Abstract
Microemulsions are thermodynamically stable dispersions of two immiscible liquids, typically oil and water, stabilized by surfactant molecules and often cosurfactants. These systems have garnered significant attention due to their unique properties, including optical transparency, high stability, and ability to solubilize both hydrophilic and hydrophobic substances. This abstract provides an overview of the fundamental principles governing the formation and stability of microemulsions, as well as their applications in various fields such as pharmaceuticals, cosmetics, food, and chemical industries. The structural characteristics, phase behavior, and factors influencing microemulsion formation are discussed, along with recent advances in formulation techniques and characterization methods. Furthermore, the potential challenges and future prospects of microemulsions in industrial applications are highlighted, emphasizing their role as promising delivery systems for enhancing the solubility, stability, and bioavailability of active compounds
Keywords
Thermodynamically, dispersion, immiscible, surfactant, cosurfactants, transperancy, emphasizing, bioavailability
Introduction
Microemulsions, comprising oil, water and a surfactant, in association with some co-surfactant, are thermodynamically stable systems. They have found applications in a large number of chemical and pharmacological processes due to their unique properties such as large interfacial area, low interfacial tension, and most importantly, the ability to solubilize and deliver hydrophobic drugs. In addition to the oral and intravenous route, they are suitable for drug delivery through the ophthalmic, vaginal, pulmonary, dental, and topical routes. This review highlights the properties and several recent developments in the use of microemulsions for medical treatment purposes including targeted drug delivery.
Microemulsions comprise a special class of “dispersion” that may be transparent or translucent in appearance They were first discovered by Hoar and Schulman (1943) in their experimental study of titration of long-chain fatty acids (soapy milky emulsions) with medium-/short-chain alcohols producing translucent or transparent system of emulsions. A schematic representation of the titration method adopted to produce is given below, which highlights the formation of transparent emulsion from water-in-oil (W/O) emulsion stabilized by long-chain fatty acids (soap)
Advantages of micro emulsion
Microemulsion offer several advantages in various applications:
- Enhanced Solubility:
Microemulsion can solubilize both polar and non-polar compounds, making them suitable for formulations requiring enhanced solubility of active ingredients.
- Improved Stability:
They have a thermodynamically stable structure, which prevents phase separation over time. This stability extends the shelf life of products, such as pharmaceuticals and cosmetics.
- Optimized Delivery:
Microemulsions enable controlled and targeted delivery of active ingredients, allowing for improved bioavailability and efficacy. This makes them valuable in pharmaceutical and topical formulations.
- Increased Absorption:
Their small droplet size enhances the surface area available for interaction with biological membranes, facilitating better absorption of drugs or nutrients.
- Ease of Manufacture:
Microemulsions are relatively easy to prepare and can be manufactured using conventional techniques, lowering production costs and time.
- Versatility:
Microemulsions can be tailored to specific applications by adjusting the composition of surfactants, co-surfactants, and oils. This versatility makes them applicable in various industries, including pharmaceuticals, cosmetics, and agriculture.
- Transparency:
Many microemulsions are transparent or translucent, making them aesthetically pleasing for use in clear formulations, such as skincare products and oral solutions.
- Environmentally Friendly:
Microemulsions can reduce the need for organic solvents, leading to environmentally friendly formulations with lower toxicity and flammability risks.
Disadvantage s of micro emulsion
- Phase separation:
Micro emulsions can be prone to phase separation over time, particularly if not properly formulated or stored under unfavorable conditions.
- Limited loading capacity:
Due to their small droplet size, micro emulsions may have limited capacity for loading high concentrations of active ingredients, which could limit their effectiveness in certain applications.
- Potential toxicity:
Some surfactants and co-surfactants used in micro emulsion formulations may have toxicity concerns, especially if they are not thoroughly removed during product manufacturing.
- Environmental impact:
Disposal of micro emulsion formulations may pose environmental concerns, particularly if they contain non-biodegradable or hazardous components.
- Energy consumption:
The production of micro emulsions typically requires high-energy processes such as high-pressure homogenization, which can increase production costs and energy consumption.
STRUCTURE OF MICROEMULSION

Types of microemulsion
Microemulsions are thermodynamically stable colloidal dispersions of two immiscible liquids (usually oil and water), stabilized by an interfacial film of surfactant molecules. There are three main types of microemulsions:
- Oil-in-water (O/W) microemulsions:
In O/W microemulsions, oil droplets are dispersed within a continuous water phase.
Surfactant molecules surround the oil droplets, with their hydrophilic ("water-loving") heads oriented towards the water phase and their hydrophobic ("water-hating") tails facing the oil droplets. This type of microemulsion is commonly used in pharmaceuticals, cosmetics, and food industries due to its stability and ease of formulation.
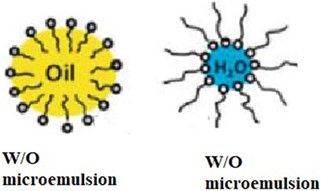
- Water-in-oil (W/O) microemulsions:
In W/O microemulsions, water droplets are dispersed within a continuous oil phase. Surfactant molecules surround the water droplets, with their hydrophobic tails oriented towards the oil phase and their hydrophilic heads facing the water droplets. W/O microemulsions are less common than O/W microemulsions but find applications in areas such as enhanced oil recovery and controlled release formulations.
- Bi-continuous microemulsions:
Bi-continuous microemulsions, also known as middle-phase microemulsions, have no clear distinction between oil and water phases. Instead, the system consists of interconnected domains of both oil and water, with surfactant molecules partitioned at the interfaces between these domains. Bi-continuous microemulsions have unique properties and find applications in areas such as drug delivery, emulsion polymerization, and soil remediation. Each type of microemulsion offers distinct advantages depending on the desired application, such as enhanced solubilization, controlled release, or improved stability. The choice of surfactants and co-surfactants, as well as the composition of the oil and water phases, can be tailored to achieve specific properties and functionalities in microemulsion formulations.
Characteristics of microemulsion
Microemulsions possess several key characteristics that make them advantageous for various applications:
- Thermodynamic stability:
Microemulsions are thermodynamically stable systems, meaning they remain dispersed over time without phase separation. This stability arises from the balance of interfacial tension and the energy of mixing between the components.
- Transparent or translucent appearance:
Microemulsions typically have a clear or translucent appearance, making them visually appealing for use in formulations such as cosmetics and pharmaceuticals.
- Nano-sized droplets:
The dispersed phase in microemulsions consists of nano-sized droplets, typically ranging from 10 to 100 nanometers in diameter. This small droplet size contributes to their stability and enhances properties such as optical clarity and surface area.
- Enhanced solubilization:
Microemulsions can solubilize hydrophobic and hydrophilic compounds simultaneously due to the presence of both oil and water phases. This property is advantageous for formulating products with poorly soluble active ingredients.
- Improved bioavailability:
Microemulsions can enhance the bioavailability of poorly soluble drugs by increasing their solubility and facilitating absorption. This is particularly beneficial for pharmaceutical formulations aimed at improving drug delivery and therapeutic efficacy.
- Tunable properties:
The properties of microemulsions, such as droplet size, viscosity, and phase behavior, can be tailored by adjusting the composition of the oil, water, and surfactant phases. This allows for precise control over formulation characteristics to meet specific application requirements.
- Ease of manufacturing:
Microemulsions are often relatively easy to prepare using simple mixing techniques, making them attractive for large-scale production in industries such as pharmaceuticals, personal care, and agrochemicals.
- Versatility:
Microemulsions have diverse applications across various industries, including pharmaceuticals, cosmetics, food, agrochemicals, and enhanced oil recovery. Their versatility stems from their ability to encapsulate and deliver active ingredients, stabilize emulsions, and improve the performance of formulated products.
METHOD OF PREPARATION
- Phase titration method
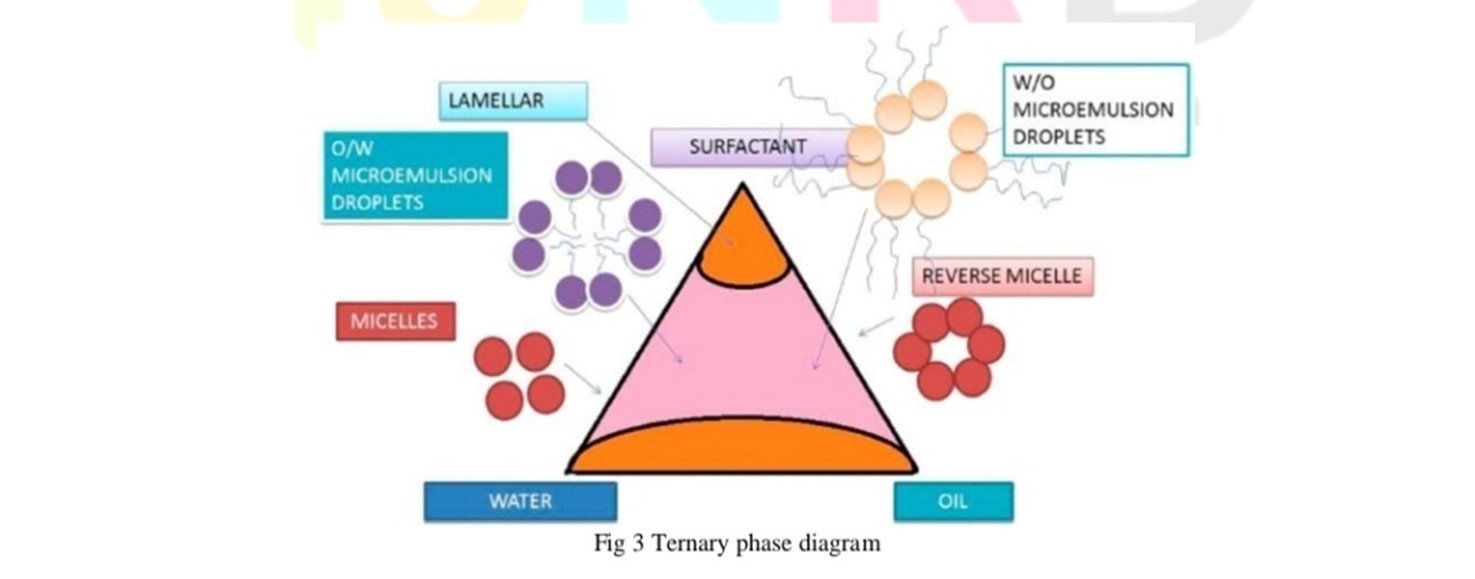
Microemulsions are prepared by the spontaneous emulsification method (phase titration method) and can be depicted with the help of phase diagrams. Construction of phase diagram is a useful approach to study the complex series of interactions that can occur when different components are mixed. Microemulsions are formed along with various association structures (including emulsion, micelles, lamellar, hexagonal, cubic, and various gels and oily dispersion) depending on the chemical composition and concentration of each component. The understanding of their phase equilibria and demarcation of the phase boundaries are essential aspects of the study. As quaternary phase diagram (four component system) is time consuming and difficult to interpret, pseudo ternary phase diagram is often constructed to find the different zones including microemulsion zone, in which each corner of the diagram represents 100% of the particular component. The region can be separated into w/o or o/w microemulsion by simply considering the composition that is whether it is oil rich or water rich. Observations should be made carefully so that the metastable systems are not included.
- Phase Inversion Method
In the phase inversion method phase inversion of microemulsions occurs by addition of excess amount of the dispersed phase. During phase inversion quick physical changes occur including changes in particle size that can affect drug release both in vivo and in vitro. For nonionic surfactants ,this can be completed by changing the temperature, forcing a transition from oil in water microemulsion at low temperatures to water in oil microemulsion at higher temperatures (transitional phase inversion). During cooling, the system crosses a point of zero spontaneous curvature and minimal surface tension, promoting the formation of finely dispersed oil droplets. This method is also known as phase inversion temperature (PIT) method Instead of the temperature, other parameters such as salt concentration or pH value may be considered as well instead of the temperature alone Additionally, a transition in the spontaneous radius of curvature can be obtained by changing the water volume fraction. By successively adding water into oil initially water droplets are formed in a continuous oil phase. Increasing the water volume fraction changes the spontaneous curvature of the surfactant from initially stabilizing a w/o microemulsion to an o/w microemulsion at the inversion locus. Short-chain surfactants form flexible monolayers at the o/w interface resulting in a bi-continuous microemulsion at the inversion point.
Formation of micro emulsion
Microemulsions are thermodynamically stable, isotropic mixtures of oil, water, and surfactant (sometimes with a co-surfactant) formed spontaneously or with minimal energy input. They typically form when mixing oil, water, and surfactant in certain proportions. The process involves several steps:
- Selection of Components:
Choosing suitable oil, water, and surfactant/co-surfactant components based on their compatibility and ability to form a stable microemulsion.
- Phase Behavior Study:
Understanding the phase behavior of the components, including the determination of phase boundaries and the identification of microemulsion regions.
- Mixing:
Combining the oil, water, and surfactant/co-surfactant components in the appropriate proportions under suitable conditions (e.g., temperature, agitation) to promote microemulsion formation.
- Energy Input:
In some cases, the system may require minimal energy input, such as gentle stirring or sonication, to facilitate the formation of the microemulsion.
- Phase Inversion:
In systems containing a co-surfactant, phase inversion may occur, leading to the formation of different types of microemulsions (e.g., oil-in-water to water-in-oil).
- Characterization:
Analyzing the formed microemulsion for stability, droplet size, viscosity, and other relevant properties to ensure it meets the desired specifications. Overall, the formation of microemulsions relies on the delicate balance between the interfacial tension reduction provided by the surfactant and co-surfactant, along with the compatibility of the oil and water phases.
THEORIES OF MICROEMULSION FORMULATION
The formulation of microemulsion is based on various theories that effect and control their stability and phase behavior. These theories are
- Thermodynamic theory
- Solubilisation theory
- Interfacial theory
- Thermodynamic theory .
Formuation and stability of microemulsion can be expressed on the basis of a simplified thermodynamic machanism. The free energy of microemulsion formation can be dependent on the extent to which surfactant lowers the surface tension of the oil–water interface and the change in entropy of the system, Thus
DG f = ?DA - T DS
Where,
DG f = Free Energy of formation,
? =Surface Tension of the oil–water interface,
DA = Change in interfacial area on microemulsification,
DS = Change in entropy of the system which is effectively the dispersion entropy,
T = Temperature.
It is found that when a microemulsion is formed, DA is changed to a large extent due to the large number of very small droplets formed. It is must to know that while the value of ? is positive at all times, it is very small, and is offset by the entropic component. The dominant favorable entropic contribution is the very large dispersion entropy arising from the mixing of one phase in the other in the form of large numbers of small droplets. However, favorable entropic contributions also come from other dynamic processes such as monomer-micelle surfactant exchange and surfactant diffusion in the interfacial layer. When large reductions in surface tension are found by significant favorable entropic change, a negative free energy of formation is achieved. In that case, microemulsification is spontaneous and the resulting dispersion is thermodynamically stable.
- Solubilisation theory
The formation of microemulsion is oil soluble phase and water phase by micelles or reverse micelles in micellar gradually become larger and swelling to a certain size range results.
- Interfacial theory
The interface mixed-film theory i.e a negative interfacial tension theory, according to this theory the micro-emulsion has been capable to form instantaneous and spontaneously generate a negative interfacial tension in the surfactant and co-surfactant in working together. The film, which may consist of surfactant and cosurfactant molecules, is considered as a liquid ‘‘two dimensional’’ third phase in equilibrium with both oil and water. Such a monolayer could be a duplex film, i.e. giving different properties on the water side and oil side. According to the duplex film theory, the interfacial tension ?T is given by the following expression
?T = ?(O/W) --- ?
Where,
? (O/W)a = Interfacial Tension( reduced by the presence of the alcohol).
? (O/W)a is significantly lower than ?(O/W) in the absence of the alcohol.
Challenges and future direction of Microemulsion
Microemulsions offer a wide range of applications such as targeted drug delivery, sustained drug delivery, controlled drug delivery, enzyme immobilization, enhancing bioavailability, and masking taste. Since orally delivered hydrophilic drugs are unstable in the gastrointestinal tract (GIT), new approaches must be found consisting of the use of biocompatible moieties for active targeting in clinical trials. Additionally, W/O microemulsions hinder the water-soluble drug molecules from being metabolized. W/O microemulsions, in addition to aqueous fluids, are converted to O/W microemulsions and, hence, release the active pharmaceutical ingredient (API), allowing the microemulsions to selectively release API to the targeted regions of GIT. A challenge in microemulsions is achieving stability over time, as they are thermodynamically unstable systems. This can involve finding the right combination of surfactants, cosurfactants, and oils to prevent phase separation and maintain the desired properties. Additionally, controlling droplet size and distribution is crucial for applications such as drug delivery or enhanced oil recovery. Overall, microemulsions offer a versatile platform for the development of stable, effective, and targeted formulations across multiple industries.
EVALUTION FACTOR OF MICROEMULSION
The microemulsions are evaluated by the subsequent techniques. They
- .Visual Inspection:
For visual inspection microemulsion is inspect visually for homogeneity, optical clarity, and fluidity.
- Examination under Cross-polarizing Microscope:
The microemulsion systems are subjected to examination under cross polarizing. microscope for the absence of birefringence to exclude liquid crystalline systems.
- Limpidity Test (Percent Transmittance):
The limpidity of the microemulsion is measured spectrophotometrically using spectrophotometer.
- Globule size and zeta potential measurements
The globule size and zeta potential of the micro emulsion may be determined by dynamic light scattering, employing a Zetasizer HSA 3000.
- Viscosity measurements Rheological
behaviour of the formulation is observed by employing a Brookfield LVDV ???+ cone and plate (CP) viscometer using rheocal software at a temperature.
- Electrical conductivity
The water phase was added drop wise a mix of oil, surfactant and co-surfactant and also the electrical conductivity of formulated samples are often measured employing a conductometer (CM 180 conductivity meter, Elico, India) at ambient temperature and at a continuing frequency of 1 Hz.
- Drug stability
The optimized microemulsion was kept under cold condition o (48 C), temperature and at elevated temperature (50 ± 2 o C). After every 2 months the microemulsion are often analyzed for phase separation, % transmittance, globule size and zippers assay.
APPLICATIONS
- Parenteral Delivery:
Both O/W and W/O microemulsion can be used for parenteral delivery. The literature contains the details of the many microemulsion systems, few of these can be used for the parenteral delivery be because of toxicity of surfactant and parental use.
- Oral Delivery:
Microemulsion formulations offer the several benefits over conventional oral formulation including increased absorption, improved clinical potency, and decreased drug toxicity. Therefore, microemulsion have been reported to be ideal delivery of drugs such as steroids, hormones, diuretic and antibiotics.
- Topical Delivery:
Topical administration of drugs have advantages like avoidance of hepatic first pass metabolism of the drug and targetability of the drug to affected area of the skin or eyes.The use of lecithin/IPP/water microemulsion for the transdermal transport of indomethacin and diclofenac has also been reported.
- Ocular and Pulmonary Delivery:
For the treatment of eye diseases, drugs are essentially delivered topically.O/W microemulsions have been investigated for ocular administration, to dissolve poorly soluble drugs, to increase absorption and to attain prolong release
- Microemulsions in cosmetics:
It is believed that microemulsion formulation will result in a faster uptake into the skin. Cost, safety, appropriate selection of ingredients are key factors in the formulation of micro emulsions. Unique microemulsions as hair care products contain an amino-functional polyorganosiloxane and an acid and/or a metal salt.
- Microemulsions in agrochemicals:
Microemulsions have a variety of applications in agrochemical industry, of which pesticide containing systems are relatively old. The ease of handling and lower requirement of smelly solvents go in favour of the use of micro emulsions. Microemulsions formulated with a hydrotope solubilizing the herbicide can be promising. The much finer droplet size of the microemulsion leads to higher penetrability, much larger contact area of the active substance to the treated surface and a much more even distribution during application
- Nasal delivery:
Microemulsions are now being studied as a delivery system to enhance uptake across nasal mucosa. Addition of a mucoadhesive polymer helps in prolonging the residence time on the mucosa.
- Tumor targeting:
The utility of microemulsions as vehicles for the delivery of chemotherapeutic or diagnostic agents to neoplastic cells while avoiding normal cells. A method for treating neoplasms, wherein neoplasms cells have an increased number of LDL (low density, lipoprotein) receptors compared to normal cells. The micro emulsion comprised of a nucleus of cholesterol esters and not more than 20% triglycerides surrounded by a core of phospholipids and free cholesterol and contained a chemotherapeutic drug. The microemulsions could then be incorporated into cells via receptors for LDL and delivered the incorporated molecules. Thus, higher concentration of anticancer drugs could be achieved in the neoplastic cells that have an increased expression of the receptors. In this way toxic effects of these drugs on the normal tissues and organs could be avoid.
CONCLUSIONS
Microemulsions are versatile and stable colloidal systems composed of water, oil, surfactant, and sometimes cosurfactants. They offer several advantages, including enhanced solubilization of hydrophobic drugs, increased bioavailability, ease of preparation, and potential for controlled drug release. Additionally, microemulsions can be tailored to specific applications due to their tunable properties, such as droplet size, viscosity, and drug loading capacity. However, challenges remain in optimizing their formulation for large-scale production and ensuring long-term stability. Further research is needed to fully understand their behavior, mechanisms of drug release, and potential toxicity before widespread application in pharmaceuticals and other industries.
REFERENCE
- Kunieda H. et al. The Journal of Physical Chemistry 1988; 92: 185.
- 1. Patel M.R. et al. Microemulsions: As Novel Drug Delivery Vehicle. Pharma infonet 2007; 5 (6).
- Danielsson I, Lindman B: The definition of microemulsion. Colloid Surf, 1981; 3: 391-392.
- Kumar. K. Senthil et al. Microemulsions as Carrier for Novel Drug Delivery: A Review. International Journal of Pharmaceutical Sciences Review and Research, 2011; 10: 37-45.
- Lawrence MJ, Rees GD. Micro emulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev., 2000; 45(1): 89-121. http://dx.doi.org/10.1016/S0169-409X(00)00103-4.
- Flanagan J, Singh H. Microemulsions: a potential delivery system forbioactives in food. Crit Rev Food Sci Nutr, 2006; 46(3): 221-37. http://dx.doi.org /10.1080/104 08690590956710;PMid:16527754.
- Jadhav KR, Shaikh IM, Ambade KW, Kadam VJ. Applications of micro emulsion based drug delivery system. Curr Drug Deliv, 2006; 3(3): 267-73. http://dx.doi.org/10.2174/156720106777731118;PM id:16848728.
- Sharma, N., Antil, V. and Jain, S., 2013. Microemulsion: A review. Asian Journal of Pharmaceutical Research and Development, pp.23 36.
- Yadav, S., Kawtikwar, P.S., Sakarkar, D.M., Gholse, Y.N. and Ghajbhiye, S.D., 2009. Microemulsion: A Review. Research Journal of Pharmacy and Technology, 2(3), pp.441-448.
- International Journal of Pharmacy and Pharmaceutical Sciences, 5(1), pp.96-102. 8.Onah chinweM., Mbah chuka J., 2019.Pharmaceutical Microemulsion Gel :Functioning as a Drug delivery system. Journal of Chemical and Pharmaceutical Reaserch, 11(10), p.p 48- 55.
- Syamasri Gupta and S.P. Moulik. Biocompatible microemulsions and their prospective uses in drug delivery. Journal of Pharmaceutical Sciences. 2008; 97: 22-45.
- Ramadan, r., Devarajan, p.v.; Micro emulsion Indian Drugs, 2003; 139-146.
- Sahu, G.K., Sharma, H., Gupta, A. and Kaur, C.D., 2015. Advancements in microemulsion based drug delivery systems for better therapeutic effects. International journal of pharmaceutical sciences and developmental research, 1(1), pp.008-015.
- Singh, V., Bushettii, S.S., Raju, A.S., Ahmad, R., Singh, M. and Bisht, A., 2011. Microemulsions as promising delivery systems: a review. Ind J Pharm Edu Res, 4, p.54.
- Shaji, J., Reddy, M.S.; Micro emulsion as drug delivery system, Parma Times, 2004; 139-146.
- Jadhav. K.R. et al. Design and Evaluation of Microemulsion Based Drug Delivery System. International Journal of Advances in Pharmaceutical Sciences. 2010; 1: 156-166.
- Malmsten. M. pharmaceuticals Microemulsions In Handbook in of Microemulsion. Science and Technology. Marcel Dekker. Inc. New York. 1999; p 755.
- Kumar, K. S., Dhachinmoorthi. D., Saravanan. R., Udaykumar. G., Shanmugam. V., 2011.Microemulsion as carrier for Novel drug delivery : A Review. International journal of pharmaceutical science review and research., vol (10), p.p 37-45


 Rutuja D. Amle*
Rutuja D. Amle*
 Vitthal Gawade
Vitthal Gawade



 10.5281/zenodo.11046034
10.5281/zenodo.11046034