Abstract
Anaphylaxis is an acute, life-threatening, multisystem condition caused by the abrupt release of mediators by mast cells and basophils. Epinephrine Autoinjectors (EAIs) are used for treating anaphylaxis. Low prescription fulfillment rates, non-carrying behavior and needle-phobia prevent them from being as useful as they could be. Growing interest has been seen in creating simple-to-use, needle-free delivery systems. Recently, a new therapeutic option for anaphylaxis has emerged: Neffy, an epinephrine nasal spray. The FDA has approved neffy, a needle-free, easy-to-carry epinephrine delivery method for severe allergies. This has the potential to reduce time of administration, leading to better clinical outcomes and improved quality of life for patients. An overview of Neffy, including its development approach and review of available clinical data is given in this review.
Keywords
Anaphylaxis, Epinephrine, Intravail, Unit Dose Spray, Severe allergic reaction, Nasal spray.
Introduction
Anaphylaxis is a severe allergic reaction that can cause breathing and circulation complications, potentially leading to death[1]. Anaphylaxis is characterised by symptoms such as erythema, pruritus, urticaria, angioedema, bronchospasm, laryngeal oedema, hypotension, cardiac arrhythmias, a sense of impending doom, unconsciousness and shock. Anaphylaxis can be triggered by an immunological response through either an IgE -mediated or immune complex-dependent pathway. Non-immunologic anaphylaxis involves direct activation of mast cells and basophils[2,3]. Basophil activation, the synthesis of vasoactive mediators, mast cell stimulation, and the initiation of further inflammatory cascades are all components of the anaphylactic pathway[4]. The mediators induce urticaria, vasodilatation, an increase in vascular permeability and vascular leakage, oedema, and bronchoconstriction. The mediators include histamine, leukotrienes, and prostaglandins.
Intranasal (IN) administration is becoming more and more popular for delivering a range of treatments, especially for use outside of hospitals. Aside from being significantly less intrusive, IN administration has several benefits over other delivery methods, including as simplicity of usage, quick absorption, and avoidance of the discomfort usually connected with intravenous (IV) or intramuscular (IM) injection. A number of medications, including midazolam, diazepam, fentanyl, naloxone, ketamine, and dexmedetomidine, among others, are routinely administered intranasally for a variety of indications. Needle-free delivery options are especially advantageous for children, who are more likely to be “needle-phobic”[5,6].
Etiology of Anaphylaxis
Foods, medicines, and insect bites are examples of common initiating sources. Injections used in immunotherapy to enhance the overall allergic response may cause a hyperacute reaction. Anaphylaxis is a risk that comes with any hypersensitivity, and latex hypersensitivity is becoming more common. Idiopathic anaphylaxis occurs when the causative cause is occasionally unknown[7,8,9]. The most frequent causes are foods (peanuts, tree nuts, fish, shellfish, milk, eggs, wheat, rye, barley, soy, red meat, and sesame), fire ant bites and bee stings. Anaphylaxis caused by alpha-gal: This is related to the mammalian galactose alpha-1,3-galactose-induced IgE antibody response.
Symptoms of anaphylaxis may include:
- Breathing difficulties
- Wheezing
- Hoarseness (changes in the way your voice Sounds)
- Hives (raised reddened rash that may itch)
- Severe itching
- Swelling of your face, lips, mouth, or tongue
- Skin rash, redness, or swelling
- Fast heartbeat
- Weak pulse
- Feeling very anxious
- Confusion
- Stomach pain
- Losing control of urine or bowel movements (incontinence)
- Diarrhoea or stomach cramps
- Dizziness, fainting, or “passing out” (unconsciousness)
Several dietary products, latex exposure, insect venom, and pharmaceuticals are known to cause anaphylaxis. Anaphylaxis is more frequently reported in people with a history of severe allergic reactions, pre-existing respiratory or cardiovascular disease, uncontrolled severe asthma, delayed adrenaline administration, history of biphasic anaphylactic reactions, mast cell disease, and advanced age.
Pathophysiology of Anaphylaxis
When basophils and mast cells degranulate in response to re-exposure to a particular antigen, a variety of chemical mediators are released, resulting in anaphylaxis, which is usually an IgE-mediated (type 1) hypersensitivity reaction. The aggregation of high-affinity receptors caused by IgE crosslinking causes the quick release of chemical mediators that had been accumulated. Proteoglycans, histamine, tryptase, and carboxypeptidase A are some examples of these chemical mediators. Phospholipase A, cyclooxygenases, and lipoxygenases are then activated to produce arachidonic acid metabolites, such as prostaglandins, leukotrienes, and factors that activate platelets[10,11]. Then, as a preformed, late-phase reactant, tumour necrosis factor (TNF-alpha) mediates the inflammatory response.
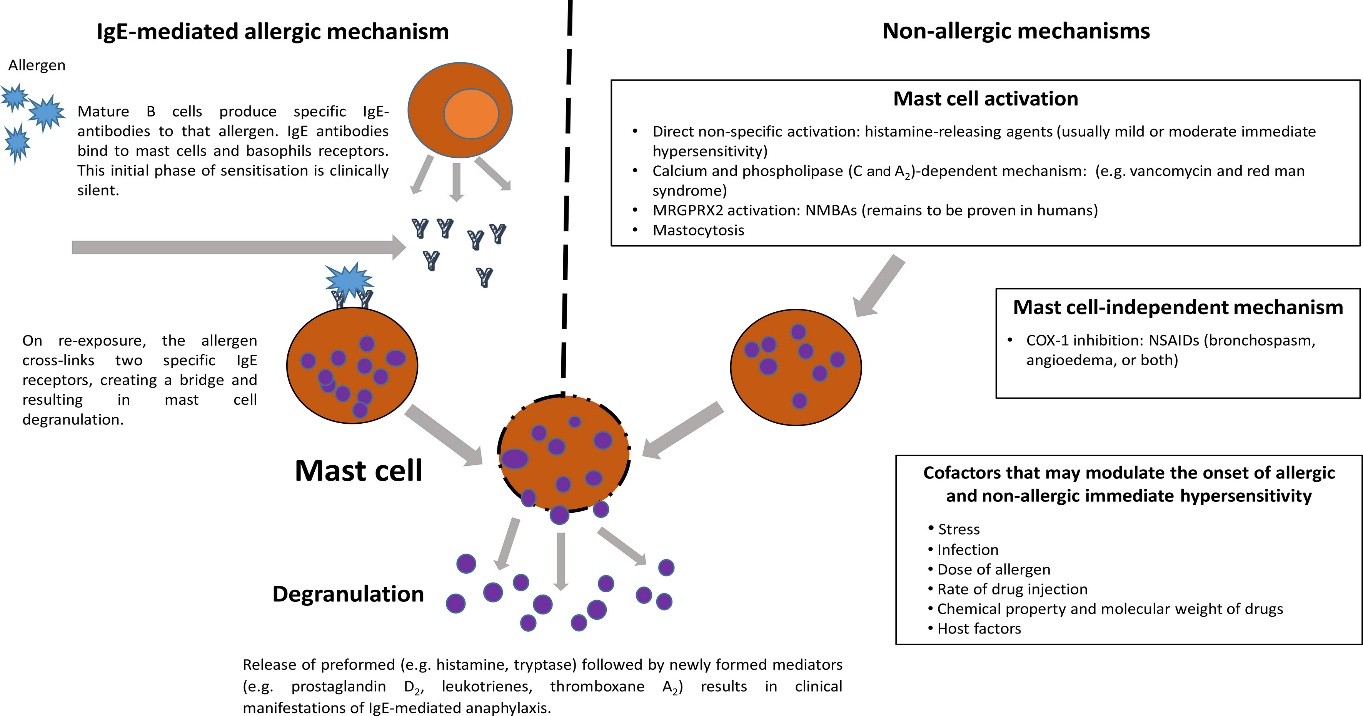
Fig. 1: Pathophysiology of Anaphylaxis
These chemical mediators have the following specific physiology:
• Tissue hypoperfusion results from histamine's increased vasodilation and vascular permeability. The body increases cardiac contraction and heart rate in response to these alterations.
• As a bronchoconstrictor, prostaglandin D constricts the pulmonary and heart arteries at the same time. Moreover, it increases peripheral vasodilation, which exacerbates the hypoperfusion of critical organs.
• Leukotrienes cause airway remodelling and increase vascular permeability and bronchoconstriction.
• In addition to increasing vascular permeability, platelet activation factor also has bronchoconstrictor properties.
• TNF-alpha promotes the synthesis of chemokines and activates neutrophils as a component of the stress response leukocytosis.
FDA Approved EAI Products
Table 1: History of FDA approved community use products
|
Device (Approved)
|
Basis of Approval
|
Pharmacokinetics (literature and other data)
|
|
EpiPen (1987)
|
No clinical or PK data
|
Significant differences (EpiPen vs. IM) only known in past 10 years
Significant blood vessel injection risk (IV bolus)only known in past 5 years
|
|
Twinject (2003)
|
No clinical or PK data
|
No PK data is currently available
|
|
Adrenaclick (2003)
|
No clinical or PK data
|
No PK data is currently available
|
|
Auvi-Q (2012)
|
Single PK study vs. EpiPen
|
Slower PK compared to EpiPen, but faster PK compared to IM
|
|
Symjepi (2017)
|
No clinical or PK data
|
Slower PK in comparison to other autoinjectors or Neffy
|
|
Teva Generic EpiPen (2018)
|
No clinical or PK data
|
No PK data is currently available
|
The majority of severe Type 1 allergic reactions take place outside of hospitals and the only first-line treatment that is generally advised is the prompt administration of epinephrine[12,13,14]. When treating severe allergic responses and anaphylaxis, epinephrine is administered by a variety of ways, including intramuscular (IM), subcutaneous (SC), intravenous (IV) infusion, and IV bolus. Many IM and subcutaneous epinephrine injection, EAI products have received FDA approval, such as SymjepiTM (Adamis Pharmaceuticals Corporation, San Diego, CA, USA), Adrenaclick® (Lineage Therapeutics Inc., Horsham, PA, USA), Auvi-Q® (Kaleo, Inc., Richmond, VA, USA), and EpiPen® (Mylan Speciality L.P., Morgantown, WV, USA), as well as several generic EAIs[15]. There were neither clinical trials nor pharmacokinetic studies carried out to support the approval of these products, with the exception of one pharmacokinetic study for Auvi-Q[16]. Rather, their endorsement was predicated on the notion that there were no notable.
NEFFY PRODUCT DESCRIPTION
Neffy (Epinephrine Nasal Spray) is made up of three FDA approved components:
? Epinephrine, the active ingredient
? Intravail (Dodecylmaltoside[DDM]), a proprietary absorption enhancer
? Unit Dose Spray (UDS)[17]
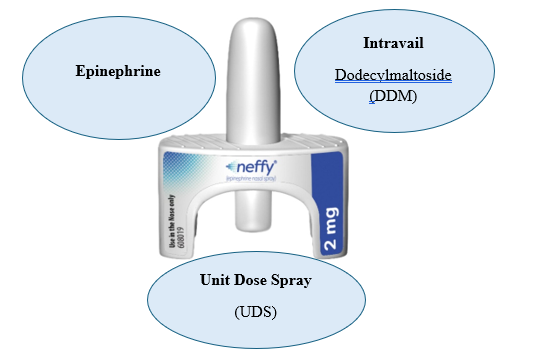
Fig. 2: Neffy Triad
Epinephrine
Epinephrine, a sympathomimetic catecholamine is present in Neffy.
Structure of Epinephrine:

Fig. 3: Structure of Epinephrine
Chemical Formula: C9H13NO3
IUPAC Name: 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol
Molecular Weight: 183.20 g/mol [18]
Since more than a century ago, epinephrine has been used to treat allergic reactions. It is the only first-line treatment for Type 1 allergic reactions. The use of epinephrine for the treatment of anaphylaxis was first reported in 1960s. Based on clinical guidelines, vast experience, case reports and few clinical trials, it is widely accepted that the most effective treatment for anaphylaxis is epinephrine[19,20,21].
Epinephrine, often known as adrenaline is a hormone and medicine that regulates the body’s acute stress response mechanism. It’s quick and strong physiological effects make it crucial in emergency medical care, where prompt action can make the difference between life and death. Epinephrine is used in a variety of medical circumstances, including treating allergies and resuscitation during cardiac arrest[22].
Mechanism of action (MOA) of Epinephrine:
Alpha and beta-adrenergic receptors are activated by epinephrine. Epinephrine is the most potent alpha receptor activator, acting on both beta and alpha receptors. Anaphylaxis causes vasodilation and increased vascular permeability, which can lead to hypotension and intravascular fluid loss. Epinephrine counteracts these effects by acting on alpha-adrenergic receptors. As a histamine antagonist and relaxant of the smooth muscle of the eye and bronchi, epinephrine is helpful in treating allergic response symptoms and related illnesses. Additionally, this medication causes a rise in blood sugar and liver glycogenolysis. Epinephrine relieves bronchospasm, wheezing, and dyspnoea that may happen during anaphylaxis by relaxing bronchial smooth muscle through its impact on beta-adrenergic receptors[23].
Adrenergic receptors decrease vasodilation and increase vascular permeability, whereas anaphylaxis causes a loss of intravascular fluid volume and hypotension. ?-adrenergic receptors serve to relieve dyspnoea, wheezing, and bronchospasm that might happen during anaphylaxis by relaxing the bronchial smooth muscle. ?-adrenergic receptors cause an increase in heart rate and contractility in order to maintain blood pressure (BP). Epinephrine ameliorates symptoms like pruritus, urticaria, and angioedema and may reduce gastrointestinal and genitourinary symptoms linked to anaphylaxis. It does this by producing relaxation effects on the smooth muscle of the stomach, colon, uterus, and bladder.
The mechanism of action (MOA) of epinephrine in treating type 1 allergic reactions, such as anaphylaxis is widely known. It involves the direct systemic agonism of ? and ?-adrenergic receptors to halt the allergic reactions, which is opposite of the pathological response brought on by exposure to an antigen and a stabilized mast cell[24].
Table 2: Pharmacological effects of adrenaline/epinephrine in the treatment of anaphylaxis
|
Adrenergic Receptor
|
Pharmacological Effect
|
|
?1 adrenergic receptor
|
• Increased vasoconstriction
• Increased peripheral vascular resistance
• Raise blood pressure
• Reduction of tissue edema
• Nasal vasoconstriction
|
|
?2 adrenergic receptor
|
• Lowering intraocular pressure
|
|
?1 adrenergic receptor
|
• Increased heart rate
• Increased cardiac contraction
• Vasoconstriction in skin and mucosa
|
|
?2 adrenergic receptor
|
• Bronchodilation
• Vasodilatation
• Inhibition of mediator release
• Lowering peripheral blood pressure
|
|
?3 adrenergic receptor
|
• Promotion of lipolysis
|
Epinephrine acts as a non-selective agonist on G-protein coupled ?- and ?-adrenergic receptors. Epinephrine inhibits the release of allergy mediators within minutes by directly counteracting immunological mediators and stabilizing mast cells[25]. Epinephrine’s major therapeutic function is to activate adenylyl cyclase and boost intracellular cyclic AMP production by directly activating ?2-adrenergic receptors.
Intravail (Dodecylmaltoside)
Intravail is a non-toxic, non-irritating agent that enhances drug absorption through nasal and other mucosal surfaces. It’s unique properties make it an attractive solution for improving bioavailability and reducing dosing requirements. Intravail, which is also known as DDM(Dodecylmaltoside) is used to improve the absorption of medications like epinephrine and is classified as Generally Recognized as Safe (GRAS) for food applications[26]. Intravail is an alkylsaccharide that changes mucosal viscosity and membrane fluidity to release cell-cell connections. It causes quick and reversible decreases in transepithelial/transendothelial electrical resistance values, resulting in modifications to tight junctions that aid absorption[27]. Alkylsaccharide absorption enhancers are soluble in water and oil and do not irritate or damage the mucosal barrier.
When an absorption enhancer such as Intravail is used, the amount of epinephrine can be lowered without compromising efficacy, and this is possible in comparison to other IN formulations that lack an equivalent absorption enhancer[28]. A high dose of epinephrine in the presence of increased permeability in the nasal mucosa, such as from an allergy response or population fluctuations, may result in excessive absorption and raise the risk of overdose[29]. Intravail’s addition to the Neffy formulation maximises effectiveness while lowering the possibility of overdosage through dose capping.
Phase 1 research indicates that adding 0.275?M to neffy resulted in an optimum bioavailability. There have been no documented safety concerns with the inclusion of DDM in the formulations of FDA-approved medications.
Unit Dose Spray (UDS)
The UDS device used for neffy is well known and proven and has a 20-year history of use with no recalls. It is a single dose device that does not require any priming or other activation. It is a simple to use mechanism that is highly reliable, with less than 1 in 100,000 chances of a failure and delivers an effective dose within specifications based on reliability testing for neffy and other products using the UDS device. In addition to the real-world experience with this device ARS has conducted multiple reliability studies with the neffy 1 mg and neffy 2 mg products[30].
Prescribing Information for neffy
Indication and Usage
Neffy is recommended for the emergency management of type I allergic responses in adults and paediatric patients weighing 30 kg or more, including anaphylaxis.
Dosage form and strength
Nasal Spray: 2mg/0.1ml of epinephrine per spray in a single dose nasal spray.
How neffy works?
A nasal spray is used to administer epinephrine to your body. The nasal spray delivers the same drug as auto-injectors, but it goes straight into the nostril rather than through a needle. An allergic reaction that is severe or potentially fatal will be stopped or slowed down by the drug. Many allergic reactions to food or insect venom carry epinephrine with them at all times. This required carrying a needle-equipped epinephrine auto-injector for many years. There's a needle-free option now available with the nasal spray.
Usually, it starts to work after five minutes. Neffy must be treated right away in order to prevent a serious allergic reaction. It can help stop potentially fatal anaphylaxis from getting worse.
Administration Instructions
- Neffy should only be used in the nose. Avoid getting any spray in your mouth or eyes.
- Each neffy contains one dose of medication and is not reusable. The gadget should not be primed or tested (pre-spray).
- Two gadgets are included in the carton that contains your Neffy. If the symptoms worsen or persist, you might need to apply a second Neffy.
- Two Neffy devices should be carried at all times.
- One dose of Neffy is administered via each nostril. If more Neffy is required, it should be administered in the same nostril and five minutes after the initial dose.
- Avoid sniffing during or after taking neffy.
Storage and Handling
Keep in storage at 20 degree Celsius to 25 degree Celsius (68°F to 77°F). Ventures were permitted to reach 122°F (50°C). Do not freeze. Neffy freezes below 5°F, or -15°C. If Neffy freezes, it won't release adrenaline.
NEFFY DEVELOPMENT APPROACH
Currently, no randomised controlled trials have been carried out to evaluate the effectiveness of epinephrine products in treating severe Type I allergic reactions, including anaphylaxis, due to ethical and practical constraints[31,32]. There are numerous explanations for the scarcity of such investigations. First, consider the unexpected clinical course. Predicting the progression, severity, and possibility of a deadly allergic reaction is challenging[33,34,35]. The clinical course’s unpredictability may put patients at danger of life-threatening, potentially deadly diseases[35,36].
Acute epinephrine administration’s pharmacokinetics have not been thoroughly studied, with prior research primarily relying on IV infusion, in addition to the dearth of efficacy trials. A pharmacokinetic trial using Auvi-Q and EpiPen was the only exception[14]. According to recent pharmacokinetic studies, which were carried out for the development of Neffy by ARS and the EMA in 2015, there are notable variations in the pharmacokinetic profiles of approved injection products[37,38,39].
Thus, over a period of eight years, ARS collaborated closely with the FDA and the EMA to establish a development route for the assessment of neffy’s safety and effectiveness. In order to assess the pharmacokinetic and pharmacodynamic response of neffy in controlled environments under a range of possible real-life conditions, such as self-administration, allergic rhinitis, infectious rhinitis, and severe hypotension in an animal model, this pathway has included numerous clinical trials.
REVIEW OF NEFFY’S CLINICAL DATA
As part of Neffy’s development program, published data from many clinical trials are included in this review. Before beginning the study, each subject provided written, informed permission, and all study methods were approved by the appropriate Institutional Review Boards or Ethics Committees. Guidelines for Good Clinical Practice from the International Conference on Harmonisation were followed in conducting the trials.
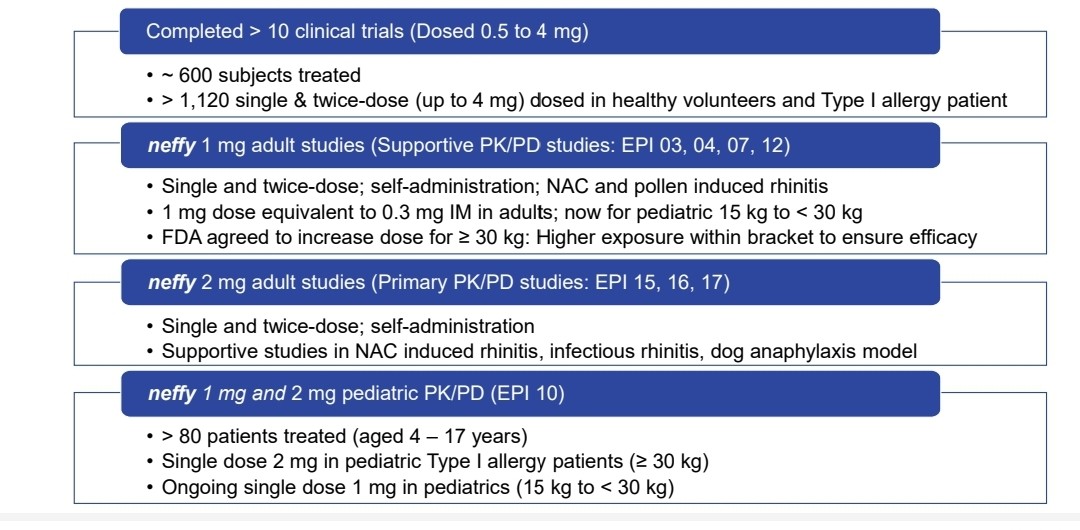
Fig. 4: Neffy Clinical Development Program
Prior to the dosage followed by 2, 4, 6, 8, 10, 12.5, 15, 20, 30, 45, 60, 90, 120, 150, 180, and 240 (360 or 480 minutes, depending on the study) minutes after the dose, blood samples were taken for pharmacokinetic analysis. Plasma epinephrine concentrations were measured using a validated liquid chromatography-mass spectrometry method. An automated system was used to measure pharmacodynamic characteristics such as systolic and diastolic blood pressure, as well as pulse rate (PR). Blood pressure and pulse rate were recorded at baseline, before dosage, and at 1, 5, 10, 15, 20, 25, 30, 45, 60, 90, and 120 min post-dosing (depending on study).
Neffy 2 mg Human Studies
Crossover Study: Neffy 2.0 mg vs EpiPen and Manual Intramuscular Injection-Dosing Once and Twice
The pharmacokinetics and pharmacodynamics were evaluated in a Phase 1 crossover study with healthy participants of neffy 2.0 mg versus 0.3 mg of EpiPen and 0.3 mg of manual intramuscular epinephrine (IM) with a needle and syringe. The study aimed to show that neffy's pharmacokinetic and pharmacodynamic profiles were comparable to licensed epinephrine injectable products. A study included 59 individuals aged 21-54 years old who received a single dose of neffy, EpiPen, and 0.3 mg of epinephrine IM, followed by a repeat dose of neffy and EpiPen[40].
The results of this study showed that the pharmacokinetic profile of neffy 2 mg is within the permissible range for epinephrine injection products, and the pharmacodynamic profile is either the same as or superior to injectable products.
Pharmacokinetics
Following an EpiPen administration, mean epinephrine concentrations peaked, and this effect lasted for around 20 minutes after delivery. The mean epinephrine concentrations following neffy were higher from 30 to 360 minutes post-dosage compared to epinephrine IM and EpiPen. The neffy treatments (R/R and L/R) showed higher mean epinephrine concentrations after many doses in comparison to the EpiPen.
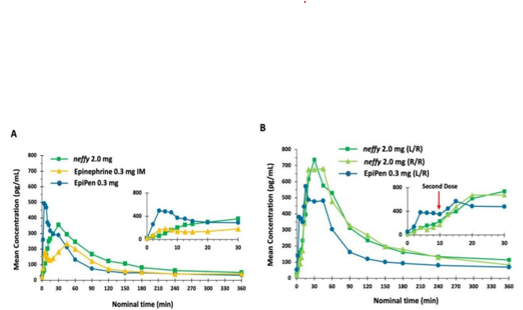
Fig. 5: Average epinephrine concentration time. The study was conducted on healthy individuals. N = 42 for Neffy 2.0 mg, Epinephrine 0.3 mg IM, and EpiPen 0.3 mg. The sample size was 39 for both neffy 2.0 mg (L/R) and neffy 2.0 mg (R/R). N = 42 for the EpiPen 0.3 mg (L/R). (A) Single dosage. (B) Repeat the dose, administering the second dose 10 minutes later.
Pharmacodynamics
neffy's pharmacodynamic effect on SBP began one minute after treatment and lasted 120 minutes. EpiPen was related with a less significant and more sudden increase in SBP compared to neffy, while epinephrine IM resulted with a minimal change. All therapies returned SBP to baseline within 120 minutes of administration. All therapies increased DBP from baseline and then decreased it. EpiPen and Epinephrine IM resulted in a more noticeable decline compared to neffy, according as the integrated analysis in Section 4.1. The PR increased from the baseline for every treatment. For both the EpiPen and epinephrine IM, the initial spike was followed by a decline, but the elevation lasted for 120 minutes after being administered.
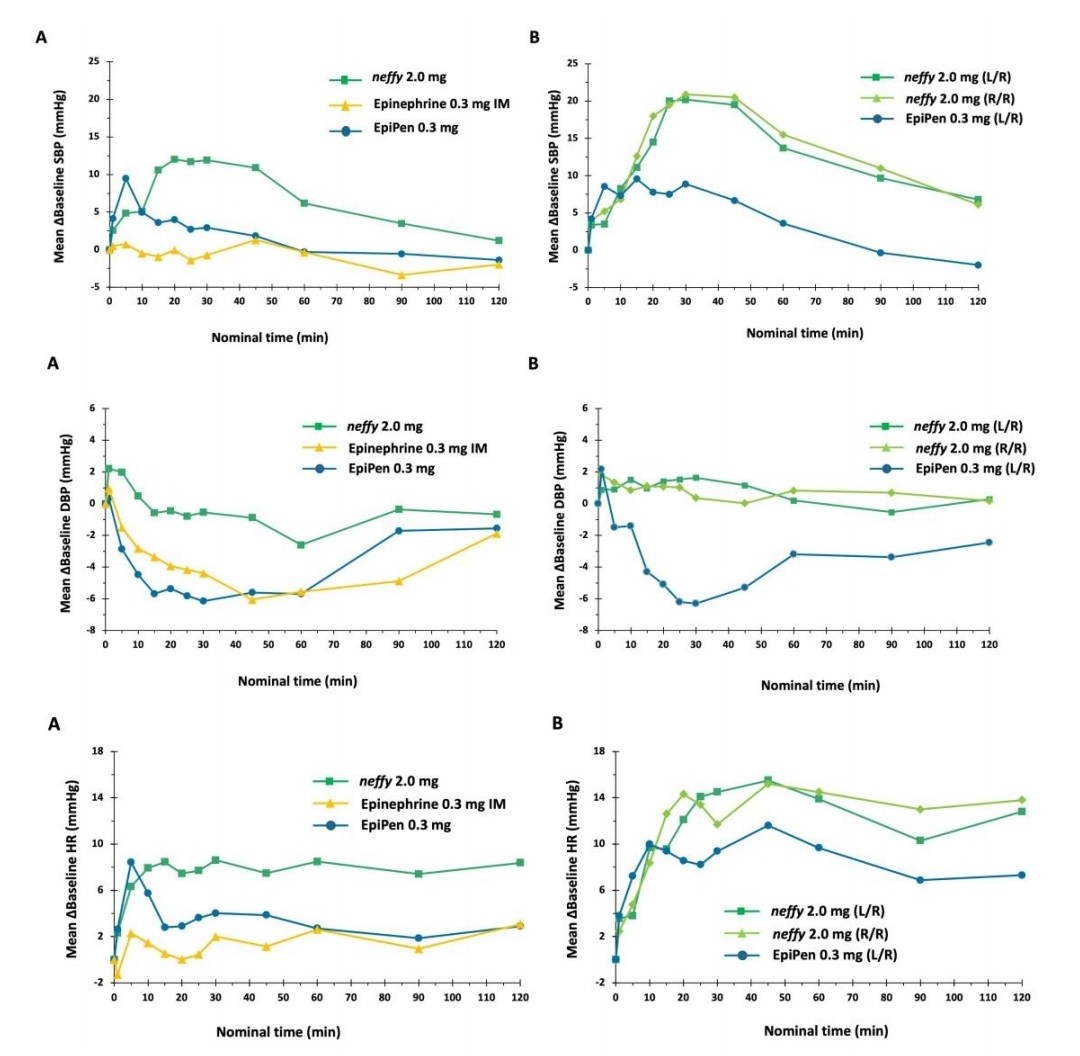
Fig. 6: Pharmacodynamic measurements vs. time after neffy, EpiPen, and epinephrine 0.3 mg IM: (A)Single doses. (B) Repeat doses.
CONCLUSION
For the treatment of anaphylaxis, the bulkiness of carrying an EAI, needle fear, and patient’s personal safety concerns are the main causes of the limits of the already approved EAIs, which highlight the need for alternative treatment choices. Intranasal (IN) administration of epinephrine is the best option for the treatment of anaphylaxis with needle-free delivery that rapidly absorbed the drug.
Neffy (Epinephrine Nasal Spray) is the needle-free alternative for EAIs which is used for emergency treatment of anaphylaxis. Over several studies, Neffy has shown a pharmacokinetic profile that falls within the current range of approved injection products, along with pharmacodynamic responses that are on equal with or better than injection. Notably, a response was observed as early as one minute after treatment, confirming the activation of ?- and ?-adrenergic receptors, which is the basis for epinephrine’s MOA for treating allergic reactions. Neffy’s goal is to decrease the unwillingness of patients to administer epinephrine to themselves, which will boost the usage of the medication earlier and lower the chance that severe anaphylaxis would develop.
REFERENCES
- Paul J. Turner, Margitta Worm, Ignacio J. Ansotegui, et al, WAO Anaphylaxis Committee. Time to visit definition and clinical criteria for anaphylaxis? WAO Journal 2019;12, 100066.
- Bilal Q. Khan, Stephen F. Kemp. Pathophysiology of anaphylaxis. Current Opinion in Allergy Clinical Immunology 2011;11:319–325.
- Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. Journal of Allergy and Clinical Immunology 2017;140:335–348.
- Blanca-López N, del Carmen Plaza-Serón M, Cornejo-García JA, et al. Drug-induced anaphylaxis. Current Treatment Options in Allergy. 2015;2:169–182.
- Abby M. Bailey; Regan A. Baum; Karolyn Horn; Tameka Lewis; Kate Morizio; Amy Schultz; Kyle Weant; Stephanie N. Justice. Review of intranasally administered medications for use in the emergency department. Journal of Emergency Medicine 2017, 53, 38–48.
- Misra, S.N.; Sperling, M.R.; Rao, V.R.; Peters, J.M.; Penovich, P.; Wheless, J.; Hogan, R.E.; Davis, C.S.; Carrazana, E.; Rabinowicz, A.L. Analyses of patients who self-administered diazepam nasal spray for acute treatment of seizure clusters. Epilepsy and Behavior Reports 2024, 25, 100644.
- Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol. 2018 Nov;121(5):594-597.
- Mota I, Gaspar Â, Benito-Garcia F, Correia M, Chambel M, Morais-Almeida M. Drug-induced anaphylaxis: seven-year single-center survey. Eur Ann Allergy Clin Immunol. 2018 Sep;50(5):211-216.
- Yue D, Ciccolini A, Avilla E, Waserman S. Food allergy and anaphylaxis. J Asthma Allergy. 2018;11:111-120.
- Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. 2018;11:121-142.
- Valenta R, Karaulov A, Niederberger V, Gattinger P, van Hage M, Flicker S, Linhart B, Campana R, Focke-Tejkl M, Curin M, Eckl-Dorna J, Lupinek C, Resch-Marat Y, Vrtala S, Mittermann I, Garib V, Khaitov M, Valent P, Pickl WF. Molecular Aspects of Allergens and Allergy. Adv Immunol. 2018;138:195-256.
- Shaker, M.S.; Wallace, D.V.; Golden, D.B.K.; Oppenheimer, J.; Bernstein, J.A.; Campbell, R.L.; Dinakar, C.; Ellis, A.; Greenhawt, M.; Khan, D.A.; et al. Anaphylaxis-a 2020 practice parameter, and grading of recommendations, assessment, development and evaluation (GRADE) analysis. J. Allergy Clin. Immunol. 2020, 145, 1082–1123.
- Panel, N.S. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 12, S1–S58.
- Golden, D.B.K.; Wang, J.; Waserman, S.; Akin, C.; Campbell, R.L.; Ellis, A.K.; Greenhawt, M.; Lang, D.M.; Ledford, D.K.; Lieberman, J.; et al. Anaphylaxis: A 2023 practice parameter update. Ann. Allergy Asthma Immunol. 2024, 132, 124–176.
- Abramowicz, M.; Zuccotti, G.; Pflomm, J.M. An epinephrine prefilled syringe (Symjepi) for anaphylaxis (reprinted from Medical Letter on Drugs and Therapeutics, volume 61, page 25-26, 2019). JAMA 2019, 321, 1306–1307.
- Edwards, E.S.; Gunn, R.; Simons, E.R.; Carr, K.; Chinchilli, V.M.; Painter, G.; Goldwater, R. Bioavailability of epinephrine from Auvi-Q compared with EpiPen. Ann. Allergy Asthma Immunol. 2013, 111, 132–137.
- Anne K. Ellis, Thomas B. Casale, Michael Kaliner, John Oppenheimer, Jonathan M. Spergel. Development of neffy, an Epinephrine Nasal Spray, for Severe Allergic Reactions. Pharmaceutics 2024, 16, 811.
- Omer A., Ahmed LÖ., Koparir P., Hama J. Structural Analysis of Epinephrine by Combination of Density Functional Theory and Hartree-Fock Methods. ECJSE 2022 (2) 760-776.
- Simons, F.E. Anaphylaxis, killer allergy: Long-term management in the community. J. Allergy Clin. Immunol. 2006, 117, 367–377.
- Ebisawa, M.; Ito, K.; Fujisawa, T. Japanese guidelines for food allergy 2020. Allergol. Int. 2020, 69, 370–386.
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 2020, 13, 100472.
- Goodall N. Guideline review: Epinephrine use in anaphylaxis (AAP guideline 2017). Arch Dis Child Educ Pract Ed. 2020 Feb;105(1):38-40.
- Worm M, Edenharter G, Rueff F, et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012;67:691–698.
- Ring. J.C.; Klimek, L.; Worm, M. Adrenaline in the acute treatment of anaphylaxis. Medicine 2018, 115, 528-534.
- Brown, J. C.; Simons, E.; Rudders, S.A. Epinephrine in the management of anaphylaxis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1186-1195.
- Boddu, S.H.S.; Kumari, S. A short review of the intranasal delivery of diazepam for treating acute repetitive seizures. Pharmaceutics 2020, 12, 167.
- Hogan, R.E.; Gidal, B.E.; Koplowitz, B.; Koplowitz, L.P.; Lowenthal, R.E.; Carrazana, E. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia 2020, 61, 455–464.
- Srisawat, C.; Nakponetong, K.; Benjasupattananun, P.; Suratannon, C.; Wachirutmanggur, L.; Boonchoo, S.; Pankaew, D.; Laocharoenkiat, A.; Pacharn, P.; Jirapongsananuruk, O.; et al. A preliminary study of intranasal epinephrine administration as a potential route for anaphylaxis treatment. Asian Pac. J. Allergy Immunol. 2016, 34, 38–43.
- Casale, T.B.; Ellis, A.K.; Tanimoto, S. Innovations in the treatment of anaphylaxis: A review of recent data. Ann. Allergy Asthma Immunol. 2024, 132, 248–249.
- FDA Adverse Events Reporting System Public Dashboard: Available online: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-A960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis (accessed on 20 May 2024).
- Sheikh, A.; Shehata, Y.A.; Brown, S.G.A.; Simons, F.E.R. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock. Cochrane Database Syst. Rev. 2008, 4, 1–15.
- Rubin, T.; Clayton, J.; Adams, D.; Jou, H.; Vohra, S. Systematic review of outcome measures in trials of pediatric anaphylaxis treatment. BMC Pediatr. 2014, 14, 158.
- Simons, F.E.R.; Ardusso, L.R.F.; Bilo, M.B.; El-Gamal, Y.M.; Ledford, D.K.; Ring, J.; Sanchez-Borges, M.; Senn, G.E.; Sheikh, A.; Thong, B.Y.; et al. World allergy organization guidelines for the assessment and management of anaphylaxis. J. Allergy Clin. Immunol. 2011, 27, e1–e22.
- Sampson, H.A.; Munoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report-Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397.
- Dinakar, C. Anaphylaxis in children: Current understanding and key issues in diagnosis and treatment. Curr. Allergy Asthma Rep. 2012, 12, 641–669.
- Lieberman, P.; Simons, F.E. Anaphylaxis-a practice parameter update 2015. Ann. Allergy Asthma Immunol. 2015, 115, 341–384.
- Tanimoto, S.; Kaliner, M.; Lockey, R.F.; Ebisawa, M.; Koplowitz, L.P.; Koplowitz, B. Pharmacokinetic and pharmacodynamic comparison of epinephrine, administered intranasally and intramuscularly-an integrated analysis. Ann. Allergy Asthma Immunol. 2023, 130, 508–514.
- Duvauchelle, T.; Robert, P.; Donazzolo, Y.; Loyau, S.; Orlandini, B.; Lehert, P.; Lecomte, J.-M.; Schwartz, J.-C. Bioavailability and cardiovascular effects of adrenaline administered by Anapen autoinjector in healthy volunteers. J. Allergy Clin. Immunol. Pract. 2018, 6, 1257–1263.
- Worm, M.; Nguyen, D.T.; Rackley, R.; Muraro, A.; Du Toit, G.; Lawrence, T.; Li, H.; Brumbaugh, K.; Wickman, M. Epinephrine delivery via EpiPen auto-injector or manual syringe across participants with a wide range of skin-to-muscle distances. Clin. Transl. Allergy 2020, 10, 21.
- Casale, T.B.; Ellis, A.K.; Nowak-Wegrzyn, A.; Kaliner, M.; Lowenthal, R.; Tanimoto, S. Pharmacokinetics/pharmacodynamics of epinephrine after single and repeat administration of neffy, EpiPen, and manual intramuscular injection. J. Allergy Clin. Immunol. 2023, 152(6): 1587–1596.


 Mayuri Lodha *
Mayuri Lodha *
 Rahul Mohan
Rahul Mohan
 Dr. Kawade Rajendra M.
Dr. Kawade Rajendra M.
 Madhuri Kshirsagar
Madhuri Kshirsagar
 Shruti Khairnar
Shruti Khairnar





 10.5281/zenodo.14212195
10.5281/zenodo.14212195