4.2 Nail Care
Nanonail care is a promising novel cosmetic and health care sector. Nanonail care particles are developed using synthetic particles and show increased protective effects against nail infections. This treatment increases nail glow and toughness in mammals[87,88]. Nanocosmeceuticals based nail care products have greater superiority over the conventional products. The nail paints based on nanotechnology have merits such as improved toughness, fast dryness, durability, chip resistance, and ease of application due to elasticity[71]. New strategies such as amalgamating silver and metal oxide nanoparticles have antifungal properties in nail paints for the treatment of toe nails due to fungal infections[72].
Nail lacquer

Figure 17: Nail Lacquer
Nail polish is a lacquer that can be applied to the human fingernail or toenails to decorate and protect the nail plates. The formula has been revised repeatedly to enhance its decorative effects and to suppress cracking or peeling.
4.3 Skin Care
Cosmeceuticals for skin care products ameliorate the skin texture and functioning by stimulating the growth of collagen by combating harmful effect of free radicals. They make the skin healthier by maintaining the structure of keratin in good condition. In sunscreen products zinc oxide and titanium dioxide nanoparticles are most effective minerals which protect the skin by penetrating into the deep layers of skin and make the product less greasy, less smelly, and transparent[73]. SLNs, nanoemulsions, liposomes, and niosomes are extensively used in moisturizing formulations as they form thin film of humectants and retain moisture for prolonged span. Marketed antiaging nanocosmeceutical products assimilating nanocapsules, liposomes, nanosomes, and nanospheres manifest benefits such as collagen renewal, skin rejuvenation, and firming and lifting the skin[74] .
Moisturizer

Figure 18: Moisturizer
Moisturizer or emollient is a cosmetic preparation used for protecting, moisturizing, and lubricating the skin[75].. These functions are normally performed by sebum produced by healthy skin. The word "emollient" is derived from the Latin verb mollire, to soften. Skin dehydration can be prevented by moisturizers, which support skin beauty and enhance skin flexibility by maintaining moisture[76]. Dehydration results in the abundant loss of water from the skin. Moisturizer creates a thin film on the outer surface of the skin maintaining skin moisture and can be effectively achieved by various nanotechnological systems[77].
Sun Screen cream

Figure 19: Sun Screen Cream
A substance that helps protect the skin from the sun's harmful rays. Sunscreens reflect, absorb, and scatter both ultraviolet A and B radiation to provide protection against both types of radiation. Commercially, sunscreens available are in the forms of creams and lotions that contain synthetic compounds, which act as a barrier when the harmful rays hit the skin. Sunscreens prevent deep UV ray penetration and inhibit irritation and other consequences. However, synthetic sunscreens have disadvantages, including formation of a chalky layer, greasiness, smelliness, decreased appeal, and toxicity[82].
Anti-aging cream

Figure 20: Anti–Aging Cream
Anti-aging creams are predominantly moisturiser-based cosmeceutical skin care products marketed with the promise of making the consumer look younger by reducing, masking or preventing signs of skin aging
Cleanser

Figure 21: Cleanser
Skin cleanser plays an active role in maintaining skin health[78,79]. The term cleanser refers to a product that cleans or removes dirt or other substances.In this case, a cleanser is a facial care product that is used to remove make-up, dead skin cells, oil, dirt, and other types of pollutants from the skin of the face. Skin cleaning directly promotes the removal of skin surface bacteria, which reduces odor production. Currently, skin cleaning products are available on the market from macro- to nanosize containing both synthetic and natural products[80,81].
Antiacne Product

Figure 22: Anti Acne Cream
Antiacne product are the medicines that help clear up the pimples, blackheads, whiteheads, and more severe forms of lesions that occur when a teen has acne. Different types of antiacne drugs are used for different treatment purposes, depending on the severity of the condition.
Eye cream

Figure 23: Eye Cream
Eye creams are formulated specifically for the delicate skin around the eye, so they tend to be thicker. They contain more oil than a regular facial lotion, and they have a lot of active ingredients aimed at the problems we see around the eyes,"n
4.4 Lip Care
Lip care products in nanocosmeceuticals comprise lipstick, lip balm, lip gloss, and lip Svolumizer. Variety of nanoparticles can be coalesced into lip gloss and lipstick to soften the lips by impeding transepidermal water loss and also prevent the pigments to migrate from the lips and maintain color for longer period of time. Lip volumizer containing liposomes increases lip volume, hydrates and outlines the lips, and fills wrinkles in the lip contour. various synthetic and metal particles are used at the nanosize for effective lip protection, enhanced color. These products last longer and have a homogenous distribution [30].The use of nanometal particles enhances the even distribution of pigments over the lips to increase protection. Since lips are most vulnerable skin-type, caution is necessary when using synthetic and metallic nanoparticles.
Lipstick

Figure 24: Lipstick
Lipstick is a cosmetic that applies color, texture, and protection to the lips. Many colors and types of lipstick exist. Some lipsticks are also lip balms, to add both color and hydration.
Lip Balm
Lip balm or lip salve is a wax-like substance applied topically to the lips to moisturize and relieve chapped or dry lips, angular cheilitis, stomatitis, or cold sores. Lip balm often contains beeswax or carnauba wax, camphor, cetyl alcohol, lanolin, paraffin, and petrolatum, among other ingredients.

Figure 25: Lip Balm
Lip gloss
Lip gloss is a cosmetic used primarily to give lips a glossy lustre, and sometimes to add a subtle color. It is distributed as a liquid or a soft solid
5.0 Toxicity of Nanocarriers Used in Cosmeceutical
1. Liposomes: Liposomes are spherical vesicles composed of lipids. They can cause toxicity due to the release of lipids, which can alter cell membrane structure and function.
2. Nanoparticles: Nanoparticles, such as metal oxide nanoparticles, can cause toxicity due to their small size, which allows them to penetrate cells and cause oxidative stress, DNA damage, and inflammation.
3. Nanoemulsions: Nanoemulsions are mixtures of two or more liquids that don't normally mix, such as oil and water. They can cause toxicity due to the release of surfactants, which can alter cell membrane structure and function
5.1 Factors Influencing Toxicity:
1. Size: The smaller size of nanocarriers can increase their toxicity due to their ability to penetrate cells and cause oxidative stress.
2. Surface Chemistry: The surface properties of nanocarriers can influence their interactions with cells and biological tissues, which can affect their toxicity.
3. Concentration: The concentration of nanocarriers can influence their toxicity, with higher concentrations potentially causing more harm.
4. Exposure Route: The route of exposure (e.g., skin, inhalation, ingestion) can affect the toxicity of nanocarriers.
5.2 Potential Toxic Effects:
1. Skin Irritation: Nanocarriers can cause skin irritation, allergic reactions, and contact dermatitis.
2. Systemic Toxicity: Nanocarriers can enter the bloodstream and distribute to various organs, potentially causing systemic toxicity.
3. Genotoxicity: Some nanocarriers have been shown to cause DNA damage and genotoxicity.
4. Environmental Toxicity: The release of nanocarriers into the environment can harm aquatic life and ecosystems.
5.4 Routes of exposure of nanoparticles:
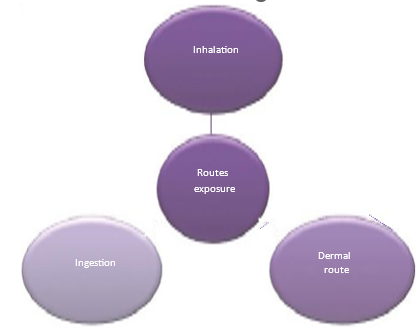
Figure 26: Routes Of Exposure of Nanoparticles
Health hazard caused by nanoparticles to the humans depends on the degree of exposure and the route through which they access the body. Inhalation, ingestion, and dermal routes are the possible routes by which humans can get exposure to the nanoparticles. Routes of exposures of nanoparticles[75]. are given in figure 26.
6.0 Ways to Minimize Toxicity of Nanocosmceuticals
Minimizing the toxicity of nanocosmeceuticals is crucial to ensure their safe use. Here are some ways to minimize toxicity:
6.1 Physical Modifications
1. Size control: Controlling the size of nanoparticles can reduce toxicity. Smaller particles may be more toxic due to their larger surface area.
2. Shape modification: Modifying the shape of nanoparticles can also reduce toxicity. For example, spherical particles may be less toxic than rod-shaped particles.
3. Surface coating: Coating nanoparticles with biocompatible materials, such as lipids or polymers, can reduce toxicity.
6.2 Chemical Modifications
1. Functionalization: Functionalizing nanoparticles with biocompatible molecules, such as PEG or amino acids, can reduce toxicity.
2. Surface charge modification: Modifying the surface charge of nanoparticles can reduce toxicity. For example, neutral or negatively charged particles may be less toxic than positively charged particles.
3. Chemical conjugation: Conjugating nanoparticles with biocompatible molecules, such as peptides or antibodies, can reduce toxicity.
6.3 Formulation Modifications
1. Encapsulation: Encapsulating nanoparticles in liposomes or other biocompatible carriers can reduce toxicity.
2. Emulsification: Emulsifying nanoparticles in oil-in-water or water-in-oil emulsions can reduce toxicity.
3. Suspension: Suspending nanoparticles in biocompatible solvents, such as water or glycerin, can reduce toxicity.
6.4 Testing and Evaluation
1. In vitro testing: Testing nanoparticles in vitro using cell cultures or other biological systems can help identify potential toxicity.
2. In vivo testing: Testing nanoparticles in vivo using animal models can help evaluate their safety and efficacy.
3. Clinical trials: Conducting clinical trials can help evaluate the safety and efficacy of nanoparticles in humans.
6.5 Regulatory Compliance
1. FDA guidelines: Following FDA guidelines for the development and testing of nanoparticles can help ensure their safety and efficacy.
2. EU regulations: Complying with EU regulations for the development and testing of nanoparticles can help ensure their safety and efficacy.
3. ISO standards: Following ISO standards for the development and testing of nanoparticles can help ensure their safety and efficacy.
6.0 CONCLUSION:
The use of nanoparticles in beauty and skincare offers numerous benefits, from improving the stability and efficacy of active ingredients to enhancing the safety and sensory experience of the products. These innovations not only improve consumer satisfaction but also open new avenues for treating complex skin issues more effectively. These advanced materials have some benefits, while their utilization in cosmetic formulation it may associated with some risk. The main aim of this dissertation work is highlight the benefits and risk of the nanomaterials used in the cosmetic products.
REFERENCES
-
-
-
- Effiong, D. Uwab, T. Jumbo, E. and Akpabio, A. (2020) Nanotechnology in Cosmetics: Basics, Current Trends and Safety Concerns – A Review. Advances in Nanoparticles, 1-22.
- Kunal Arora et al. / International Journal of Research in Pharmaceutical and Nanosciences. 7(5), 2018, 161-169
- Nidhi Kushwaha, International Journal of Pharmaceutical Science Invention ISSN (Online): 2319-6718, ISSN (Print) 2319-670x www.ijpsi.org Volume 9 Issue 1 || Jan 2020 || PP.43-51
- Vividha Dhapte- Pawar1, Shivajirao Kadam2, Shai Saptarshi1 & Prathmesh P Kenjale Future Sci. OA (2020) FS0613 ISSN 2056-5523)
- Banerjee R. Nanocosmetics. The good, the bad and the beautiful. Trichol Cosmetol Open J. 2017, 1(1)
- P. Tripura and H. Anushree, “Anushree novel delivery systems: current trend in cosmetic industry,” European Journal of Pharmaceutical and Medical Research, vol. 4, no. 8, pp. 617–627, 2017.
- N. Arora, S. Agarwal, and R. S. R. Murthy, “Latest technology advances in cosmaceuticals,” International Journal of Pharmaceutical Sciences and Drug Research, vol. 4, no. 3, pp. 168–182, 2012.
- P. Tripura and H. Anushree, “Anushree novel delivery systems: current trend in cosmetic industry,” European Journal of Pharmaceutical and Medical Research, vol. 4, no. 8, pp. 617–627,
- N. Arora, S. Agarwal, and R. S. R. Murthy, “Latest technology advances in cosmaceuticals,” International Journal of Pharmaceutical Sciences and Drug Research, vol. 4, no. 3, pp. 168–182, 2012.
- M. J. Hope and C. N. Kitson, “Liposomes: a perspective for dermatologists,” Dermatologic Clinics, vol. 11, no. 1, pp. 143–154, 1993.
- G. Bhupendra, K. Prajapati Niklesh, M. Manan, and P. P. Rakesh, “Topical Liposomes in Drug Delivery: A Review,” International journal of pavement research and technology, vol. 4, no. 1, pp. 39–44, 2012.
- A. Tasleem, N. Nuzhatun, S. A. Syed, S. Sheikh, M. Raheel, and R. S. Muzafar, “herapeutic and Diagnostic Applications of Nanotechnology in Dermatology and Cosmetics Nanomedicine & Biotherapeutic,” Discovery Journal of Nanomedicine & Biotherapeutic Discovery, vol. 5, no. 3, pp. 1–10, 2015.
- K. Egbaria and N. Weiner, “Liposomes as a topical drug delivery system,” Advanced Drug Delivery Reviews, vol. 5, no. 3, pp. 287– 300, 1990
- M. M. Rieger, Skin Lipids and Teir Importance to Cosmetic Science, vol. 102, Cosmetics Toiletries, 1987.
- G. Blume, E. Teichmuller, and E. Teichm ¨ uller ¨ , “New evidence of the penetration of actives by liposomal carrier system,” Cosmetics & Toiletries Manufacture Worldwide, pp. 135–139, 1997.
- M. Ghyczy, H.-P. Nissen, and H. Biltz, “Te treatment of acne vulgaris by phosphatidylcholine from soybeans, with a high content of linoleic acid,” Journal of Applied Cosmetology, vol. 14, no. 4, pp. 137–145, 1996.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran et al., “Liposome: classifcation, preparation, and applications,” Nanoscale Research Letters, vol. 8, article 102, 2013.
- Dermosome,https://www.ulprospector.com/en/la/PersonalCare/Detail/1832/44044/Dermosome.
- Decorte,https://www.decortecosmetics.com/skincare/liposome
- K. Karim, A. Mandal, N. Biswas et al., “Niosome: a future of targeted drug delivery systems,” Journal of Advanced Pharmaceutical Technology & Research, vol. 1, no. 4, pp. 374–380, 2010.
- S. Duarah, K. Pujari, R. D. Durai, and V. H. B. Narayanan, “Nanotechnology-based cosmeceuticals: A review,” International Journal of Applied Pharmaceutics, vol. 8, no. 1, pp. 8–12, 2016.
- A. Gandhi, “Suma oomen sen, abhijit paul. current trends in niosome as vesicular drug delivery system,” Asian Journal of Pharmacy and Life Science, vol. 2, no. 2, pp. 339–352, 2012.
- K. M. Karim et al., “A future of targeted drug delivery systems,” Journal of Advanced Pharmaceutical Technology & Research, vol. 1, no. 4, pp. 374–380, 2010.
- V. Pola Chandu, A. Arunachalam, S. Jeganath, K. Yamini, and K. Tarangini, “Niosomes: a novel drug delivery system,” International Journal of Novel Trends in Pharmaceutical Sciences, vol. 2, no. 1, pp. 2277–2782, 2012.
- G. P. Kumar and P. Rajeshwarrao, “Nonionic surfactant vesicular systems for efective drug delivery—an overview,” Acta Pharmaceutica Sinica B (APSB), vol. 1, no. 4, pp. 208–219, 2011.
- S. Biswa, P. N. Murthy, J. Sahu, and F. Amir, “Vesicles of nonionic surfactants (niosomes) and drug delivery potential,” International journal of pharmaceutical sciences and nanotechnology, vol. 1, no. 1, pp. 1–8, 2008.
- P. Sudheer and K. Kaushik, “Review on Niosomes - a Novel Approach for Drug Targeting,” Journal of Pharmaceutical Research, vol. 14, no. 1, pp. 20–25, 2015.
- P. Tripura Sundari and H. Anushree, “Novel delivery systems: current trend in cosmetic industry,” European Journal of Pharmaceutical and Medical Research, vol. 4, no
- V. Takur, S. Arora, B. Prashar, and P. Vishal, “Niosomes and liposomes-vesicular approach towards transdermal drug delivery,” International Journal of Pharmaceutical and Chemical Sciences, vol. 1, no. 3, pp. 981–993, 2012.
- A. Lohani, A. Verma, H. Joshi, N. Yadav, and N. Karki, “Nanotechnology-based cosmeceuticals,” ISRN Dermatology, vol. 2014, Article ID 843687, 14 pages, 2014
- A. Gupta, S. K. Prajapati, M. Balamurugan, M. Singh, and D. Bhatia, “Design and Development of a Proniosomal Transdermal Drug Delivery System for Captopril,” Tropical Journal of Pharmaceutical Research, vol. 6, no. 2, 2007.
- D. Puri, A. Bhandari, P. Sharma, and D. Choudhary, “Lipid nanoparticles (SLN, NLC): A novel approach for cosmetic and dermal pharmaceutical,” Journal of Global Pharma Technology, vol. 2, no. 9, pp. 1–15, 2010.
- M. S. Bangale, S. S. Mitkare, S. G. Gattani, and D. M. Sakarkar, “Recent nanotechnological aspects in cosmetics and dermatological preparations Dm2,” International Journal of Pharmacy and Pharmaceutical Sciences, vol. 4, no. 2, pp. 149–165, 2012.
- H. H. Mohamed and N. G. Omaima, Rice Bran Solid Lipid Nanoparticles: Preparation and Characterization, vol. 1, Universal Research Publications, 2 edition, 2011.
- Herbal massage cream, http://herbalmassagecream.buy.peerfix .com/pz6cc822b-facial-lifing-cream-anti-aging-face-creamsln-technology-3d-tightener.html.
- Chanel Fragrance, https://www.chanel.com/en US/fragrancebeauty/allure-138049.
- K. Dilip, T. Surendra, K. N. Suresh, and K. Roohi, “Nanostructured lipid carrier (Nlc) a modern approach for topical delivery: a review,” World Journal of Pharmacy and Pharmaceutical Sciences, vol. 2, no. 3, pp. 921–938, 2013.
- A. Saupe, S. A. Wissing, A. Lenk, C. Schmidt, and R. H. Muller, ¨ “Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) - Structural investigations on two diferent carrier systems,” Bio-Medical Materials and Engineering, vol. 15, no. 5, pp. 393–402, 2005.
- L. Shailesh, R. Patwekar Snehal, P. Ashwini, S. Manoj, and B. Arvind, “Nanostructured lipid carriers in stability improvement forcosmetic nanoparticles,” International Journal of Pharmacy & Pharmaceutical Resarch, vol. 6, no. 1, pp. 168–180, 2016.
- S. Khan, S. Baboota, J. Ali, S. Khan, R. Narang, and J. Narang, “Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs,” International Journal of Pharmaceutical Investigation, vol. 5, no. 4, p. 182, 2015.
- R. H. Muller, M. Radtke, and S. A. Wissing, “Nanostructured ¨ lipid matrices for improved microencapsulation of drugs,” International Journal of Pharmaceutics, vol. 242, no. 1-2, pp. 121– 128, 2002
- K. Sarabjot, N. Ujjwal, S. Ramandeep et al., “Nanostructure lipid carrier (NLC)the new generation of lipid nanoparticles,” Asian Pacifc Journal of Health Sciences, vol. 2, no. 2, pp. 76–93, 2015.
- C. L. Fang, S. A. Al-Suwayeh, and J. Y. Fang, “Nanostructured lipid carriers (NLCs) for drug delivery and targeting,” Recent Patents on Nanotechnology, vol. 7, no. 1, pp. 41–55, 2013.
- N. Dragicevic and H. I. Maibach, “Howard I. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement”.
- http://Drrimpler.Com/Dr-Rimpler-Professional-Treatments/Nanocare-Q10-Treatment/V=920f83e594a1.
- P. Shah, D. Bhalodia, and P. Shelat, “Nanoemulsion: A pharmaceutical review,” Systematic Reviews in Pharmacy, vol. 1, no. 1, pp. 24–32, 2010.
- S. Ozgun, “Nanoemulsions in cosmetics,” Nanomaterials and Nanotechnology.
- C. Padmadevi, F. Diana Arifn, M. Ahmad, A. Asaad, and A. Nagib, “Nanoemulsion for Cosmetic Application,” European Journal of Biomedical and Pharmaceutical Sciences, vol. 3, no. 7, pp. 8–11, 2016.
- Patel and Joshi, “An overview on nano emulsion: a novel approach,” International Journal of Pharmaceutical Sciences and Research, vol. 3, no. 12, pp. 4640–4650, 2012.
- https://www.chanel.com/enWW/fragrancebeauty/fragrance/p/women/coco-mademoiselle/cocomademoisellefresh-moisture-mistp116850.html#skuid0116850.
- K. Lata, K. J. Arvind, N. Laxmana, and S. Rajan, “Gold nanoparticles: preparation, characterization and its stability in bufer,” A Journal of Nanotechnology and Its Applications, vol. 17, no. 1, pp. 1–10, 2014.
- A. K. Khan, R. Rashid, G. Murtaza, and A. Zahra, “Gold nanoparticles: Synthesis and applications in drug delivery,” Tropical Journal of Pharmaceutical Research, vol. 13, no. 7, pp. 1169–1177, 2014.
- H. N. Verma, P. Singh, and R. M. Chavan, “Gold nanoparticle: synthesis and characterization,” Veterinary World, vol. 7, no. 2, pp. 72–77, 2014.
- Y.-C. Yeh, B. Creran, and V. M. Rotello, “Gold nanoparticles: Preparation, properties, and applications in bionanotechnology,” Nanoscale, vol. 4, no. 6, pp. 1871–1880, 2012.
- A. S. Takor, J. Jokerst, C. Zavaleta, T. F. Massoud, and S. S. Gambhir, “Gold nanoparticles: a revival in precious metal administration to patients,” Nano Letters, vol. 11, no. 10, pp. 4029–4036, 2011. [97] F. K. Alanazi, A. A. Radwan, and I. A. Alsarra, “Biopharma http://www.totalbeauty.com/reviews/product/6184661/chantecaille-nano-gold-energizing-cream.
- URLhttps://www.aliexpress.com/item/AMEIZII-NanoGold-Original-Liquid-Skin-Care-Face-Day-creams-Facial-Whitening-Moisturizing-Anti-Aging, Serum/32823859689.html.
- https://www.amazon.com/Nanogold-Silk-Skincare-Set-Cleansing/dp/B011HDPFCG.
- https://www.consumerhealthdigest.com/wrinkle-cream-reviews/nuvoderm-nano-gold.html.
- https://www.orogoldcosmetics.com/24k-nano-ultra-silk-serum .html.
- S. Fruchon and R. Poupot, “Pro-infammatory versus antiinfammatory efects of dendrimers: Te two faces of immunomodulatory nanoparticles,” Nanomaterials, vol. 7, no. 9, article no. 251, 2017.
- B. Klajnert and M. Bryszewska, “Dendrimers: Properties and applications,” Acta Biochimica Polonica, vol. 48, no. 1, pp. 199– 208, 2001.
- R. Awani K., T. Ruchi, M. Priyanka, and Y. Pooja, “Dendrimers: a potential carrier for targeted drug delivery system,” Pharmaceutical and Biological Evaluations June, vol. 3, no. 3, pp. 275– 287, 2016.
- E. A. Yapar and O. Inal, “Nanomaterials and cosmetics,” ¨ Journal of Pharmacy of Istanbul University, vol. 42, no. 1, pp. 43–70, 2012
- K. Tilekar, K. Prashant, K. Sujit, K. Sachin, and P. Ravindra, “Cubosomes- a drug delivery system,” International Journal of Pharmaceutical, Chemical and Biological Sciences, vol. 4, no. 4, pp. 812–824, 2014.
- R. B. Rohit, A. O. Riyaz, R. H. Bhargav, and P. G. Prasanna, “Cubosomes: the inimitable nanoparticulate drug carriers,” Scholars Academic Journal of Pharmacy, vol. 2, no. 6, pp. 481– 486, 2013.
- T. Madhurilatha, S. K. Paruchuri, and K. Suria Prabha, “Overview of cubosomes: a nano particle,” International Journal of Research in Pharmacy and Chemistry, vol. 1, pp. 535–541, 2011.
- R. R. Bhosale, R. A. Osmani, B. R. Harkare, and P. P. Ghodake, “Cubosomes: the inimitable nanoparticulate drug carriers,” Scholars Academic Journal of Pharmacy, vol. 2, no. 6, pp. 481– 486, 2013.
- J. Rosen, A. Landriscina, and A. Friedman, “Nanotechnology Based Cosmetics for Hair Care,” Cosmetics, vol. 2, no. 4, pp. 211– 224, 2015.
- Z. Hu, M. Liao, Y. Chen et al., “A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone,” International Journal of Nanomedicine, vol. 7, pp. 5719–5724, 2012.
- H. Bethany, “Zapping nanoparticles into nail polish,” Laser Ablation Method Makes Cosmetic and Biomedical Coatings in a Flash, vol. 95, no. 12, p. 9, 2017.
- L. Pereira, N. Dias, J. Carvalho, S. Fernandes, C. Santos, and N. Lima, “Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum,” Journal of Applied Microbiology, vol. 117, no. 6, pp. 1601–1613, 2014.
- T. G. Smijs and S. Pavel, “Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and efectiveness,” Nanotechnology, Science and Applications, vol. 4, no. 1, pp. 95–112, 2011.
- D. A. Glaser, “Anti-aging products and cosmeceuticals,” Facial Plastic Surgery Clinics of North America, vol. 12, no. 3, pp. 363– 372, 2004.
- Shim JH, Park JH, Lee JH, Lee DY, Lee JH, Yang JM. Moisturizers are effective in the treatment of xerosis irrespectively from their particular formulation: results from a prospective, randomized, doubleblind controlled trial. J Eur Acad Dermatol Venereol. 2016;30(2): 276–281.
- Berardesca E, Barbareschi M, Veraldi S, Pimpinelli N. Evaluation of efficacy of a skin lipid mixture in patients with irritant contact dermatitis, allergic contact dermatitis or atopic dermatitis: a multicenter study. Contact Dermatitis. 2001;45(5):280–285.
- Ribeiro RC, Barreto SM, Ostrosky EA, da Rocha-Filho PA, Veríssimo LM, Ferrari M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) mill extract as moisturizing agent. Molecules. 2015;20(2):2492–2509
- Lee BM, An S, Kim SY, et al. Topical application of a cleanser containing extracts of folium, and var. reduces skin oil content and pore size in human skin. Biomed Rep. 2015;3(3):343–346.
- Werschler WP, Trookman NS, Rizer RL, Ho ET, Mehta R. Enhanced efficacy of a facial hydrating serum in subjects with normal or self perceived dry skin. J Clin Aesthet Dermatol. 2011;4(2):51–55.
- Coret CD, Suero MB, Tierney NK. Tolerance of natural baby skin-care products on healthy, full-term infants and toddlers. Clin Cosmet Investig Dermatol. 2014;7:51–58.
- Mitani K, Takano F, Kawabata T, et al. Suppression of melanin synthesis by the phenolic constituents of sappanwood (Caesalpinia sappan). Planta Med. 2013;79(1):37–44.
- Pollack AZ, Buck Louis GM, Chen Z, et al. Bisphenol A, benzophenonetype ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res. 2015;137:101–107
- Goodier M, Hordinsky M. Normal and aging hair biology and structure ‘aging and hair’. Curr Probl Dermatol. 2015;47:1–9.
- Perera E, Yip L, Sinclair R. Alopecia areata. Curr Probl Dermatol. 2015; 47:67–75.
- Tobin DJ. Age-related hair pigment loss. Curr Probl Dermatol. 2015; 47:128–138.
- Trüeb RM. Effect of ultraviolet radiation, smoking and nutrition on hair. Curr Probl Dermatol. 2015;47:107–120.
- Golubovic-Liakopoulos N, Simon SR, Shah B. Nanotechnology use with cosmeceuticals. Semin Cutan Med Surg. 2011;30(3):176–180.
- Meghea A. Pharmaceuticals and cosmeceuticals based on soft nanotechnology techniques with antioxidative, immunostimulative and other therapeutic activities. Recent Pat Nanotechnol. 2008;2(2):137–145.


 Sharda Gaikwad *
Sharda Gaikwad *
 Pranali Karande
Pranali Karande
 Rani Chavan
Rani Chavan
 Dr. Sadhana Shahi
Dr. Sadhana Shahi



























 10.5281/zenodo.15221709
10.5281/zenodo.15221709