Abstract
Quality Indicators in molecular microbiology laboratories are following the strict maintenance of working guidelines for the purpose of reducing laboratory induced infections and adherence to the laboratory standards. Regular monitoring of the quality indicators in the pre-analytical, analytical and post-analytical phases is essential to maintain the credibility of the tools and maintain the records. This study is aimed to study the quality indicators in molecular microbiology laboratory specific to respiratory samples. The quality indicators were recorded, maintained and analyzed for a period of 2 years. The present study indicated the error values in pre-analytical (3.99), analytical (2.66) and post-analytical (3.32) phases. One of the challenging issues is monitoring the laboratory quality throughout the day. The current study emphasizes the monitoring of the Quality Indicators in molecular microbiology laboratories where the quality indicators should be specific and sensitive which helps to improve the accuracy and precision. In order to maintain the internal quality of the laboratory, setting institutional standards as benchmark for laboratory performance including interlaboratory comparisons, external quality assurance are mandate.
Keywords
Analytical phases, Molecular Microbiology, Quality Indicators
Introduction
The quality indicator is one of the essential measures which are used to determine hospital or laboratory standards for diagnosis and patient outcome. On the basis of operational performances and testing process in different phases, we can classify quality indicators, which should be analyzed periodically (every month). Data which is collected should be documented and analyzed by auditing for evaluation of performances in the laboratory for monitoring trends and correcting the deviations. Further, the laboratory can produce quality report to help the clinician for the better clinical outcome and to minimize hospital stay of the patient. (1) A quality indicator is one of the main tools that can enable us to quantify the quality of a selected aspect of laboratory reports by comparing with benchmarks. A comprehensive approach can address the stages in the laboratory total testing process that focus on the areas that are considered important to patient care and the overall health outcomes of the institution. (2) The use of quality indicators is effective in reducing errors, increasing patient safety, and helping to meet standard quality requirements. (3) The laboratory management and its real-time working team have to address the overall quality, outcome, safety of laboratory workers and patients through documentation, presentation, discussion, route cause analysis, and corrective and preventive actions. (1) The quality indicators, including sample rejection and loss, internal and external quality, reporting errors, and critical value reporting on time, are the major factors described in the pre-analytical, analytical, and post-analytical phases.[4] The main objective of this study is to review quality indicators for respiratory tract infection samples with special reference to errors in quality.
MATERIALS AND METHODS
The respiratory samples were received by technical staff round the clock and processed subsequently without delay. Samples were processed on a first-come, first-served basis, except in cases of emergency. The samples were numbered at the reception counter accordingly, and a unique barcode was given for all samples, which is generated by the laboratory information system (LIS), which records the data, including patient details (name, gender and age), referring department, full name of the referring consultant, specimen type, date and time of collection and sample tests to be done. All procedures in the study were performed in accordance with the ethical standards of the Institutional Ethics Committee granting approval (211/TSRMMCH&RC/ME-1/2022 – IEC No. 064 dated 14.03.2022). The samples were screened at all three different levels: pre-analytical, analytical, and post-analytical error. The table 1 describes the details of the error recorded.
Table 1: Details of error in quality indicator
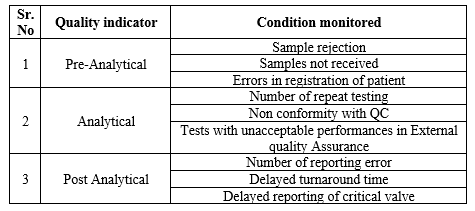
Once the samples were received for processing, it is screened for pre-analytical errors according to acceptance and rejection criteria. When sample was rejected based on the criteria, repeat samples were requested and the reasons for rejection were documented. All the reports were reviewed by an authorized person from the laboratory before dispatch for clinical evaluation. Due to technical errors, the test has to perform again. Further it is defined as repeat the procedures and names as repeat testing. The main reasons for this error was analyzed thereby the route cause error identified, documented and rectified. Inadequate sample, chip error, error in extractor and analyzer due to biomedical issues and, power failure and fluctuation were recorded as reason for error.
In the laboratory, internal and external quality assurance (EQA)/ proficiency testing (PT) programs are actively used to identify the analytical phase accuracy. The laboratory conducts monthly internal quality control (IQC) and EQA/PT on a regular basis. The EQA specimens are processed in the same manner as routine specimens. In case of unavailability of EQA/PT for a particular test, the laboratory performs interlab comparison for accuracy of results. Documentation of all the steps in the analytical process were done for reducing errors.[2,3] Sample rejection consists of requests without clinical diagnosis, unintelligible requests, and samples with insufficient volumes, improperly transported and stored samples, etc. Registers are maintained for the documentation of all three pre analytical error, the analytical error and post analytical error.
RESULTS
During the two-year period, a total of 1503 samples were received. In this single-centric Microbiology laboratory quality analysis for molecular respiratory specimens, pre-analytical errors were estimated at 3.99 per 1000 patients for the years 2021 and 2022. Inadequate samples, requests without clinical diagnosis, and improper or incomplete registration were the most common causes of sample rejection (3 per 1000 samples).
The major issues described in the analytical quality indicators including IQC failure, repeat testing due to chip and instrument errors and unacceptable performance in EQAS. In this study, the EQAS report was 100% but had issues in IQC due to chip failure, may happen in any lot of the kit. The analytical errors evaluation showed 2.66 per 1000 patients for the period of two years. The post analytical error management is crucial due to 24 hours monitoring is cumbersome. Due to manual despatch of reports, some time delay in reporting occurred. The benchmark of turnaround time for molecular respiratory samples is three hours, but only due to repeating the procedure, the TAT cannot be achieved. The post analytical error description highlighted 3.32 per 1000 patients. The overall errors get reduced from 2021 to 2022 indicated the quality management enhanced mainly due to the continuous training specially to sampling, procedures, discarding, documentation and reporting, strict adherence to procedures and protocols, and supervision of technical experts, quality managers and Clinical Microbiologists.
Table 2: Quality indicators of Pre-analytical, Analytical and Post-Analytical error
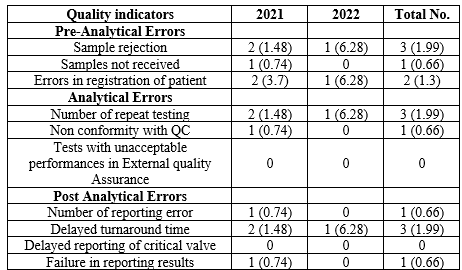
The data for this study was taken after the peak pandemic period; thus, a delay in reports was not observed. Release of duplicate reports was possible in three circumstances due to a request from the patients. Chip invalidity, transcription error, and failure to report were recorded, and these were the main reasons for not maintaining TAT.
DISCUSSION
The scientific, technical, and administrative performances and related documentation in laboratory medicine play a vital role in quality enhancement. The quality improvements in the routine activities of the technical persons involved in various laboratory procedures required proper and frequent training and reorientation related to updates, reporting to the quality manager, documentation of the errors, assessing the route cause by 5 whys analysis, and appropriate corrective and preventive actions by the quality and laboratory teams. (4) The diagnostic management of respiratory specimens is crucial in order to concentrate on self, environmental, and specimen safety and is considered a routine and commendable challenge for Clinical Microbiologists. This is mainly due to improper sampling, high sample loading during procedures including biomedical issues, proper sample storage for further references, proper discarding of used materials, report delivery time, trained technicians, and skilled personnel availability. (5)
Preparedness deals with the strict adherence to standard operating procedures at every step of sample collection, transporting, handling, processing, storing, reporting, and discarding. The managerial responsibilities deal with budget and finance, appropriate infrastructure, working and maintenance of equipment, adequate reagents and consumables, skilled staff with updates, the feasibility of the laboratory information system, and constant evaluation and audit of both internal and external factors. (6)
Among the various phases, the errors were found to be 3.99, 2.66, and 3.32% per 1000 patients for pre-analytical, analytical, and post-analytical errors. While comparing with other studies that included all the clinical laboratories, our study showed higher error rates. (4,7,8,9) In our study, we included only the Molecular Biology Laboratory with special reference to respiratory samples. In some studies, the overall error rate was observed to be more than 4% in the pre-analytical phase, where we have lower pre-analytical error values. (5,10) Similar results were found, which had a higher than average repeat testing error rate of 4.37% in the analytical phase, which is significantly higher than the present study. (11)
In post-analytical errors, our study showed less error compared to other studies, where 16 duplicate reports were recorded, compared with two duplicate reports in this study. To finish, transcription errors were seen in one report in this study, whereas it was recorded as 14 reports in the other. (5) In order to improve the quality of the Molecular Biology Laboratory with special reference to infectious disease diagnosis, standard operating procedures comprised of various quality indicators subjected to the institution make the overall quality superior. Also, referencing the regional centers enhances the updating of the indicators for subjecting and reduces errors. We are lacking references in the study zone, thus making the comparison minimized, and reporting the error data in the public domain of all accredited laboratories will upgrade the laboratory quality.
Providing better-quality laboratory services yields a high standard for comparison and demands the supply of reports on time and with appropriateness to clinicians, making the overall health care delivery with accuracy and reliability. To achieve overall performances of good quality, a team of laboratory management comprising visceral disciplines must strictly adhere to quality indicators for measurement and evaluation of various sets of assessments in polygonal patterns.
REFERENCES
- Agarwal, R, Chaturvedi S, Chhillar, N, Pant I, Kaushik S and Tripathi C: A trend analysis of quality indicators of patient safety in the clinical laboratory over 21 months. Laboratory Medicine (2012), 43: 300-306.
- Bhumika G, Roli S, Komal P, Puruv P and Parul DS: Analysis of quality indicators in Molecular laboratory of a tertiary care hospital in India. International Journal of Pharmaceutical and Clinical Research (2023), 15: 1511-1519.
- Chawla R, Goswami B, Singh B, Chawla A, Gupta VK and Mallika V: Evaluating laboratory performance with quality indicators. Laboratory Medicine (2010), 41: 297-300.
- ISO 15189:2012 Medical laboratories - Requirements for quality and competence. Medical Laboratories - Requirements for quality and competence. Available from: http://www.tecmoh.com/mypages/guides/CwgXKF9s1c.pdf.
- Krithikaa S. Description of the quality indicators used in Microbiology laboratory – methodological approach to quality improvement of the clinical lab reports generated. International Journal of Advance Research and Innovative Ideas in Education (2022), 8: 118-124.
- Lundberg GD: Acting on significant laboratory results. JAMA (1981),245: 1762-1763.
- Madhuri D, Swathi A and Nagamani K: Impact of quality indicators on the performance of clinical serology laboratory. Journal of Evolution in Medical and Dental Sciences (2016), 5: 6300.
- MoHFW - Ministry for Health and Family Welfare: Training module - Labs for life project (Management requirements for laboratory quality management system); 2018.
- Priyanka PG, Ravindra SP, Shilpa AV, Seema UK, Shridhan AP and Shrinivas SK: Evaluation of quality indicators in the laboratory: a three year experience. International Journal of Scientific Research Methodology (2020), 16: 92-102.
- Sakyi AS, Laing EF, Ephraim RK, Asibey OF and Sadique OK. Evaluation of analytical errors in a clinical chemistry laboratory: A 3 year experience. Annals of Medical and Health Science Research (2015); 5: 8-12.
- Savitha AK, Prashanth J, Shilpa P and Ajai K. Evaluation of Quality Indicators in a Laboratory Supporting Tertiary Cancer Care Facilities in India. Laboratory Medicine (2014); 45: 272-277.


 Anitha.k*
Anitha.k*
 Vallab ganesh Bharadwaj
Vallab ganesh Bharadwaj


 10.5281/zenodo.11079630
10.5281/zenodo.11079630