Abstract
The largest organ in the body, the skin, may be used to administer drugs for both local and systemic effects. However, the stratum corneum (SC), the skin's outermost layer, serves as a strong barrier that prohibit hydrophilic and high molecular weight medications from penetrating. One simple and easy solution is to incorporate medications in formulations with elastic vesicles or skin enhancers. The vesicular-shaped structures known as ethosomes are composed primarily of ethanol and phospholipids. Since they are nanometres in size and have a higher quantity of ethanol in their structure, they have demonstrated exceptionally significant drug penetration whether applied topically. The hot method and the cold method are the two main methods used to formulate the ethosomes. Furthermore, recently new method was developed for formulating ethosomes including classical mechanical dispersion method. Then formulated ethosomes incorporated into gel to carry ethosomes, deep penetration of drug into skin, stability and sustained release of ethosomes. Ethosomal gels offer a promising strategy for enhancing the delivery of drugs, improving their therapeutic efficacy and improve patient compliance. In this review article various aspect of ethosomes including their mechanism of penetration, advantages, composition, application, method of preparation, characterization of ethosomes and in ethosomal gel aspect like gelling agent, importance, examples of gelling agent and Characterization of ethosomal gel. These carriers pose new challenges and potential for the development of novel, better treatment.
Keywords
Topical delivery, Ethosomes, Ethosomal gel, Ethanol, Permeation, Patient compliance.
Introduction
Advancements in drug delivery methods are happening much more quickly now than they did twenty years ago. Enhancing patient adherence and sustainability are the primary goals of the novel drug delivery methods.[1] The skin is one of the broadest and easily accessible organs in the human body, so utilizing it as a route of medication delivery can provide numerous advantages over standard drug delivery systems. The skin provides an excellent barrier to molecular transport since the corneum layer is the most formidable barrier to the passage of most medications, except for lipophilic and low molecular weight drugs.[2] This method is called transdermal drug delivery, which uses a person's skin as a route for a drug molecule to enter their body and be delivered systemically. The transdermal drug delivery system (TDDS) is one of the systems under the category of controlled drug delivery, in which the goal is to deliver the drug through the skin at a predetermined and controlled rate. The transdermal drug delivery system (TDDS) is having much more benefits, particularly for the drugs that had very poor permeation in the stratum corneum of the skin.[3] Transdermal drug delivery system (TDDS) showed promising results in comparison to the oral drug delivery system as it eliminates gastrointestinal interferences and first-pass metabolism of the drug, but the main drawback of TDDS is that it encounters the barrier properties of the stratum corneum, i.e., only lipophilic drugs having a molecular weight < 500>
Skin
The skin is the largest organ of the body and is readily available organs of human body system; simply a modest quantity of a millimetre of tissue isolates the skin surface. The skin on a typical adult body measures about 20 square feet and receives roughly one-third of total accessible blood. The epidermis is the skin's outermost layer, which acts as a waterproof barrier and affects our skin tone. Dermis, beneath epidermis, contains tough connective tissue, hair follicles, and sweat glands and deeper subcutaneous tissue (hypodermis) is made of fat and connective tissue.[6] A perception of the nature and base of the deterrent properties of skin, and of the physicochemical characteristics of substances which choose their ability to penetrate through the skin and enter the stream, would have enormous incentive to ecologists and toxicologists, stressed over the dangers of skin exposure to air and water pollutants, and currently most of the doctors and pharmacologists are keen on the use of skin as a course of section of meds for the treatment of or dermatological and a lot more infections.[7]
Layer of Skin
There are three layers of skin: The epidermis (Outer layer), The dermis (Middle layer), The hypodermis or subcutaneous tissue (Bottom layer).[8]
A. Epidermis
The layers of the epidermis include four layers, the stratum basale (the deepest portion of the epidermis), stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum (the most superficial portion of the epidermis).
a. Stratum basale: The innermost layer of skin is called stratum basale, or stratum germinativum. Hemidesmosomes connect it to the basement membrane, which separates it from the dermis. This layer contains cuboidal to columnar stem cells that are constantly generating keratinocytes through mitosis. Also, melanocytes present in this layer.
b. Stratum spinosum: The prickle cell layer, or stratum spinosum, is made up of 8–10 layers of irregular, polyhedral cells with cytoplasmic processes—sometimes referred as "spines" that extend outward and make contact with neighbouring cells by desmosomes. This layer contains dendritic cells.
c. Stratum granulosum: Diamond-shaped cells with keratohyalin and lamellar granules are found in the stratum granulosum, which is composed of three to five cell layers. Keratin precursors seen in keratohyalin granules eventually combine, crosslink, and form bundles. Lamellar granules include glycolipids, which are released to the cell surface and act as a glue to hold the cells together.
d. Stratum lucidum: The thicker skin of the palms and soles contains two to three cell layers. The thin, transparent layer is composed of up of eleidin, a by-product of keratohyalin transformation.
e. Stratum corneum: The topmost layer, known as the stratum corneum, is composed of 20–30 cell layers of keratin and hairy scales, which are composed of anucleate squamous cells, which are dead keratinocytes. The thickness of this layer fluctuates the most, particularly in callused skin. Defensins, a component of our initial line of defense, are secreted by the dead keratinocytes in this layer.[9]
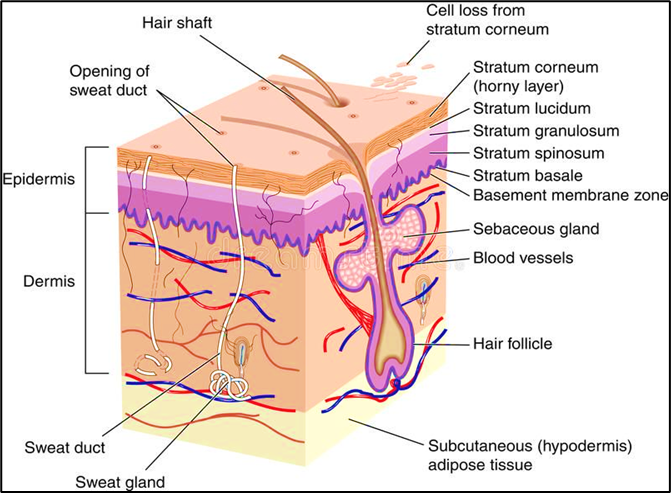
Fig 1 - Human Skin
B. Dermis
The second layer of skin underneath the epidermis in the integumentary system is called the dermis. The two layers that constitute the dermis are the reticular and papillary layers. Adipocytes, blood arteries, and lymphatic capillaries are all located in the dermal papillary layer. The reticular layer is much denser than the papillary layer due to its high concentration of collagen fiber, which provides it the flexibility it needs to move.[10]
C. Hypodermis
The skin's deepest layer, the hypodermis, is located underneath the dermis. Because it acts as a connective tissue between the skin, muscles, and bones, the hypodermis—also known as the superficial fascia or the subcutaneous layer is abundant in glycosaminoglycans and proteoglycans. Adipose tissue, which is abundant in the hypodermis, acts as thermal insulation to keep the body warm.[11]
Vesicular approaches for topical drug delivery[12]
It has been discovered that drugs encapsulated in lipid vesicles made of non-ionic surfactants and phospholipids can pass through and enter the skin. Skin lipids help maintain the skin's barrier function and stop medications from being absorbed systemically. Lipid vesicles may be used as a non-toxic medication penetration enhancer because of their amphiphilic nature. Moreover, hydrophilic and lipophilic medications with varying molecular weights can be encapsulated in vesicles. Thus, it is theorized that these lipid-rich vesicles carry a significant quantity of medication through the skin, improving the absorption of the medication throughout the body. Although transdermal formulations of liposomes have been researched for a variety of uses, but their unstable nature and low skin penetration restrict their use in topical distribution. The idea of proliposome came up to improve the stability of liposomes. Niosomes, which showed better stability than liposomes, were subjected to this method. However, liposomes and niosomes were only effective for topical application because of their low skin permeability, which prevented them from being effectively utilized for systemic drug administration. Cevc et al. and Touitou et al. recently developed two novel vesicular carrier systems ethosomes and transferosomes, respectively for the non-invasive transport of medications into or across the skin in order to solve issues with poor skin permeability. Transferosomes and ethosomes include edge activators (surfactants) and penetration enhancers (alcohols and polyols), which impact the characteristics of vesicles and the stratum corneum. For several decades, the vesicles' significance in particle transportation and cellular communication has been widely recognized. In order to tag the vesicle for cell selectivity, researchers have figured out how to use the structure of vesicles to improve medication delivery within their cavities. The discovery of a vesicle derivative called an ethosomes was one of the significant developments in vesicle research.
Ethosomes
Ethosomes are invented by Touitou et al in 1997.[13] Ethosomes are specially tailored vesicular vehicle able to efficiently deliver various molecules with different physicochemical properties into deep skin layers. Ethosomes are ethanolic liposomes that contain high amount of ethanol. Ethosomes are liposomes that contain ethanol. Ethosomes are non-invasive delivery vehicles that allow drugs to reach the bloodstream or penetrate deep into the skin's layers.[14]. They are soft and flexible nano vesicles. Because of their unique structure, they can pass through the skin's natural barrier and distribute medications through its layers.[15] Ethosomes are lipid vesicles consist of phospholipids, alcohol (ethanol or isopropyl alcohol) in relatively high concentration and water. Size of vesicles varies from 10 nanometers to microns.[16] Deeper distribution and penetration in the lipid bilayers of the skin have been attributed to the synergistic effects of the phospholipids and high ethanol concentration in formulations. It disrupts the structure of the lipid bilayer in the skin since it is non-invasive. Drug release may occur at different stages along the penetration pathway as a result of the fusion of the ethosomes system with the epidermal lipids.[17] Delivery of medications through Ethosomes can be modified not only for better skin permeation but localizes the drug at the site of action which enables drugs to reach the deep skin layers. A wide range of molecules, including hydrophilic, lipophilic, and amphiphilic ones, can be effectively entrapped in ethosomes. Due to factors such as the solubility of many drugs in ethanol, large drug loading in ethosomes is possible. A high ethanol concentration boosts their flexibility and penetrating capability by increasing thermodynamic activity from ethanol evaporation and improving penetration by lowering the stratum corneum's barrier property. In addition, by protecting the drug from the immune system and other removal mechanisms, vesicles could give long-term control over the drug's release rate, allowing it to release exactly the proper quantity of medicine while keeping that concentration for prolonged time.[18]
Scope of Ethosomes [19,20]
• Ethosomal delivery methods minimize the interaction between food and drugs through preventing enzymatic breakdown.
• These are helpful in the disorders like vomiting and diarrhea for conventional drug administration without loss of drug.
• Ethosomes deliver the drug direct into the systemic circulation without entering into the portal circulation and also it skips mechanical and chemical digestion thus drug loss is minimized.
• The Ethosomal system of drug delivery is non-invasive, is convenient compared to that parenteral route which is inconvenient.
• As they provide continuous drug supply, thus frequency of dose can be avoided.
• Thus, Single application gives better therapy than other dosage forms.
• They act as drug reservoir and help to increase the half-life or the continuous,
controlled release of drug leading to better therapy.
• Due to their physical features, they can be rapidly identified in emergencies (for example, patients who are unconscious, unresponsive and in coma).
Composition of Ethosomes [21]
Table No 1: Composition of Ethosomes
|
Class
|
Example
|
Uses
|
|
Phospholipid
|
Soya phosphatidyl choline, Egg phosphatidyl choline,
Dipalmitoyl phosphatidyl choline, Distearoyl phosphatidyl choline
|
Vesicles forming component
|
|
Polyglycol
|
Propylene glycol
|
As a skin penetration enhancer
|
|
Alcohol
|
Ethanol, Isopropyl alcohol
|
For providing the softness for vesicle membrane
As a penetration enhancer
|
|
Cholesterol
|
Cholesterol
|
For providing the stability to vesicle membrane.
|
|
Dye
|
Rhodamine-123 Rhodamine red Fluorescence Isothiocyanate (FITC)
|
For characterization study
|
|
Vehicle
|
Carbopol 934
|
As a gel former
|
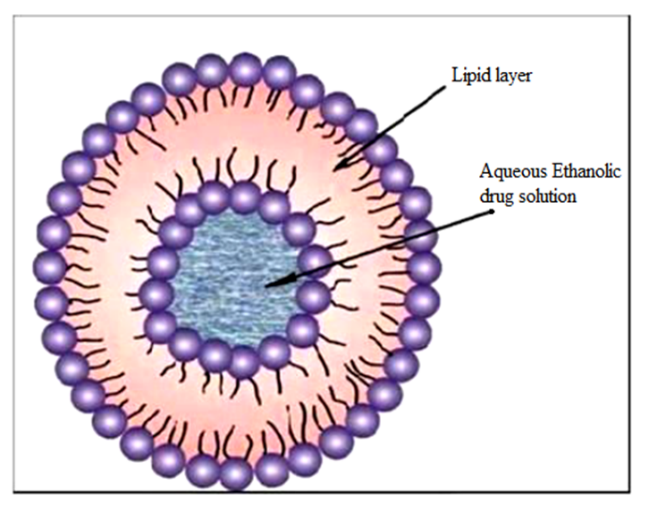
Fig.2: Structure of Ethosome
Advantages of Ethosomes [22,23]
1. It enhance permeation of drugs through skin for dermal, transdermal and intracellular delivery.
2. It transport a variety of hydrophilic and lipophilic compounds, proteins, peptides, and other macromolecules that have different physicochemical characteristics into the skin.
3. The ethosomes constituents are non-toxic, Generally Recognized as Safe (GRAS), and approved for use in medicine and cosmetics.
4. Low risk profile- Since ethosomes toxicity profiles are well-established in the scientific literature, there is no major risk associated with ethosomes structure in drug development.
5. The ethosomal system is suitable for instant marketing because it is non-invasive and passive.
6. Ethosomal drug delivery system can be utilized widely in Pharmaceutical, Biotechnology, Veterinary, Cosmetic & Nutraceutical fields.
7. High patient compliance- The ethosomal drug is administered in a semi-solid form (gel or cream) can achieve high patient compliance.
8. Simple drug delivery technique as compared to complicated techniques like sonophoresis and iontophoresis.
9. Ease of industrial scale-up- Relatively easy to manufacture with no complicated technical investments required for production of ethosomes.
10. Multi liter amounts can be conveniently prepared for ethosomal formulation.
11. Ethosomes improve permeation of drugs across/through the skin in an efficient manner, thereby the drug able to reach the targeted site in the skin or to the blood.
12. Higher amount of drug entrapment efficiencies when compared to liposomes can be observed.
13. Excellent stability over extended periods can be observed.
14. Alcohol in the ethosomes acts as natural preservative, hence there is no need to add any other preservative.
15. The cost of manufacturing ethosomes is very low.
Disadvantages of Ethosomes [24]
1. Allergic reaction can be detected if the patients has allergy to ethanol or any of the ethosomal components.
2. Ethosomal carriers are mainly for topical application, compared to other carriers (solid lipid nanoparticles, polymeric nanoparticles, etc.) that can be utilized for a multiple routes.
3. Low yield so may not be cost-effective.
4. Loss of product during moving from organic to aqueous phase.
5. Excipients and penetration enhancers used in drug delivery systems can cause dermatitis or skin irritation.
6. If shell locking is not effective, ethosomes can bind together and disintegrate when submerged in water.
Mechanism of drug penetration
While the precise mechanism of drug delivery via ethosomes is still a topic of debate, the improved drug transport facilitated by ethosomes is believed to result from their interaction with skin lipids. At physiological temperature, the lipid multilayers of the stratum corneum are tightly packed and exhibit a high degree of conformational order. It is hypothesized that there are two potential mechanisms underlying the effective drug delivery provided by ethosomes. The "ethanol effect" represents the first part of the mechanism. The ethosomes are unique because of the high concentration of ethanol, which is known to disrupt the organization of the lipid bilayer in the skin. As a result, when ethanol is incorporated into a vesicle membrane, it allows the vesicles to pass through the stratum corneum. Also, because of their high ethanol content, the lipid membrane is less tightly packed than that of traditional vesicles but is just as stable, allowing for a more flexible structure that gives it more flexibility and the capacity to pass through narrow spaces like the openings created when the stratum corneum lipid is disrupted.[25]
1. Ethanol effect
Ethanol serves as a penetration enhancer when applied to the skin. The process by which it enhances penetration is well understood. Ethanol infiltrates the intercellular lipids, enhancing the fluidity of the cell membrane lipids and reducing the density of the lipid multilayer in the cell membrane.
2. Ethosome effect
Skin permeability is increased as a result of the ethanol in ethosomes enhancing the lipid fluidity of cell membranes. Thus, the ethosomes easily penetrate the deep layers of the skin, where they fuse with skin lipids and release the medications.[26]
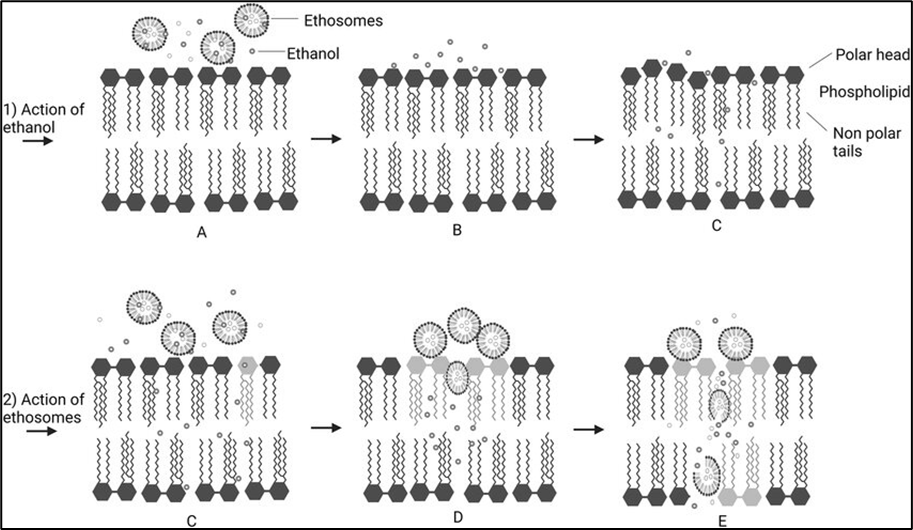
Fig 3: Proposed permeability mechanism of ethosomes: (A) Stratum corneum lipid layer; (B) ethanol interacts with lipid molecules; (C) ethanol increases lipid layer density; (D) interaction of ethanol and ethosomes on stratum corneum; (E) penetration and release of ethosomes into deep layers of skin.
Application of Ethosomes
There are numerous applications for ethosomes in drug delivery. Their primary purpose is to substitute for liposomes. Adopting the transdermal route for medication administration is primarily preferred. Drugs that are hydrophilic or lipophilic can be delivered through the skin by ethosomes. Ethosomal carriers utilize similar medications.
1. Delivery of Problematic Drug Molecules
Transdermal administration in the GIT tract is a safer choice due to the complete breakdown of broad biogenic substances, such as insulin and peptides or proteins. However, there are permeation problems with the conventional formulation of biogenic molecules like insulin and peptides or proteins. Permeation and therapeutic efficacy are significantly increased when the above mentioned compounds developed into ethosomes.[27]
2. Cosmeceutical Applications of ethosomes
Adding ethosomes to cosmeceutical substances has several benefits, including increasing the stability of cosmetic chemical substances and reducing skin discomfort caused by irritating cosmetic chemicals. The structure and scale of the vesicles are therefore the most important factors to be taken into account in order to achieve these advantages of elastic vesicles.[28]
3. Delivery of Anti-Viral drugs
Horwitz et al. found that a 5?yclovir ethosomal preparation significantly improved the treatment of herpes infections when compared to a 5?yclovir cream.[29] Jain et al. The effect of different formulation variables on skin permeation of Zidovudine was studied using locally fabricated Keshry-Chien type of diffusion cell. A vesicle interaction with the skin study was conducted in order to comprehend the mechanism of improved skin penetration of ethosomes. To confirm the better skin permeability of ethosomes, fluorescence microscopy using Rhodamine-123 as fluorescence probe was performed. The optimized ethosomes showed transdermal flux of 78.5± 2.5 Qg/cm2 /hr across the rat skin. A study on the interaction between vesicles and their skin revealed that ethosomes had an impact on the stratum corneum's ultrastructure because different areas with lamellar stacks originating from the vesicles were seen in the intercellular spaces. Thus, ethosomes can enhance the transdermal flux, prolong the release and present an attractive route for sustained delivery of Zidovudine.[30]
4. Treatment of Parkinsonian Syndrome
Dayan et al. studied the delivery of Trihexyphenidyl HCl (THP) between ethosomes and classic liposomes. As the THP concentration was increased from 0 to 3%, the size of the vesicles decreased from 154 to 90 nm. This is most likely due to the surface activity of THP (critical micelle concentration of 5.9 mg/ml), as measured in this work. In addition, the ethosomes zeta potential value increased as a function of THP concentration, from -4.5 to +10.4 when the THP concentration was increased from 0 to 3%. THP liposomes, on the other hand, were significantly bigger and THP had no effect on their charge. Ethosomes were better at delivering entrapped fluorescent probe to the deeper layers of skin and had a higher entrapment capacity than regular liposomes. The flux of THP through nude mouse skin from THP ethosomes (0.21 mg/cm2 h) was 87, 51 and 4.5 times higher than from liposomes.[31]
5. Delivery of Antibiotics
Godin et al. looked into a novel method of applying erythromycin topically in an ethosomal carrier to treat bacterial infections of the deep dermis and soft tissues. The effectiveness of local application of hydroethanolic Erythromycin solution and intraperitoneal Erythromycin administration was contrasted with that of ethosomal Erythromycin applied to the skin-infected region. Histological analysis and bacterial counts of the ethosomal antibiotic-treated skin showed normal skin structure and no bacterial growth. However, when topical hydroethanolic Erythromycin solution was applied to diseased mice, no subdermal healing was seen.
Godin et al. investigated the intracellular and cutaneous transport of Bacitracin derived from ethosomes. It has been noted that ethosomal administration of antibiotics effectively delivers them to the deep dermis, minimizing the possibility of adverse effects.[32]
6. Improved Anti-Inflammatory Activity
Lodzki et al. used ethosomal carriers to create a transdermal administration method for cannabidol. In the same animal model, the sub-plantar injection of carrageenan caused inflammation and edema, which were avoided by transdermal administration of ethosomal cannabidol. Ethosomes thereby made it possible for cannabidol to penetrate the skin and accumulate in a depot at quantities that were shown.[33]
7. Transdermal delivery of Insulin
In an experimental study, Touitou et al. examined the effects of an ethosomal insulin formulation applied topically on blood glucose levels. In both normal and diabetic rats, the ethosomal formulation reduced blood glucose levels by up to 60% and maintained the level for at least eight hours.[34]
8. Transdermal delivery of Hormonal agents
Kaplun and Touitou et al. have shown that the results of this investigation indicate that menthol affects skin permeation by a dual mechanism: by forming a eutectic with the penetrating compound, thereby increasing its solubility, and by altering the barrier properties of the stratum corneum. Moreover, this study indicates that both types of interactions must be taken into consideration when using chemical enhancers and that decreasing the melting temperature of the permeant through formation of a eutectic could be one approach for increasing solubility and permeation rates.[35]
9. Improved Pharmacokinetic release profile
Esposito et al. examined the fundamental characteristics and the release kinetics and in vitro release rate of azelaic acid, which was also carried in various phospholipid based vesicles like liposomes or ethosomes. Using a Franz cell made with synthetic membranes, the diffusion of azelaic acid from ethosomal or liposomal dispersions, as well as from ethosomes and liposomes integrated in a viscous gel, was examined. Compared to liposomal systems, ethosomal systems had a faster rate of release.[36]
10. Improved Skin retention of Minoxidil
Kim et al. developed three different topical dosage forms of minoxidil: inclusion complexes with hydroxypropylg-cyclodextrin (HP-g-CD), vesicles, and double emulsions. Using hairless mouse skins, the preparations' ability to retain skin was assessed in vitro. When the medication was enclosed in cationic vesicles, retention was at its peak. Nonionic vehicle, the double emulsion and HP-g-CD left no substantial amount of medication penetration through the skin. Each dosage form's effect on promoting hair growth in vivo was examined by applying the sample to female mice's clipped backs and then rinsing the backs once daily for 30 days. Because of the considerable skin retention, only minoxidil in the cationic vesicles showed a hair growth stimulation effect.[37]
11. Improved permeation activity
The USA-based Osmotics Inc. announced Lipoduction, a novel cellulite treatment that smoothes skin and contains ethosomes, a breakthrough permeation technology that allows chemicals to enter fat cells directly and breach the skin's lipid barrier. Compared to the existing cream, the introduction of flexible nanospheres (ethosomes) in lipoduction cream enables a cocktail of fat-metabolizing chemicals to reach the fat cells 700% more efficiently. Within 60 days, the appearance of cellulite was improved by up to 80% thanks to ingredients in lipoduction.
12. Newer potentials
Ethosomes have shown promise as transdermal dosage forms for migraine prophylaxis, and their capacity to deliver compounds to cultured cells has been studied. Transdermal absorption of polypeptides is currently being studied. The high interest of ethosomes in the design of new therapies has been investigated with other drugs such as Propranolol.
13. Topical Delivery of DNA
Numerous infections in the environment try to enter the body through the skin. As a result, skin has developed into a superb barrier of defense that is also immunologically active and gene-expressible. Based on the data provided above, topical delivery of DNA molecules to skin cells to express genes is another important purpose for ethosomes. The GFP-CMV-driven transfecting construct was encapsulated in an ethosomal formulation in their study. For a period of 48 hours, they applied this formulation to the dorsal skin of male CD-1 nude mice that were 5 weeks old. Following a 48-hour period, CLSM observed the penetration into the green fluorescent protein (GFP) formulation after the treated skin was removed. It was shown that ethosomes-GFP-CMV-driven transfecting constructs applied topically allowed for effective gene transport and expression in skin cells. Ethosomes have been suggested as potential carriers for gene therapy applications that need temporary gene expression. These findings also suggested that ethosomes could be used for transdermal vaccination. Using a transferosomal formulation, Gupta et al. have revealed immunization potential. Therefore, ethosomes improved skin penetration capability makes it possible to use these dosage forms to administer vaccinations.[38]
Table 2: Application of Ethosomes
|
Drug
|
Applications
|
|
Acyclovir
|
Treatment of Herpetic Infection
|
|
Zidovudine
|
Treatment of Aids
|
|
Erythromycin
|
Efficient healing of S. Aureus – Induced deep dermal infection
|
|
Cannabidol
|
Prevent inflammation and edema
|
|
Minodixil
|
Hair growth promotion effect
|
|
Bacitracin
|
Treatment of dermal infection
|
|
Cyclosporine
|
Treatment of inflammatory skin disease
|
Table 3: Marketed product of Ethosomal Drug Delivery System [39,40]
|
Sr. No
|
Name of the
Product
|
Uses
|
Manufacture
|
|
1
|
Decorin cream
|
Noticeable skin aging signs including lines,sagging, age spots, loss of elasticity, and over pigmentation are treated, restored and postponed.
|
Genome
Cosmetics,
Pennsylvania,
US
|
|
2
|
Noicellex
|
Topical anti-cellulite cream
|
Novel Therapeutic
|
|
3
|
Supravir cream
|
For the treatment of herpes virus
|
Trima,Israel
|
|
4
|
Skingenuity
|
Powerful cellulite buster, Reduces orange peel
|
Physonics,
Nottingham, UK
|
|
5
|
Nanominox
|
The first medication to contain ethosome was minoxidil. It contains 4% minoxidil, a proven hair growth agent that is converted to the active ingredient by sulfurization.
|
Sinere, Germany
|
|
6
|
Cellutight EF
|
Topical cellulite cream has a potent combination of ingredients that speed up metabolism and reduce fat.
|
Hampden Health,
USA
|
Safety of Ethosomal systems
Elements of ethosomes are often considered healthy (GRAS). The protection of ethosomal systems applied topically to the skin was examined in a number of studies, both in vitro and in vivo. Ethosomal structures have been shown to be beneficial to cells in in vitro cell culture tests.[41] The ethosomal systems containing various compounds (e.g., BH, ibuprofen, testosterone, CBD, etc.) did not improve the horny layer's composition and thickness, nor did they cause any inflammatory cells to penetrate the skin at the treatment site. Both acute and regular ethosomal patch treatments for 14 days did not cause any signs of skin inflammation in the rabbits. In a study by the Paolino and Fresta Community, reflectance spectrophotometry was used to evaluate the skin tolerability of ethosomal systems in healthy individuals.[42]
Methods of Preparation of Ethosomes:
The ethosomal formulation can be made using three method, as explained below. All method don't require any complex equipment, practical and cost-effective. The preparation process is simple to scale up on an industrial scale.
1. Cold Method
This is the method that is most frequently used to preparation of ethosomal formulation. In this method, phospholipids, drugs, and other lipid components are dissolved in ethanol in a covered vessel at room temperature with vigorous stirring using a mixing instrument like magnetic stirrer. While stirring, propylene glycol is added. On magnetic stirrer or water bath, this mixture is heated to 30°C. In a different vessel the water was heated to 30°C is added, the mixture is covered and stirred for five minutes. Using the sonication or extrusion methods, the ethosomal formulation vesicle size can be reduced to the desired extent. Finally, the mixture is refrigerated for storage.[43]
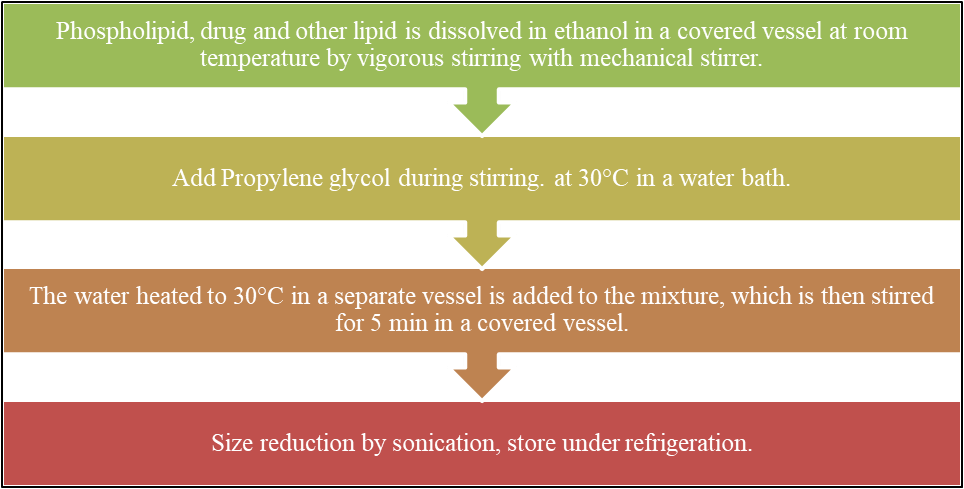
Fig 4: Method of preparation of Ethosomes by Cold method
2. Hot method
This process involves heating phospholipid in a water bath at 40°C till a colloidal solution is produced. Propylene glycol and ethanol are combined and heated to 40°C in another container. The organic phase is added to the aqueous phase when both solutions are at 40°C. Based on if the medicine is hydrophilic or hydrophobic, it dissolves in either water or ethanol. The extrusion or sonication methods can be used to reduce vesicle size to the desired extent.[44]
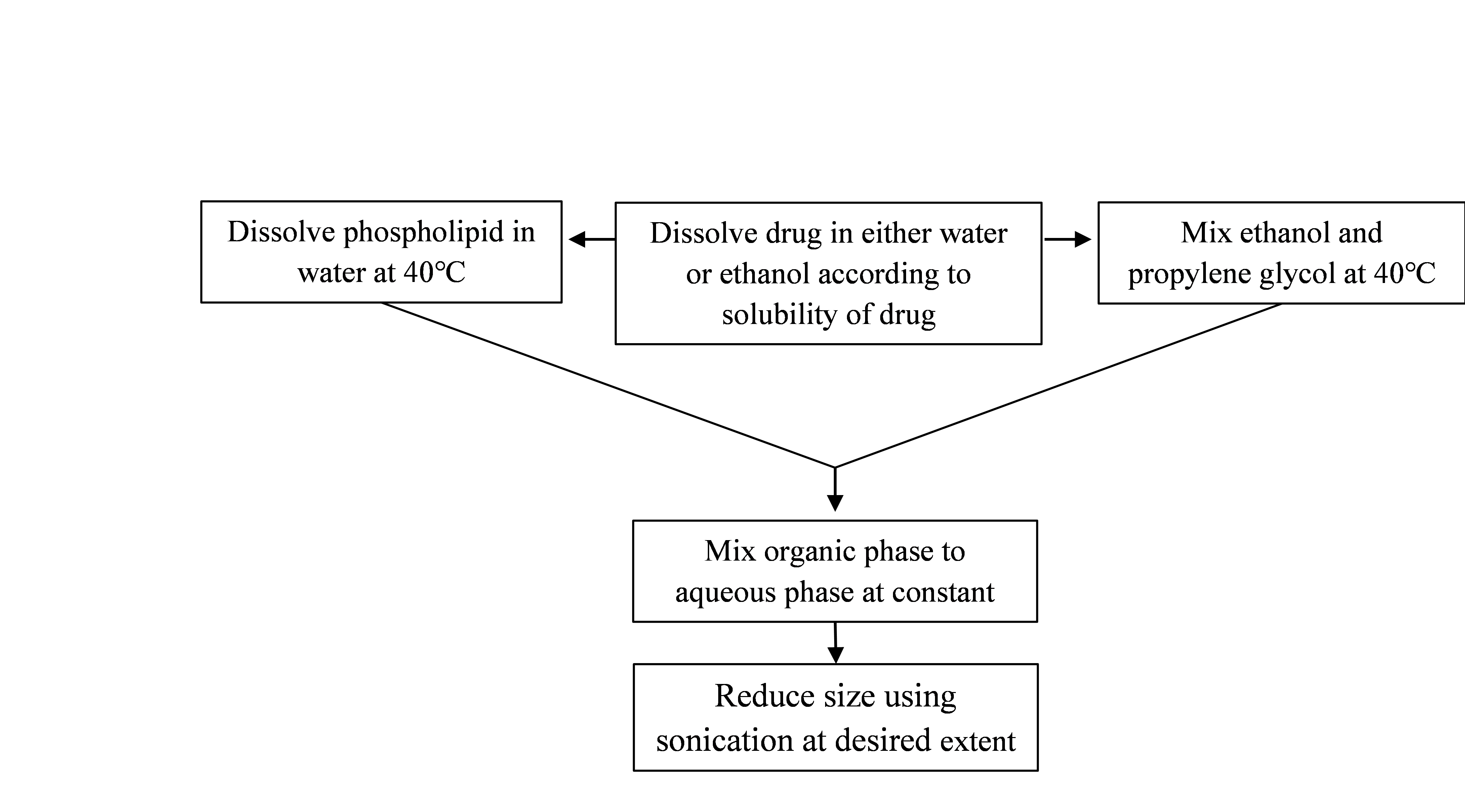
Fig 5: Method of preparation of Ethosomes by Hot method
3. Classic mechanical dispersion method
Phospholipid can be dissolved in an organic solvent or in a mixture of organic solvents in a round bottom flask (RBF). To produce a thin lipid film on the RBF wall, the organic solvent is removed using a rotary vacuum evaporator above the lipid transition temperature. Any leftover solvent should be separated from the accumulated lipid film by keeping it in a vacuum overnight. At the proper temperature, rotate the flask with or without sonication to hydrate the lipid layer with the drug's hydro ethanol solution. Finally, let the ethosomal suspension to settle at the room temperature. It is recommended that the mixture be kept refrigerated.[45]
Evaluation of Ethosomes [46,47]
1. Vesicle shape
Transmission electron microscopy (TEM) or scanning electron microscopy (SEM) are two methods for visualizing ethosomes. Electron microscope visualization shows a vesicular structure with a diameter of 300–400 nm in an ethosomal formulation. The vesicles imperfect circular form suggests that they are flexible.
2. Vesicle size and Zeta potential
Particle size and zeta potential can be determined by dynamic light scattering (DLS) using a computerized inspection system and photon correlation spectroscopy (PCS).
3. Entrapment efficiency
The ultracentrifugation technique can be utilized to determine the drug's entrapment efficiency in ethersomes. Because lipid produces a bilayer structure that holds the drug properly, the chemical nature of the lipid has a significant role in determining the EE of the drug in the ethersomes. The vesicles undergo a 30-minute centrifugation process at 2000 rpm while they are kept at room temperature. The clear supernatant was collected to calculate the amount of drug analysed by UV spectroscopy for drug content after dilution using suitable solvent.
% Entrapment = Actual content/Theoretical content x 100
Where, DE - Amount of drug in the ethosomal sediment
DT- Theoretical amount of drug used to prepare the formulation
(Equal to amount of drug in supernatant liquid and in the sediment).
4. Transition temperature
DSC measures the transition temperature (T) of vesicular lipids in duplicate in an aluminum pan with a steady nitrogen stream and a heating rate of 10°C per minute between 20°C -300°C.
5. Drug content
A UV spectrophotometer can be used to determine the drug content of the ethosomes. A modified high performance liquid chromatographic method can also be used to measure it.
6. Surface tension measurement
A Du Nouy ring tensiometer can be used to evaluate a drug's surface tension activity in aqueous solution using the ring method.
7. Stability studies
Ethosomal preparations drug-retentive behaviour can be evaluated by storing them at various temperatures, such as 25 ± 2°C (room temperature, RT) and 37 ± 2°C, over various periods of time (1, 20, 40, 60, 80, and 120 days). After being flushed with nitrogen, the ethosomal preparations were stored in sealed vials with a capacity of 10 ml. By employing DLS and TEM to track the vesicles size and shape, the stability of ethosomes was also measured.
8. Skin permeation studies
The method of confocal laser scanning microscopy (CLSM) is employed to determine the depth of ethosomes penetration into skin. The combined action of ethanol and phospholipid, ethosomes exhibit noticeably greater skin deposition, offering a delivery method for both dermal and transdermal application.
9. In vitro drug release study
In vitro drug release study and drug deposition of Ethosomal preparation can be performed by Franz diffusion cell with artificial or biological membrane or egg membrane, Dialysis bag diffusion at room temperature 37 ±0.5?. Then collect sample at specific time interval, dilute it with suitable solvent and analysed using UV spectrophotometer.
Incorporation of Ethosomes into Gel
By adding ethosomes to gel formulations with the right gelling agents, medication transport through the skin can be greatly improved. The method used is determined by the specific medication properties and desired features of the final gel product. Every method has benefits and can be improved for improved transdermal application performance. Ethosomes are added to gel compositions to increase their stability and effectiveness. Gels offer a practical and easy-to-use application form that can enhance patient compliance, making them the perfect medium for ethosome delivery. Furthermore, the gel matrix can protect ethosomes from external influences, maintaining their integrity and extending the time that the medication is released from the encapsulation.
Gelling Agents used in Ethosomal Gels
Ethosomal gels are novel transdermal drug delivery formulations that make use of ethosomes, which are lipid-based carriers boosted with ethanol. Gelling agents are essential for stabilizing these formulations, increasing their viscosity, and enhancing the drug release and skin adhesion qualities. They produce a gel matrix that makes it easier for the drugs to pass through the skin. Every gelling agent has distinct benefits, and the choice of one can be determined by the formulation's particular needs.
Types of Gelling Agents
a. Synthetic Gelling Agents
These agents are often highly efficient at increasing viscosity and stabilizing formulations.
Examples: Carbomer
b. Semisynthetic Gelling agents
These are modified natural polymers that combine properties of both natural and synthetic gelling agents.
Examples: HPMC
c. Natural Gelling Agents
Natural gelling agents are biocompatible and often preferred for their safety and lower irritation potential.
Examples: Agar, Xanthan Gum, Sodium Alginate.
Importance of Gelling Agents in Ethosomal Gels
a. Enhanced Stability: The structural integrity of ethosomes is preserved by gelling agents, which stop them from leaking or aggregating.
b. Controlled Release: The viscosity of gelling agents can slow down the release of the active ingredient and allowing for sustained therapeutic effects.
c. Improved Skin Penetration: Gelling agents can enhance the permeation of ethosomal formulations through the skin by modifying the diffusion properties of the gel.
d. Improved Adhesiveness: Drug effectiveness and contact time are increased when gels prepared with the right gelling agents adhere to the skin better.
e. Patient Experience: Patients frequently prefer gels over liquid formulations, and the incorporation of efficient gelling agents can improve the gel's visual qualities, including texture, spreadability, and appearance. It could have a major impact on patient compliance and acceptance.
Examples of Common Gelling Agents
1. Carbomer (Carbopol)[48]
Properties: Carbomers are high molecular weight polyacrylic acids that can swell in water, creating a thick gel.
Mechanism: They undergo a neutralization process that leads to increased viscosity, improving the stability and spreadability of ethosomal gels.
Applications: Widely used in topical formulations, it enhances the residence time of ethosomal gels on the skin, allowing for prolonged drug release.
2. Hydroxypropyl Methylcellulose (HPMC)[49]
Properties: HPMC is a non-ionic, cellulose-based polymer that provides gel-like consistency and enhances the viscosity of ethosomal formulations.
Mechanism: It forms a gel network through hydrogen bonding, which helps in the controlled release of the drug.
Applications: Used in ethosomal gels to improve viscosity and enhance the stability of the formulation.
3. Xanthan Gum [50]
Properties: Xanthan gum is a polysaccharide that is highly effective at thickening and stabilizing aqueous solutions.
Mechanism: It increases the viscosity of the ethosomal gel and enhances its bioadhesive properties, promoting better skin penetration.
Applications: Commonly used in ethosomal gels to enhance the viscosity and stability of the formulation.
4. Sodium Alginate [51]
Properties: Sodium alginate is a natural polysaccharide that can form gels in the presence of divalent cations like calcium.
Mechanism: It interacts with ethosomes, providing a gel matrix that can regulate drug release profiles.
Applications: Often combined with ethosomes to improve drug delivery and retention, particularly in topical applications.
Characterization of Ethosomal Gels [52,53,54]
1. Appearance
The appearance have to check visually. After gelling the clarity of the formulations, colour, is determine by visual examination of the formulations under light, alternatively against white and black background.
2. pH
Mix 1g of gel in 100ml of water and pH was checked using pH meter. The electrode was dip into the formulation at room temperature and the readings were noted.
3. Viscosity
Viscosity was determined by Brookfield viscometer spindle S63 at specific rpm and the values were noted.
4. Drug content
1g equivalent of gel was taken and dissolved in suitable solvent and filtered using Whatman filter paper. The volume was made up to 10ml with solvent. The resultant solution was suitably diluted and absorbance is measure using UV spectroscopy.
5. Spreadability
Spreadability of gel is determine by weight and glass slide apparatus. A measured amount of gel was placed on fixed glass slide, and other glass slide is place over the fixed glass slide then weight put on glass slide such that the gel is placed between two glass slides for 5 mins. The weight was continuously removed. Spreadability was determined using the formula.
S= M/T
Where, S - spreadability in g/s, M - mass in grams & T – time in sec.
5. Washability
After applying ethosomal gel to the skin, the ease of water washing were physically evaluated.
6. Extrudability
In order to determine the extrudability of ethosomal gel, it was loaded into either aluminum or metal collapsible tubes. The formulation's extrudability was examined after the tubes were compressed to extrude the material.
7. Invitro drug release
The Invitro drug release studies of ethosomal gels were studied using Franz diffusion cell with artificial or biological membrane or egg membrane, at room temperature 37 ±0.5?. Then collect sample at specific time interval, dilute it with suitable solvent and analysed using UV spectrophotometer.
8. Release kinetics
In order to elucidate mode and mechanism of drug release, the invitro data was transformed and interpreted at graphical interface constructed using various kinetic models. The zero order release, First order release, Higuchi model, Peppas model.
9. Stability studies
The stability studies of optimized batch were performed as per ICH guidelines Q1(R2). Samples were analysed at periodic time intervals for 3 months for the estimation of appearance, phase separation, pH and drug content, record the observation.
CONCLUSION
A conclusion can be drawn from the above review that ethosomes is a promising drug delivery system. The introduction of ethosomes has created an entirely novel path for the successful distribution of medications with varying physicochemical properties across the skin for both local and systemic effects. Ethosomes are specially tailored lipid vesicles containing high ethanol, which provides better skin permeability than the other lipid vesicles and targeting to deeper skin layers for various skin diseases. Ethosomes do offer a decent chance for the non-invasive drug delivery of little, medium, and for the large size of drug molecules. Ethosomes are easy to prepare, stable, and safe for use. After two decades of development, ethosomes have proved their potential to transport medicinal substances via the skin without causing adverse reactions.
Incorporation of ethosomes in suitable vehicles such as gels offers better skin permeability, improve bioavailability and therapeutic effects. Various ethosomal preparations are available in the market. However, Further studies like stability and human subject study are to be done to have this vesicular system to be more effective and then could increase ultimately the patient compliance
REFERENCES
- Rastogi V, Yadav P. Transdermal drug delivery system: An overview. Asian Journal of Pharmaceutics (AJP). 2012;6(3).
- Udapurkar PP, Kamble SR, Biyani KR. Ethosomes novel vesicular carriers for enhancing transdermal drug delivery. International journal of pharmaceutical and chemical sciences. 2015;4(1):170-90.
- Nurleni N, Iskandarsyah AA, Aulia A. Formulation and penetration testing of ethosome azelaic acid on abdominal skin white male rats (Rattus norvegicus) with franz diffusion cell. Asian J Pharm Clin Res. 2018 Apr;11(4):327-30.
- Bhalaria MK, Naik S, Misra AN. Ethosomes: a novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian journal of experimental biology. 2009 May 1;47(5):368-75.
- Jadhav PK, Kapadnis KA, Shinkar DM, Pathan VT, Jadhav AG. Ethosomes as Novel Drug Delivery System: International Journal of Pharmaceutical Sciences Review & Research. Int. J. Pharm. Sci. Rev. Res. 2020 May;62(1):173-82.
- Michaels AS, Chandrasekaran SK, Shaw JE. Drug permeation through human skin: theory and in vitro experimental measurement. AIChE journal. 1975 Sep;21(5):985-96.
- Maheswary T, Nurul AA, Fauzi MB. The insights of microbes’ roles in wound healing: A comprehensive review. Pharmaceutics. 2021 Jun 29;13(7):981.
- Gilaberte Y, Prieto-Torres L, Pastushenko I, Juarranz Á. Anatomy and Function of the Skin. InNanoscience in dermatology 2016 Jan 1 (pp. 1-14). Academic Press.
- Yousef H, Alhajj M, Sharma S. Anatomy, skin (integument), epidermis.(2017)
- Shirshin EA, Gurfinkel YI, Priezzhev AV, Fadeev VV, Lademann J, Darvin ME. Two-photon autofluorescence lifetime imaging of human skin papillary dermis in vivo: assessment of blood capillaries and structural proteins localization. Scientific reports. 2017 Apr 26;7(1):1171.
- Ramadon D, McCrudden MT, Courtenay AJ, Donnelly RF. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug delivery and translational research. 2021 Jan 20:1-34.
- Vijayakumar KS, Parthiban S, Senthilkumar GP, Mani TT. Ethosomes-A new trends in vesicular approaches for topical drug delivery. Asian J. of Res Pharm Sci and Biotech. 2014;2(1):23-30.
- Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes—novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. Journal of controlled release. 2000 Apr 3;65(3):403-18
- Bhalaria MK, Naik S, Misra AN. Ethosomes: a novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian journal of experimental biology. 2009 May 1;47(5):368-75.
- Geetha K, Harshika A, Nandini KG, Rao TR. Formulation and Evaluation of Bifonazole Ethosomal Gel for Enhanced Topical Delivery. Journal of Coastal Life Medicine. 2023 May 29;11:1747-56.
- Ainbinder D, Paolino D, Fresia M, Touitou E. Drug delivery applications with ethosomes. Journal of biomedical nanotechnology. 2010 Oct 1;6(5):558.
- Kumar GP, Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta pharmaceutica sinica B. 2011 Dec 1;1(4):208-19.
- Pradhan M, Singh D, Singh MR. Novel colloidal carriers for psoriasis: current issues, mechanistic insight and novel delivery approaches. Journal of controlled release. 2013 Sep 28;170(3):380-95.
- Maheshwari RG, Tekade RK, Sharma PA, Darwhekar G, Tyagi A, Patel RP, Jain DK. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: a comparative assessment. Saudi pharmaceutical journal. 2012 Apr 1;20(2):161-70.
- Touitou E, Godin B, Weiss C. Enhanced delivery of drugs into and across the skin by ethosomal carriers. Drug development research. 2000 Jul;50(3?4):406-15.
- Patrekar PV, Inamdar SJ, Mali SS, Mujib MT, Ahir AA, Hosmani AH. Ethosomes as novel drug delivery system: A review. The pharma innovation. 2015 Nov 1;4(9, Part A):10.
- Pawar P, Kalamkar R, Jain A, Amberkar S. Ethosomes: a novel tool for herbal drug delivery. IJPPR Human. 2015;3:191-202.
- Aggarwal D, Nautiyal U. Ethosomes: A review. Int J pharm med res. 2016 Aug 10;4(4):354-63.
- Vijayakumar KS, Parthiban S, Senthilkumar GP, Mani TT. Ethosomes-A new trends in vesicular approaches for topical drug delivery. Asian J. of Res Pharm Sci and Biotech. 2014;2(1):23-30.
- Upadhyay N, Mandal S, Bhatia L, Shailesh S, Chauhan P. A review on ethosomes: an emerging approach for drug delivery through the skin. Recent Research in Science and Technology. 2011;3(7):19-24.
- Kesharwani R, Patel DK, Sachan A, Kumar V, Mazumdar B. Ethosomes: A novel approach for transdermal and topical drug delivery. Research Journal of Topical and Cosmetic Sciences. 2015;6(1):15-20.
- Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug development and industrial pharmacy. 2003 Jan 1;29(9):1013-26.
- Manosroi A, Jantrawut P, Khositsuntiwong N, Manosroi W, Manosroi J. Novel elastic nanovesicles for cosmeceutical and pharmaceutical applications. Chiang Mai J Sci. 2009 May 1;36(2):168-78.
- Horwitz E, Pisanty S, Czerninski R, Helser M, Eliav E, Touitou E. A clinical evaluation of a novel liposomal carrier for acyclovir in the topical treatment of recurrent herpes labialis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1999 Jun 1;87(6):700-5.
- Jain S, Umamaheshwari RB, Bhadra D, Jain NK. Ethosomes: a novel vesicular carrier for enhanced transdermal delivery of an antiHIV agent. Indian journal of pharmaceutical sciences. 2004;66(1):72-81.
- Dayan N, Touitou E. Carriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. liposomes. Biomaterials. 2000 Sep 1;21(18):1879-85.
- Godin B, Touitou E. Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. Journal of controlled release. 2004 Feb 10;94(2-3):365-79.
- Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E. Cannabidiol—transdermal delivery and anti-inflammatory effect in a murine model. Journal of controlled release. 2003 Dec 12;93(3):377-87.
- Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, Levi-Schaffer F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials. 2001 Nov 15;22(22):3053-9.
- Jain NK, editor. Controlled and novel drug delivery. New Delhi: CBS publishers & distributors; 1997.
- Esposito E, Menegatti E, Cortesi R. Ethosomes and liposomes as topical vehicles for azelaic acid: a preformulation study. International Journal of Cosmetic Science. 2004 Oct;26(5):270-1.
- Kim JC, Lee MH, Rang MJ. Minoxidil-containing dosage forms: skin retention and after-rinsing hair-growth promotion. Drug Delivery. 2003 Jan 1;10(2):119-23.
- Touitou E, Godin B, Wiess C. Novel therapeutic technology-ethosmal drug delivery system. Drug Development Research. 2000;50:406-15.
- Mohanty D, Mounika A, Bakshi V, Haque MA, Sahoo CK. Ethosomes: a novel approach for transdermal drug delivery. Int. J. ChemTech Res. 2018;11(8):219-26.
- Zahid SR, Upmanyu N, Dangi S, Ray SK, Jain P, Parkhe G. Ethosome: a novel vesicular carrier for transdermal drug delivery. Journal of drug delivery and therapeutics. 2018 Nov 15;8(6):318-26.
- Godin B, Touitou E. Erythromycin ethosomal systems: physicochemical characterization and enhanced antibacterial activity. Current drug delivery. 2005 Jul 1;2(3):269-75.
- Paolino D, Lucania G, Mardente D, Alhaique F, Fresta M. Ethosomes for skin delivery of ammonium glycyrrhizinate: in vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. Journal of controlled release. 2005 Aug 18;106(1-2):99-110.
- Aggarwal D, Nautiyal U. Ethosomes: A review. Int J pharm med res. 2016 Aug 10;4(4):354-63.
- Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: An overview. Journal of advanced pharmaceutical technology & research. 2010 Jul 1;1(3):274-82.
- Mishra D, Mishra PK, Dabadghao S, Dubey V, Nahar M, Jain NK. Comparative evaluation of hepatitis B surface antigen–loaded elastic liposomes and ethosomes for human dendritic cell uptake and immune response. Nanomedicine: Nanotechnology, Biology and Medicine. 2010 Feb 1;6(1):110-8.
- Dhurve R, Kashyap N, Mishra A, Pathak AK. A holistic review on ethosome: a promising drug delivery system for topical fungal disease. Int J Pharm Biol Arch. 2014;5(05):13-26.
- Chauhan AS, Pandey K, Girijesh AJ, Dubey B, Jain P. A review on Ethosome: a novel drug delivery system for topical fungal disease. The pharma innovation journal. 2018;7(12):355-62.
- Bisht D, Verma D, Mirza MA, Anwer MK, Iqbal Z. Development of ethosomal gel of ranolazine for improved topical delivery: in vitro and ex vivo evaluation. Journal of molecular liquids. 2017 Jan 1;225:475-81.
- Abdallah MH, Lila AS, Unissa R, Elsewedy HS, Elghamry HA, Soliman MS. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech. 2021 Nov 11;22(8):269.
- Nsengiyumva EM, Alexandridis P. Xanthan gum in aqueous solutions: Fundamentals and applications. International Journal of Biological Macromolecules. 2022 Sep 1;216:583-604.
- Tanwar YS, Jain AK. Formulation and evaluation of topical diclofenac sodium gel using different gelling agent. Asian J Pharm Res Health Care. 2012;4(1):1-6.
- Manjanna KM, Krishna GS, Keerthana PH. Studies on Development and Evaluation of Topical Ethosomal Gel Embedded Antifungal Agent for Athlete's Foot. RGUHS Journal of Pharmaceutical Sciences. 2023;13(2).
- Khan WA, Sharma V, Maurya P, Bijauliya RK. Development and characterization of oxiconazole nitrate loaded ethosomal gel for treating fungal infections. World J Pharmacol Res. 2019 Jul 8;8(10):1341-56.
- Paroliya R, Jain NP, Banke A. Formulation development and evaluation of ethosomal gel of amphotericin b for treatment of fungal infections. Int J Pharm Drug Res. 2019;7:10-8


 Jaysing Giram*
Jaysing Giram*
 Rashmi Mahabal
Rashmi Mahabal
 Namita Bhosale
Namita Bhosale
 Vijay Jagtap
Vijay Jagtap





 10.5281/zenodo.14275673
10.5281/zenodo.14275673