Abstract
A robust and efficient Reverse Phase High Performance Liquid Chromatography (RP-HPLC) method was developed and validated for the simultaneous estimation of Metformin and Telmisartan in tablet dosage form. The method employed a C18 column Xterra (150 x 4.6 mm, 5mm) with a mobile phase consisting of phosphate buffer (pH 4.0) and acetonitrile in a 60:40 v/v ratio. The flow rate was set at 1.0 mL/min, and detection was performed at 210 nm. The method demonstrated excellent resolution and reproducibility, with retention times of Metformin and Telmisartan being 2.70 and 3.64 minutes, respectively. Validation studies confirmed the specificity, linearity, accuracy, and precision of the method, with a linear range of 62.5-375ppm & 5-30 ppm for both drugs. The limits of detection (LOD) and quantification (LOQ) were determined to be 0.02 mcg/ml and 0.07 mcg/ml for Metformin, and 0.02mcg/ml and 0.06 mcg/ml for Telmisartan, respectively. Recovery studies indicated high accuracy, with recoveries ranging from 99% to 100%. The method was also found to be robust under varied conditions. This validated RP-HPLC method is suitable for routine quality control analysis of Metformin and Telmisartan in pharmaceutical formulations, ensuring compliance with regulatory standards.
Keywords
Metformin, Telmisartan, HPLC, Validation.
Introduction
Metformin:
Metformin is an oral antidiabetic drug in the biguanide class, first-line drug of choice for the treatment of type 2 diabetes, in peculiar, in over weight and obese people and those with normal kidney function. Metformin’s use in gestational diabetes has been limited by safety matters. It is also used in the treatment of polycystic ovary syndrome, has been investigated for other diseases where insulin resistance may be an important factor, it works by suppressing glucose production by the liver.
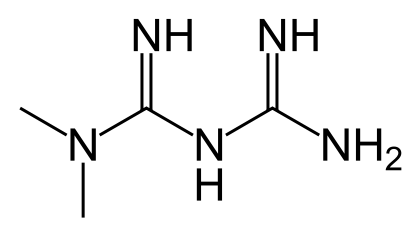
Fig 1: Structure of Metformin
IUPAC Name: 1-Carbamimidamido-N,N-Dimethyl methanimidamide
Molecular formula: C4H11N5
Molecular weight: 129.1636 g/moll
Half-life: 6.2 hours. Duration of action is
8-12 hours.
Category: Antidiuretic Agents
TELMISARTAN:
Telmisartan is an angiotensin II receptor antagonist (ARB) used in the management of hypertension. Generally, angiotensin II receptor blockers (ARBs) such as telmisartan bind to the angiotensin II type 1 (AT1) receptors with high affinity, causing inhibition of the action of angiotensin II on vascular smooth muscle, ultimately leading to a reduction in arterial blood pressure. Recent studies suggest that telmisartan may also have PPAR-gamma agonistic properties that could potentially confer beneficial metabolic effects.
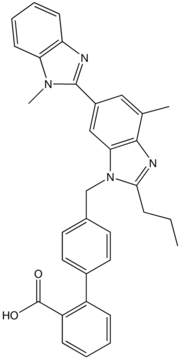
Fig: 2 Structure of Telmisartan
IUPAC Name: 2-(4-{[4-methyl-6-(1-methyl-1H-1,3- benzodiazol-2-yl)-2-propyl-1H-1,3-
benzodiazol-1-yl]methyl}phenyl)benzoicacid
Molecular formula: C33H30N4O
Molecular weight: 5 14.64gm/mol
Half-life: Bi-exponential decay kinetics with a terminal elimination half-life of approximately 24 hours.
Category: Antihypertensive
MATERIALS AND METHODS
Materials:
Metformin and Telmesartan, Combination Metformin and Telmesartan tablets, distilled water, acetonitrile, phosphate buffer, ammonium acetate buffer, glacialacitic acid, methanol, potassium dihydrogen phosphate buffer, tetra hydrofuran, tri ethyl amine, ortho-phosphoric acid etc.
Instrument:
- HPLC instrument used was of WATERS HPLC 2965 SYSTEM with Auto Injector and PDA Detector. Software used is Empower
- UV-VIS spectrophotometer PG InstrumentsT60 with special bandwidth of 2mm and 10mm and matched quartz was be used for measuring absorbance for Metformin andTelmesartan solutions.
Methods:
Preparation of buffer:
Buffer: 1 ml OPAin a 1000mlof Volumetric flask add about 900ml of milli-Q water added and degas to sonicate and finally make up the volume with water then PH adjusted to 3.6 with dil. Orthophosphoric acid solution.
Standard Preparation: (250µg/ml Metformin and 20µg/ml Telmisartan)
Accurately Weighed and transferred 25mg&5mg of Metformin and Telmisartan working Standards into 10 ml and 25ml clean dry volumetric flasks seperately, add 3/4th ml of diluent, sonicated for 30 minutes and make up to the final volume with diluents. From the above each stock solutions, 1 ml was pipeted out in to a 10ml Volumetric flask and then make up to the final volumewith diluent.
Sample preparation: 5 tablets were weighed and calculate the average weight of each tablet then the weight equivalent to 5 tablets was transferred into a 100ml volumetric flask, 70ml of diluent added and sonicated for 30 min, further the volume made up with diluent and filtered. From the filtered solution 0.1ml was pipeted out into a 10 ml volumetric flask and made upto 10ml with diluent.
Method Development:
Trial 1
Column Used : Kromosil 150 x 4.6 mm, 5m.
Mobile phase : Buffer methanol (50:50)
Flow rate : 1ml/min
Wavelength : 210nm
Temperature : 30 ? C
Injection Volume : 10µl
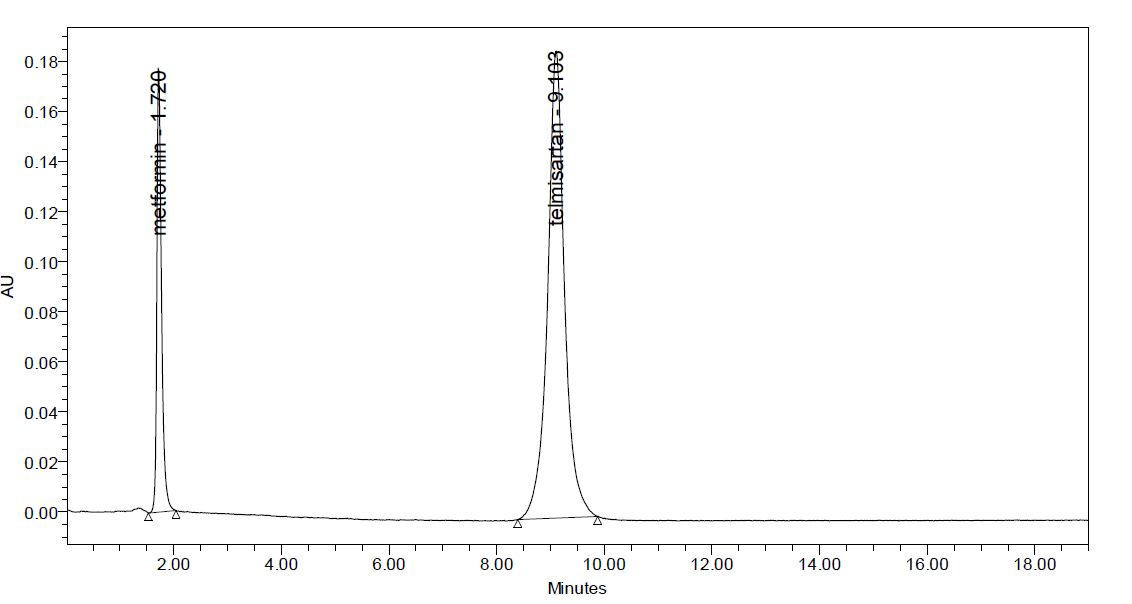
Fig: 3 Trial chromatogram 1
Trial 2
Column Used : Xterra 150 x 4.6 mm, 5m.
Mobile phase : Buffer: Acetonitrile methanol (50:40:10)
Flow rate : 1ml/min
Wavelength : 210nm
Temperature : 30 ? C
Injection Volume : 10µl
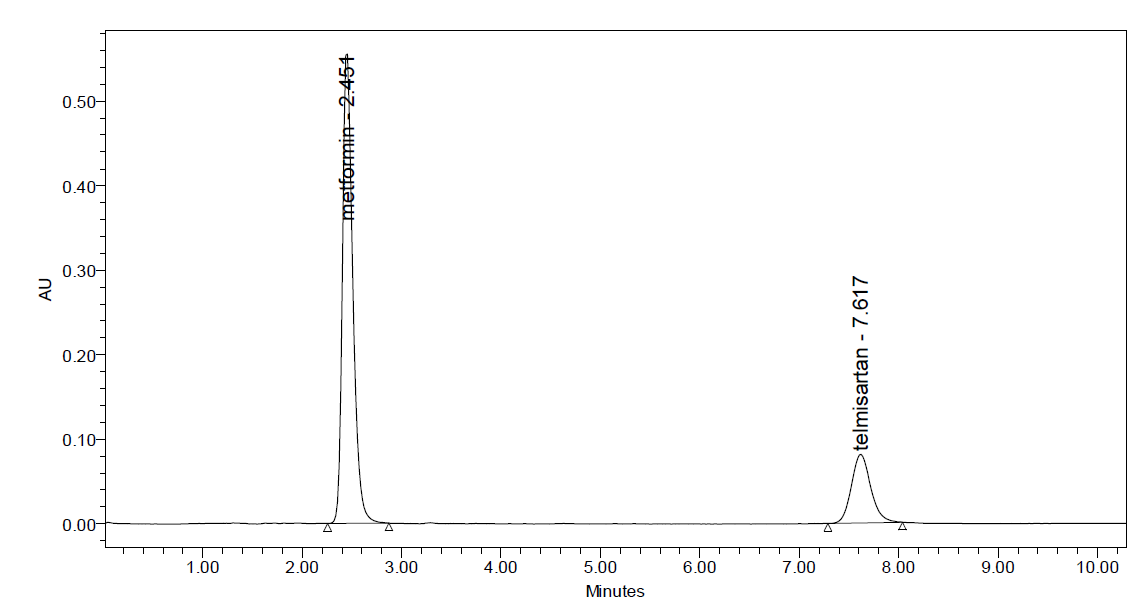
Fig: 2 Trial chromatogram 2
Trial 3:
Column Used : Xterra 150 x 4.6 mm, 5m.
Buffer used : 0.1% OPA
Mobile phase : Buffer: acetonitrile (55:45A)
Flow rate : 1ml/min
Wavelength : 210nm
Temperature : 30 ? C
Injection Volume : 10µl
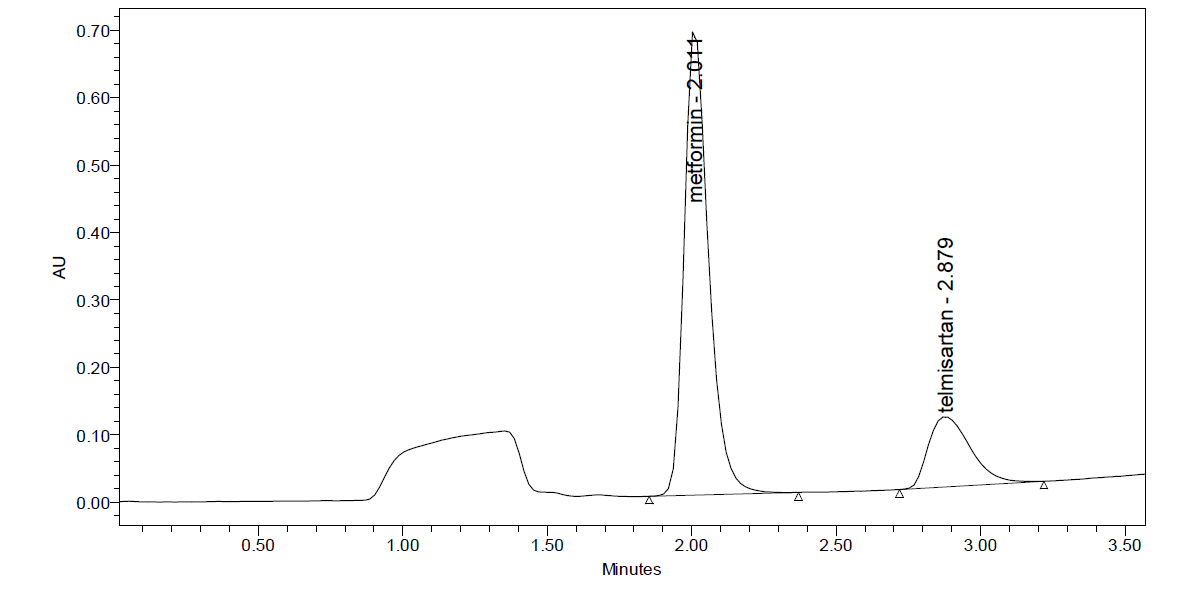
Fig: 3 Trial chromatogram 3
Optimized Method: Drugs were eluted with good retention time, resolution; all the system suitable parameters like Plate count and Tailing factor were within the limits.
Column Used : Xterra150 x 4.6 mm, 5m.
Buffer used : 0.1% OPA
Mobile phase : Buffer: Acetonitrile (60:40A)
Flow rate : 1ml/min
Diluent : Water: methanol 50:50
Wavelength : 210nm
Temperature : 30 ? C
Injection Volume : 10µl
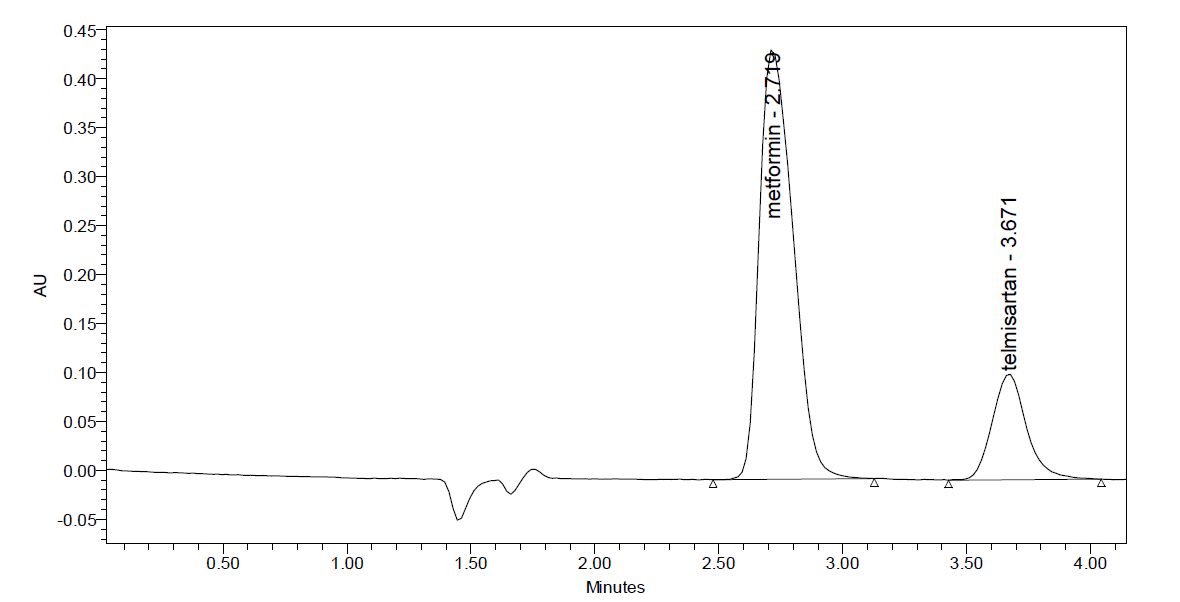
Fig: 4. Optimized Chromatogram
RESULTS AND DISCUSSIONS
System suitability: All the system suitability parameters are within range and satisfactory as per ICH guidelines.
Table: 1 System suitability studies of Metformin and Telmesartan
|
Property
|
Metformin
|
Telmesartan
|
|
Retention time (tR)
|
2.701± 0.3 min
|
3.640±0.3min
|
|
Theoretical plates(N)
|
2713± 163.48
|
3705± 163.48
|
|
Tailing factor (T)
|
1.35± 0.117
|
1.20± 0.117
|
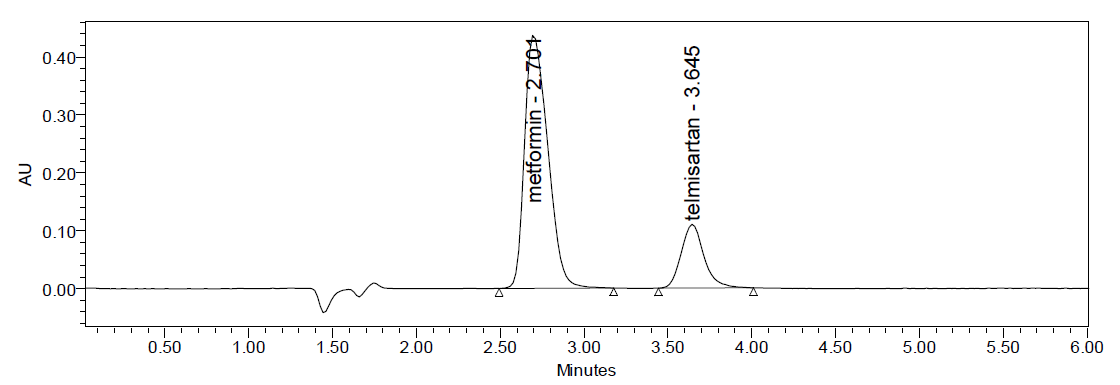
Fig: 5 Typical chromatogram of Metformin and Telmesartan
Linearity: Six Linear concentrations 50ppm - 300ppm of Metformin and 5ppm - 30ppm of Telmesartan are prepared and Injected.Regression equation of theMetformin and Telmesartan are found to bey = 16786x + 673.7 and y = 40365x + 1626 and regression co-efficient was 0.999.
Table:2 Calibrationdata of Metformin and Telmesartan
|
S. No
|
Metformin Concentration
Metformin (µg/ml) Response (mV)
|
Telmesartan Concentration
Telmesartan Response
|
|
CONC
|
RESPONSE
|
CONC
|
RESPONSE
|
|
1
|
0
|
0
|
0
|
0
|
|
2
|
62.5
|
997842
|
5
|
202266
|
|
3
|
125
|
2102553
|
10
|
393075
|
|
4
|
187.5
|
3247781
|
15
|
626474
|
|
5
|
250
|
4151914
|
20
|
812422
|
|
6
|
312.5
|
5286514
|
25
|
1010212
|
|
7
|
375
|
6249487
|
30
|
1205306
|
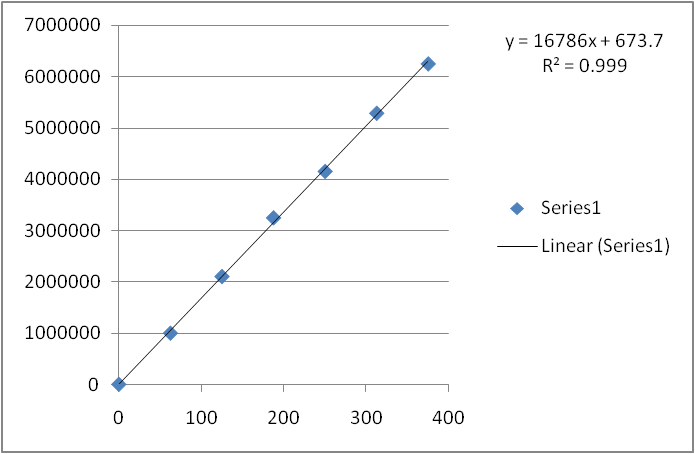
Fig: 6 Calibration curve of Metformin
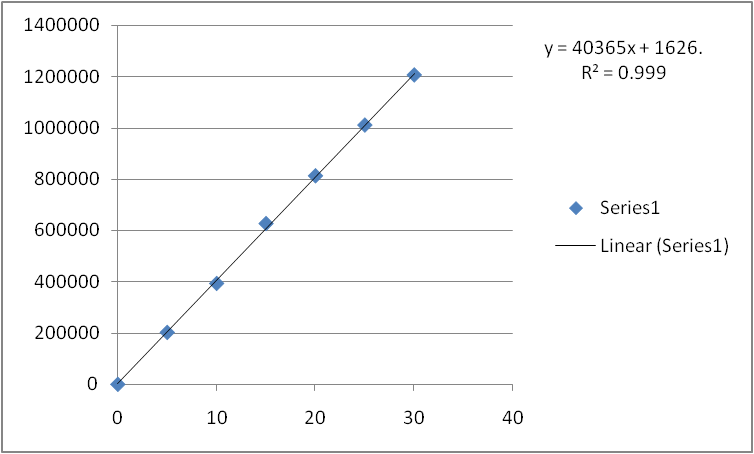
Fig:6 Calibration curve of Telmesartan
Precision:
Intraday precision (Repeatability): Intraday Precision was performed and % RSD for Metformin and Telmesartan were found to be 0.43% and 1.04% respectively.
Table: 3 Repeatability data forMetformin and Telmesartan
|
S. No.
|
Metformin
|
Telmesartan
|
|
1
|
4194425
|
809790
|
|
2
|
4198279
|
817824
|
|
3
|
4177547
|
810827
|
|
4
|
4165200
|
828601
|
|
5
|
4150690
|
829452
|
|
6
|
4172834
|
822917
|
|
Mean
|
4176496
|
819902
|
|
Std. Dev.
|
17911.0
|
8542.7
|
|
%RSD
|
0.43
|
1.04
|
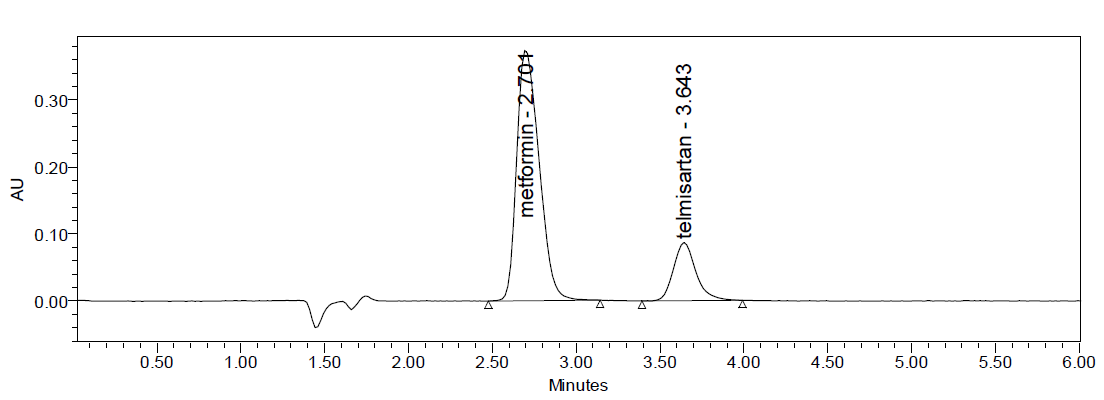
Fig: 7 Repeatability Chromatogram of Metformin and Telmesartan
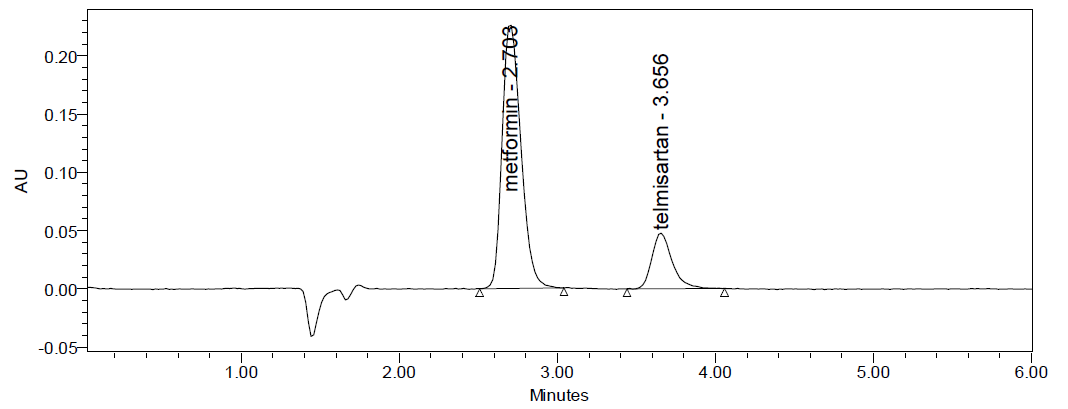
Accuracy: Three concentrations 50%, 100%, 150%, were injected in a triplicate manner and amount Recovered and % Recovery were displayed in
Table 4. Accuracy data of Metformin and Telmesartan
|
Sample
|
Concentration (%)
(µg/ml)
|
Amount Recovered (µg/ml)
|
Recovery (%)
|
%RSD
|
|
Metformin
|
125
|
125.59
|
100.48
|
0.22
|
|
250
|
252.59
|
101.04
|
0.59
|
|
375
|
377.87
|
100.76
|
0.25
|
|
Telmesartan
|
10
|
10.12
|
101.24
|
0.72
|
|
20
|
20.05
|
100.26
|
0.79
|
|
30
|
30.21
|
100.70
|
0.10
|
Robustness: Small deliberatechanges in method like Flow rate, mobile phase ratio, and temperature are made but there were no recognized change in the result and are within range as per ICH Guide lines.
Table 5. Robustness data of Metformin and Telmesartan
|
S.NO
|
Robustness condition
|
Metformin
%RSD
|
Telmesartan
%RSD
|
|
1
|
Flow minus
|
0.2
|
0.35
|
|
2
|
Flow Plus
|
0.6
|
0.8
|
|
3
|
Mobile phase minus
|
0.0
|
0.33
|
|
4
|
Mobile phase Plus
|
0.2
|
0.3
|
|
5
|
Temperature minus
|
0.9
|
0.2
|
|
6
|
Temperature Plus
|
0.4
|
1.7
|
Assay: Standard preparations are made from the API and Sample Preparations are from Formulation. Both sample and standards are injected six homogeneous samples. Drug in the formulation was estimated by taking the standard as the reference. The Average %Assay was calculated and found to be 99.79 and 100.51 forMetformin and Telmesartan respectively.
Table :6 Assay of Tablet
|
S. No.
|
Metformin
%Assay
|
Telmesartan
%Assay
|
|
1
|
100.22
|
99.27
|
|
2
|
100.31
|
100.25
|
|
3
|
99.82
|
99.40
|
|
4
|
99.52
|
101.58
|
|
5
|
99.17
|
101.68
|
|
6
|
99.70
|
100.88
|
|
AVG
|
99.79
|
100.51
|
|
STDEV
|
0.428
|
1.05
|
|
%RSD
|
0.43
|
1.04
|
Table: 7. Summary Table
|
Parameters
|
Metformin
|
Telmesartan
|
|
Calibration range(mcg/ml)
|
62.5-375ppm
|
5-30ppm
|
|
Optimized wavelength
|
210nm
|
210nm
|
|
Mobilephase
(Acetonitrile: Buffer)
|
60:40
|
60:40
|
|
Column
|
Xterra 150 x 4.6 mm, 5m.
|
Xterra 150 x 4.6 mm, 5m.
-
|
|
Retention time
|
2.701min
|
3.640min
|
|
Regression equation(Y*)
|
y = 16786x + 673.7
|
y = 40365x + 1626
|
|
Precision (%RSD*)
|
0.43
|
1.04
|
|
% Recovery
|
99.79
|
100.51
|
|
LOD(mcg/ml)
|
0.02
|
0.02
|
|
LOQ(mcg/ml)
|
0.07
|
0.06
|
CONCLUSIONS
A simple, Accurate, precise method was developed for the simultaneous estimation of the Metformin and Telmesartan in tablet formThe retention times (Rt’s) of Metformin and Telmesartan were found to be 2.701min and 3.640min. Percentage RSD of the Metformin was found to be 0.43 and for Telmesartan was 1.04 and the %Recoveries were gained as 99.79% for Metformin and 100.51% for Telmesartan.
LOD, LOQ values are from regression equations of Metformin and Telmesartan were 0.02, 0.07ppm and 0.02, 0.06ppm resp.
Regression equation of Metformin is y = 16786x + 673.7,Telmesartan is and y = 40365x + 1626 .In this proposed method retention times(Rt’s) were decreased so the method developed was simple and economical that can be adopted in regular Quality control test in Industries.
REFERENCES
- Anushaakula, n. Prajwala, m. Sandhya, dr. Uma maheswararao, development and validation of rp-hplc method for simultaneous estimation of metformin hydrochloride and gliclazide in bulk and combined doasage form, International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 5, Suppl 4, 2013.
- A. Korolkovas. “Essentials of Medicinal Chemistry”, 2nd edition, Wiley Interscience, New Jersey, 1988.
- B. Bhoomaiah, a. Jaya shree Development and validation of rp-hplc method for simultaneous Determination of metformin and miglitol in bulk and pharmaceutical Formulation. International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 6, Issue 6, 2014.
- “Burger’s Medicinal Chemistry and drug discovery”, 6 th edition, Wiley Interscience, New Jersey, 2007.
- GattuMadhavaPrathap, Muthukumaran M, Krishnamoorthy B, Development and Validation of Simultaneously Estimation of Vildagliptin& Metformin Hydrochloride by RP-HPLC in Bulk and Oral Dosage Form, IJAPGR,2014..
- “Goodman and Gilman’s The Pharmacological Basis of Therapeutics”, 9th edition, McGraw-Hill health professions division, New york, 1996.
- G.Alekya , Naira Nayeem, T.Mahati , RP-HPLC Method Development and Validation of Metformin and Vildagliptin in Bulk and Its Pharmaceutical Dosage form and their Bio-Analytical Studies, American journal of pharmatech research.
- Ganipisetty Lakshmi Aswini*1, D.Dachinamoorthy2, J.V.L.N.Seshagirirao Development and validation of stability indicating rp-hplc method for simultaneous estimation oftelmisartancilinidipine and chlorthalidone in bulk and their combined tablet dosage form with forced degradation studies.World Journal of Pharmaceutical Research SJIF Impact Factor 5.045 Volume 4, Issue 3, 1373-1387
- ICH, Q2B, Harmonized Triplicate Guideline, Validation of analytical Procedure Methodology, IFPMA, Proceeding of the International Conference on Harmonization, Geneva, March 1996.
- ICH, Q2A, Harmonized Tripartite Guideline, Validation of Analytical Procedure Methodology, IFPMA, Proceeding of the International Conference on Harmonization, Geneva, March1994.
- KanijFatema, Md. ZakiurRahman, TasnuvaHaque, MohammadAbulKalam Azad and Md. Selim Reza, Development and Validation of a Simple Method for Simultaneous Estimation of Metformin Hydrochloride and Gliclazide in Tablets by using Reversed Phase High performance Liquid Chromatography
- Madhukar.A,Prince.A ,vijaykumar.A simple and sensitive analytical method development and validation of metformineHcl by RP-HPLC ,International Journal of Pharmacy and Pharmaceutical Sciences , 3, 3, 2011.
- MohdIzzathullahSiddiqui, MedidiSrinivaS, Simultaneous estimation of telmisartan and cilnidipine in bulk and in tablet formulation using rp-hplc. An International Journal of Advances in Pharmaceutical SciencesVolume 5|Issue 3|May-June 2014|Pages 2142-2148.
- N. Mukuntha Kumar1*, Sumathi V rao1, Konde Abbulu2, B.Venkata Narayana1, I. Sukumar 1. Simultaneous Estimation of Telmisartan and Chlorthalidone in Tablet Dosage Form by Using Reversed Phase High Performance Liquid Chromatographic Method.AJPTR Fri, 17 Apr 2015.


 Battu Rama Madhuri*
Battu Rama Madhuri*











 10.5281/zenodo.14020904
10.5281/zenodo.14020904