Abstract
Smart nanocarriers have become a pivotal innovation in cancer therapy, enhancing the delivery of therapeutic agents to tumor sites while minimizing systemic toxicity. These nanocarriers, including liposomes, polymeric nanoparticles, gold nanoparticles, exosomes, and carbon-based materials, have revolutionized the way drugs are administered in oncology. Their ability to overcome limitations of traditional treatments, such as poor drug solubility, limited bioavailability, and off-target effects, makes them a promising approach for targeted drug delivery. The mechanisms of targeting, such as passive targeting via the enhanced permeability and retention (EPR) effect and active targeting through receptor-mediated endocytosis, further enhance the specificity of drug delivery. Recent advances in stimuli-responsive nanocarriers and theranostic platforms have opened new avenues for personalized medicine, where treatment can be adjusted based on the tumor environment and patient characteristics. However, despite the considerable progress, there are still challenges in terms of scalability, regulatory approval, and long-term safety that need to be addressed. This review explores the current state of smart nanocarriers in cancer therapy, their mechanisms of action, clinical applications, and the future prospects that continue to shape this dynamic field.
Keywords
Smart nanocarriers, revolutionized, gold nanoparticles, toxicity
Introduction
Cancer remains a significant global health burden, necessitating innovative therapeutic strategies to enhance treatment specificity and efficacy. Conventional cancer therapies, including chemotherapy and radiotherapy, often lack selectivity, leading to damage to healthy tissues and severe side effects. This non-specificity limits their therapeutic potential, driving the need for targeted drug delivery systems[1].
The Role of Targeted Drug Delivery
Targeted drug delivery systems are designed to deliver therapeutic agents directly to tumor sites, minimizing systemic toxicity and enhancing drug efficacy. These systems leverage unique characteristics of the tumor microenvironment, such as the enhanced permeability and retention (EPR) effect, overexpressed receptors, and acidic pH. Among these systems, smart nanocarriers stand out due to their ability to carry and release drugs in a controlled manner in response to specific stimuli, such as pH changes, temperature, or enzymatic activity.
Advantages of Smart Nanocarriers
Smart nanocarriers, including liposomes, polymeric nanoparticles, and dendrimers, have demonstrated significant potential in oncology due to their versatile properties:
- Enhanced Drug Targeting: Functionalized nanocarriers with ligands (e.g., folic acid, transferring, or peptides) enable selective interaction with cancer-specific receptors.
- Controlled Drug Release: Stimuli-responsive systems ensure that drugs are released only within the tumor microenvironment, reducing off-target effects.
- Reduced Multi-Drug Resistance: By bypassing traditional drug resistance mechanisms, nanocarriers ensure better drug accumulation in tumor tissues.
Recent Advances
Emerging technologies have enhanced the functionality of smart nanocarriers. For example, theranostic platforms now integrate diagnostic imaging capabilities with therapeutic functions, enabling real-time monitoring of treatment efficacy. Additionally, combining nanocarriers with immunotherapy and gene therapy is opening new avenues in personalized cancer treatment[2].
Future Directions
Despite their promise, challenges such as manufacturing scalability, stability, and regulatory hurdles need to be addressed. Future research focuses on improving biocompatibility, reducing production costs, and expanding clinical translation of smart nanocarriers.
This review delves into recent advancements in smart nanocarrier technologies, highlights their clinical applications, and discusses future prospects to address existing limitations [3].
Fig 1: Nanocarriers in Drug Delivery
Types of Smart Nanocarriers for Targeted Cancer Therapy
1. Liposomes
Liposomes are spherical vesicles formed from a phospholipid bilayer encapsulating an aqueous core. They can carry hydrophilic drugs within the core and hydrophobic drugs in the bilayer. Functionalized liposomes, such as PEGylated liposomes, extend circulation time and reduce immune clearance. Stimuli-responsive liposomes can release drugs under specific conditions, such as acidic pH in tumors. Recent developments include ligand-targeted liposomes for enhanced tumor specificity.
2. Polymeric Nanoparticles
Constructed from biodegradable polymers like PLGA or PCL, polymeric nanoparticles provide controlled and sustained drug release. They can be tailored with targeting ligands or stimuli-responsive coatings to release drugs in the tumor microenvironment. For instance, nanoparticles designed with pH-sensitive properties enable drug release specifically in acidic tumor sites, improving therapeutic outcomes [4].
3. Dendrimers
Dendrimers are highly branched, nanoscale polymers with a central core and multiple surface functional groups. These groups can be modified to carry drugs, targeting ligands, or imaging agents. Their precise structure allows for multifunctionality, enabling simultaneous drug delivery and imaging in cancer treatment.
4. Micelles
Micelles are formed from amphiphilic molecules, with a hydrophobic core for drug encapsulation and a hydrophilic shell for stability in aqueous environments. They are particularly effective for delivering poorly water-soluble drugs. Stimuli-responsive micelles, such as redox-sensitive micelles, are gaining attention for targeted drug release in tumor tissues [5]
5. Quantum Dots (QDs)
Quantum dots are nanoscale semiconductor particles with unique optical properties. They combine drug delivery with imaging capabilities, enabling real-time tracking of therapeutic responses. While their use in cancer therapy shows promise, concerns regarding long-term toxicity and biocompatibility need to be addressed.[6]
6. Hydrogel-Based Nanocarriers
Hydrogels are three-dimensional polymeric networks that can absorb water and encapsulate therapeutic agents. As nanocarriers, they are effective for controlled drug delivery, especially for large biomolecules like proteins or nucleic acids. Stimuli-responsive hydrogels are being developed to release drugs in response to environmental cues like pH or enzymes.
7. Exosomes and Biomimetic Nanocarriers
Exosomes are natural nanocarriers secreted by cells, used in intercellular communication. They are biocompatible and can be engineered to carry therapeutic agents. Similarly, biomimetic nanocarriers, such as nanoparticles cloaked with cell membranes, mimic natural biological processes to evade the immune system and enhance targeting.[7]
8. Hybrid Nanocarriers
Hybrid nanocarriers combine features of different systems, such as liposomes and polymeric nanoparticles, to achieve multifunctionality. These carriers can encapsulate drugs and imaging agents simultaneously, offering both therapeutic and diagnostic capabilities. They represent a cutting-edge approach to theranostic applications in cancer therapy.[8]
Mechanisms of Targeting in Smart Nanocarriers for Cancer Therapy
Smart nanocarriers are engineered to deliver therapeutic agents specifically to tumor cells while minimizing side effects to healthy tissues. These carriers use several mechanisms to achieve precise targeting, which can be broadly classified into passive targeting, active targeting, and triggered targeting.[9]
1. Passive Targeting (Enhanced Permeability and Retention Effect - EPR Effect)
The most widely studied mechanism for targeting is passive targeting, which relies on the tumor's unique characteristics. Tumor tissues often have irregular blood vessels with larger pores (200-800 nm), making them more permeable than normal tissue. This allows nanoparticles to accumulate within tumors through the Enhanced Permeability and Retention (EPR) effect. Once nanoparticles enter the tumor interstitial space, they tend to remain there due to limited lymphatic drainage, leading to an increased concentration of the therapeutic agent at the site. Liposomes, polymeric nanoparticles, and dendrimers have been designed to exploit this effect.[10]
2. Active Targeting
Active targeting involves functionalizing nanoparticles with ligands or antibodies that specifically bind to overexpressed receptors or antigens on cancer cells. This targeted approach improves the specificity of drug delivery to tumor cells and reduces off-target effects.
Common ligands include:
Antibodies and Antibody Fragments: These can be attached to the surface of nanocarriers to recognize tumor-associated antigens, such as HER2 for breast cancer or EGFR for various cancers.
Folate Receptors: Cancer cells often overexpress folate receptors, particularly in cancers like ovarian and lung cancer. Folate-functionalized nanoparticles exploit this overexpression for targeted drug delivery.
Peptides and Aptamers: These small biomolecules can be selected to bind to specific proteins overexpressed on cancer cells. They offer high specificity and can be engineered to enhance the targeting ability of nanocarriers.[11]
3. Triggered (Stimuli-Responsive) Targeting
Triggered targeting relies on the tumor microenvironment to release the therapeutic payload from the nanocarrier. Various environmental stimuli, including pH, temperature, and enzyme activity, can trigger drug release in cancer cells. For instance, the tumor environment is often acidic due to the rapid proliferation of cells, and pH-sensitive nanoparticles can release their contents under these conditions. Similarly, redox-sensitive nanoparticles can release their payloads in response to the high levels of reactive oxygen species (ROS) present in tumor tissues. [12]
Examples include:
pH-Sensitive Nanocarriers: Polymeric nanoparticles or liposomes designed to release their drug contents at low pH, common in tumor tissues. Enzyme-Sensitive Nanocarriers: Some nanocarriers are designed to release drugs upon cleavage by tumor-specific enzymes, such as matrix metalloproteinases (MMPs), which are upregulated in many cancers . Thermal and Light-Triggered Nanocarriers: Nanocarriers, like gold nanoparticles, can be activated by external stimuli such as near-infrared (NIR) light or temperature, which enhances localized drug release or induces tumor cell death.
4. Multifunctional Targeting
Recent advancements combine multiple targeting strategies, such as integrating both passive and active targeting or combining active targeting with stimuli-responsive release. Multifunctional carriers can carry diagnostic agents (for imaging) alongside therapeutic agents, enabling "theranostics" (therapeutic + diagnostic) for real-time monitoring of treatment efficacy.[13]
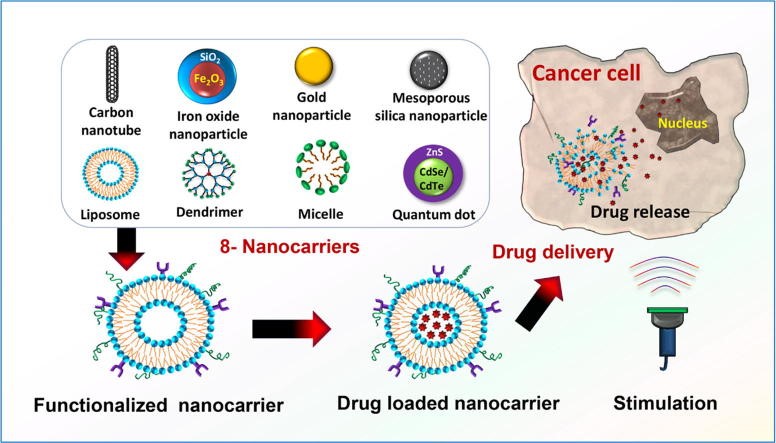
Fig 2: Mechanism of Nanocarriers
Recent Advances in Smart Nanocarriers for Targeted Cancer Therapy
Recent advancements in nanocarriers for cancer therapy have been driven by innovations in targeting mechanisms, materials, and delivery systems. Here are some of the most notable developments:
1. Hybrid Nanocarriers for Enhanced Targeting and Drug Delivery
Hybrid nanocarriers combine two or more materials to harness the advantages of each, such as the combination of liposomes and nanoparticles for enhanced drug loading, stability, and controlled release. For instance, liposome-nanoparticle hybrids have been developed to improve the targeting ability of chemotherapy agents by integrating the stability and drug-loading capacity of nanoparticles with the bioavailability and surface modification of liposomes. These hybrid systems have shown promise in increasing the accumulation of therapeutic agents in tumor tissues and improving treatment outcomes.[14]
2. Stimuli-Responsive Nanocarriers
Stimuli-responsive systems that can release drugs in response to changes in the tumor microenvironment (e.g., pH, temperature, or redox) are a major breakthrough. For instance, pH-sensitive nanoparticles have been developed using polymers that undergo conformational changes at low pH, commonly found in tumor environments. These carriers release their drug payload only when they reach the acidic tumor site, thereby minimizing side effects and improving therapeutic efficacy. This approach is being expanded to include dual- or multi-stimuli-responsive systems that react to multiple signals like both pH and temperature or light and magnetic fields.[15]
3. Nanocarriers for Personalized Medicine
Personalized cancer therapy has greatly benefited from the development of nanocarriers tailored to individual tumor characteristics. Recent advances include the design of nanoparticles that are functionalized with specific ligands or antibodies that recognize unique tumor markers on individual patients’ cancer cells. For example, nanoparticles conjugated with HER2 antibodies for breast cancer or folate receptors for ovarian cancer are enabling more precise targeting. These strategies are supported by diagnostic tools such as biomarkers and imaging techniques, enhancing the effectiveness of precision medicine.[16]
4. Nanocarriers for Combination Therapy
Recent innovations in nanocarriers are facilitating combination therapies, where multiple therapeutic agents, including chemotherapy, immunotherapy, and gene therapy, are delivered simultaneously to cancer cells. For example, some nanocarriers combine chemotherapeutic agents with immune checkpoint inhibitors to enhance the immune response against tumors. These combination therapies, delivered by multifunctional nanocarriers, aim to overcome drug resistance and enhance overall therapeutic efficacy.
5. Biomimetic Nanocarriers
Biomimetic nanocarriers, designed to mimic the properties of natural biological systems, are an emerging field in cancer therapy. These nanocarriers, such as exosomes or liposomes with biological coatings, exploit the body's natural systems for better interaction with cancer cells. For instance, red blood cell-derived nanoparticles have been shown to evade the immune system while targeting tumors effectively. Similarly, exosome-based carriers are gaining attention due to their natural ability to interact with tumor cells and facilitate drug delivery.[17]
6. Advancements in Nanocarrier Imaging and Monitoring
Integrating imaging agents into smart nanocarriers has been a significant development, enabling real-time tracking of drug delivery and monitoring treatment responses. Nanocarriers that are combined with fluorescent dyes or magnetic resonance imaging (MRI) contrast agents allow clinicians to visualize the distribution of drugs within the body. This advance enables more precise dosing and provides immediate feedback on the effectiveness of the therapy.[18]
Clinical Applications of Smart Nanocarriers in Cancer Therapy
Smart nanocarriers have transitioned from research laboratories to clinical trials and therapeutic applications, significantly advancing cancer treatment. These nanocarriers provide precision in drug delivery, reducing systemic toxicity and improving efficacy. Below are key clinical applications:
1. Liposome-Based Drug Delivery Systems
Liposomes are among the earliest nanocarriers to reach clinical application. Drugs like Doxil® (doxorubicin liposomal formulation) have been approved for treating ovarian cancer, Kaposi’s sarcoma, and multiple myeloma. Liposomes encapsulate chemotherapeutic agents, minimizing their systemic toxicity while enhancing accumulation in tumor tissues via the EPR effect.[19]
2. Polymeric Nanoparticles in Targeted Therapy
Polymeric nanoparticles like Abraxane®, an albumin-bound paclitaxel, are used in clinical settings to treat breast, pancreatic, and non-small cell lung cancers. This formulation improves the solubility and bioavailability of paclitaxel and facilitates active targeting.[20]
3. Gold Nanoparticles in Imaging and Therapy
Gold nanoparticles (AuNPs) are being explored for photothermal therapy and drug delivery. AuroShell® is in clinical trials for photothermal ablation in head and neck cancers. AuNPs enable localized tumor heating upon exposure to near-infrared light, effectively killing cancer cells.[21]
4. Carbon-Based Nanocarriers
Graphene oxide and carbon nanotubes are gaining attention for their ability to deliver drugs and genes. Although still largely in clinical trials, carbon nanocarriers have demonstrated potential in delivering cisplatin and doxorubicin with high efficiency in preclinical studies.
5. Exosome-Based Drug Delivery
Exosome-mimicking nanocarriers are being investigated for delivering RNA and protein-based therapies. These biomimetic carriers naturally evade immune clearance and are ideal for delivering small interfering RNA (siRNA) and microRNA (miRNA). Clinical trials using exosomes for targeted delivery are ongoing for diseases like pancreatic and colorectal cancers.[22]
6. Theranostics for Cancer Management
Theranostic nanocarriers integrate therapy with diagnostics, enabling real-time tracking of drug delivery. Quantum dots and magnetic nanoparticles are among the most explored in clinical settings for combined imaging and treatment, particularly in breast and prostate cancers.[23]
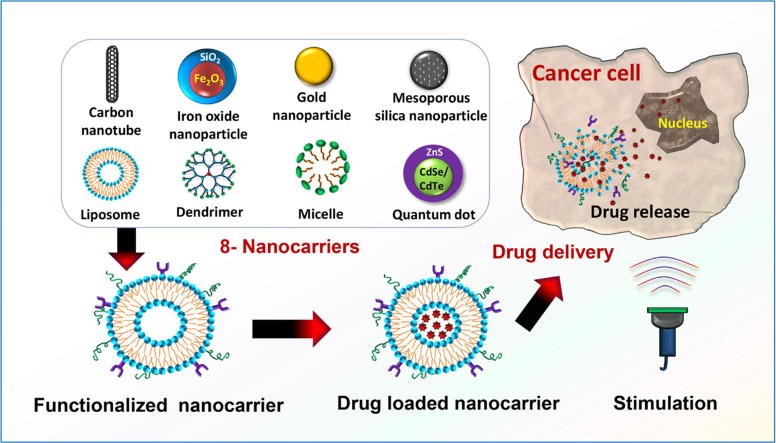
Fig 3: Applications of Nanocarriers
Future Prospects of Smart Nanocarriers in Cancer Therapy
The field of smart nanocarriers is rapidly evolving, with a strong focus on overcoming current limitations and expanding their applications in cancer therapy. Below are some key future directions:
1. Integration with Artificial Intelligence (AI)
AI-driven algorithms are expected to optimize the design and synthesis of nanocarriers by predicting their biological behavior and improving their targeting efficiency. Machine learning can aid in creating patient-specific delivery systems tailored to unique tumor profiles.[24]
2. Advances in Multi-Stimuli Responsive Systems
Future nanocarriers will combine multiple stimuli responses (e.g., pH, temperature, redox potential) to enhance targeted drug release. These carriers can be programmed to release drugs in a sequential manner, mimicking combination therapy approaches.[25]
3. Development of Hybrid Nanocarriers
Hybrid nanocarriers that combine organic (e.g., lipids, polymers) and inorganic (e.g., silica, gold) materials are gaining attention. These systems synergize the advantages of both types, offering improved stability, functionalization, and payload capacity.[26]
4. Expansion of RNA Nanomedicine
Delivery of RNA-based therapeutics (e.g., siRNA, mRNA) using smart nanocarriers is anticipated to revolutionize cancer treatment. Lipid nanoparticles (LNPs) are leading this progress, especially after their success in COVID-19 vaccines.[27]
5. Immunotherapy and Smart Nanocarriers
Nanocarriers designed to enhance immune responses, such as delivering immune checkpoint inhibitors or cytokines, are a growing area. This includes innovations in cancer vaccines that combine antigen delivery with adjuvants.[28]
6. Enhancing Biodegradability and Safety
Improving the biocompatibility and biodegradability of smart nanocarriers is critical for their long-term clinical use. Efforts include the development of fully biodegradable systems that minimize accumulation and toxicity.[29]
CONCLUSION
Smart nanocarriers have demonstrated considerable potential in transforming cancer therapy by enabling targeted, efficient, and controlled drug delivery. These advanced delivery systems allow for enhanced specificity in tumor treatment, reducing the side effects commonly associated with traditional chemotherapy. Various types of nanocarriers, including liposomal systems, polymeric nanoparticles, and gold-based platforms, have shown promising results in both preclinical and clinical settings. The targeting mechanisms employed by these nanocarriers, such as utilizing the enhanced permeability and retention (EPR) effect or receptor-targeted strategies, ensure that therapeutic agents are delivered directly to cancer cells, minimizing damage to healthy tissues. Recent advancements, particularly in the development of stimuli-responsive nanocarriers and theranostic platforms, offer a high level of customization, allowing for dynamic treatment based on real-time tumor characteristics, thereby advancing personalized medicine. Despite these advancements, challenges remain. Issues related to large-scale production, cost-effective manufacturing, and stringent regulatory frameworks hinder the widespread application of these systems in clinical practice. Moreover, long-term safety concerns, such as potential toxicity and immune system interactions, must be carefully addressed to ensure the broader use of nanocarriers in routine cancer therapy. Nonetheless, the integration of artificial intelligence and machine learning in the design and development of nanocarriers is expected to further optimize their functionality, paving the way for innovative treatments. As research progresses and technological hurdles are overcome, smart nanocarriers hold immense promise in revolutionizing the treatment landscape for cancer, offering more effective, safer, and personalized therapeutic options for patients.
REFERENCES
- Singh, A. et al. (2023). Role of Smart Nanocarriers in Cancer Therapy. Pharmaceutical Advances. DOI:10.1039/D3RA02969G
- Li, J. et al. (2023). Recent Advances in Targeted Drug Delivery Strategies for Enhancing Oncotherapy. Pharmaceutics, 15(9), 2233. DOI:10.3390/pharmaceutics15092233.
- Peng, Z.-H. et al. (2022). Targeted Drug Delivery to Improve Cancer Therapy. Pharmaceutics, 14(8), 1725. DOI:10.3390/pharmaceutics14081725.
- Kumar, P., et al. (2023). Smart Nanocarriers for Targeted Cancer Therapy: Recent Developments and Challenges. Journal of Drug Delivery Science and Technology, 85, 104223.This paper highlights the design and functionalization of liposomes, polymeric nanoparticles, and dendrimers for active targeting in oncology.
- Ghosh, S., et al. (2023). Advances in Stimuli-Responsive Nanocarriers for Targeted Cancer Drug Delivery. Materials Today Chemistry, 29, 101255. Focuses on stimuli-sensitive carriers like polymeric nanoparticles and mesoporous silica nanoparticles with mechanisms to enhance tumor-specific drug release.
- Xie, X., et al. (2022). Functionalized Gold Nanoparticles for Photothermal and Chemotherapy Applications in Cancer. Nanoscale Advances, 4(3), 674-689.Discusses the dual role of gold nanoparticles in drug delivery and photothermal therapy.
- Ali, M. R. K., et al. (2023). Carbon-Based Nanocarriers for Cancer Therapy: A Comprehensive Review. ACS Nano, 17(5), 4231-4254.Covers the advancements in carbon nanotubes and graphene oxide for delivering therapeutics and diagnostics.
- Chakraborty, S., et al. (2023). Emerging Applications of Exosomes and Biomimetic Nanocarriers in Cancer Therapy. Journal of Controlled Release, 354, 211-226.Explores the application of biomimetic nanocarriers like exosomes for precision medicine in cancer treatment.
- Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36-48. A comprehensive discussion on the role of passive and active targeting in liposomal drug delivery.
- Hare, J. I., et al. (2017). Challenges and strategies in anti-cancer nanomedicine development: A critical appraisal. Nature Reviews Cancer, 17, 20–37. Explores targeting mechanisms including the EPR effect and receptor-mediated active targeting.
- Danhier, F., et al. (2010). Targeting the tumor microenvironment with nanocarriers: A potential strategy to overcome drug resistance. Journal of Controlled Release, 141(2), 141-152.
- Torchilin, V. P. (2014). Passive and active drug targeting: Drug delivery to tumors as an example. Handbook of Experimental Pharmacology, 197-210. Examines both passive and active targeting mechanisms in cancer therapy.
- Shi, J., et al. (2016). Cancer nanomedicine: Progress, challenges and opportunities. Nature Reviews Cancer, 16, 20–37.
- Wang, H., et al. (2023). Hybrid nanoparticles for synergistic cancer therapy. Nano Today, 51, 101641.
- Rosenblum, D., et al. (2018). Progress and challenges towards targeted cancer nanotherapies. Nature Communications, 9(1), 1410. Discusses the integration of multifunctional targeting and advanced imaging capabilities in nanocarriers.
- Peer, D., et al. (2007). Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology,2(12),751-760.
- Sun, T., et al. (2022). Recent advances in stimuli-responsive nanocarriers for cancer therapy. Journal of Controlled Release, 348, 457-473. Focuses on pH-sensitive and multi-stimuli-responsive nanocarriers for controlled drug release.
- Blanco, E., et al. (2015). Nanomedicine in cancer therapy: Innovative trends and emerging challenges. Cancer-Science,106(8),993-1001. Explores cutting-edge trends in theranostics and personalized nanocarriers for cancer therapy.
- Barenholz, Y. (2012). Doxil®—the first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release, 160(2), 117-134.
- Geraldes, C., et al. (2023). Current and emerging nanocarriers for breast cancer therapy. Journal of Nanobiotechnology, 21, 54.
- Zhang, Z., et al. (2020). Gold nanoparticles in cancer theranostics. Frontiers in Oncology,10,610499.
- Smyth, T., et al. (2015). Exosomes and nanotechnology: Emerging roles in cancer therapy. Molecular Therapy, 23(10), 1498-1508.
- Mitchell, M. J., et al. (2021). Engineering precision nanoparticles for drug delivery. Nature-Reviews,Drug-Discovery,20,101–124.
- Kulkarni, J. A., et al. (2021). Lipid nanoparticles enabling gene therapies: From concepts to clinical applications. Nature Reviews Drug Discovery, 20(9), 620-637.
- Torchilin, V. P. (2020). Multifunctional and stimuli-sensitive pharmaceutical nanocarriers for targeted therapy. Nature Reviews Drug Discovery, 19(9), 801-821.
- Ge, Z., et al. (2022). Recent advances in multi-stimuli-responsive nanocarriers for targeted cancer therapy. Chemical Society Reviews, 51(15), 6387-6417.
- Kang, H., et al. (2021). Hybrid nanocarriers for cancer-targeted drug delivery. Advanced Drug Delivery Reviews, 171, 83-101.
- Verbeke, R., et al. (2021). The dawn of mRNA vaccines: The COVID-19 case. Journal of Controlled Release, 333, 511-520.
- Goldberg, M. S. (2022). Nanoparticle platforms for cancer immunotherapy. Journal of Controlled Release, 348, 775-789


 Sachin Adsule* 1
Sachin Adsule* 1



 10.5281/zenodo.14267817
10.5281/zenodo.14267817