Abstract
The study aimed to establish stability assays for Diloxanide Furoate using both High-Performance Liquid Chromatography (HPLC) and High-Performance Thin-Layer Chromatography (HPTLC) methods, covering crucial aspects of method development and validation. Detailed specifications were provided for the HPLC and HPTLC systems to ensure proper setup and functioning. Chromatographic conditions were optimized, including parameters like stationary phase, mobile phase composition, flow rate, and detection wavelength, to achieve optimal separation and detection of Diloxanide Furoate. The identity of pure Diloxanide Furoate samples was confirmed using UV-visible wavelength scans and FT-IR spectra. Calibration curves demonstrated strong linearity, emphasizing method robustness. Method development involved optimizing quantitative analysis techniques, experimenting with various chromatographic conditions. Validation studies assessed specificity, linearity, accuracy, precision, and robustness, ensuring reliability for pharmaceutical analysis. System suitability testing evaluated parameters like retention time and peak symmetry, ensuring consistent chromatographic performance. The methods were successfully applied to analyze Diloxanide Furoate in active pharmaceutical ingredients (API) and formulations, demonstrating high accuracy. Forced degradation studies assessed stability under different conditions. The study provides comprehensive insights into stability assays for Diloxanide Furoate, affirming the suitability of HPLC and HPTLC methods for pharmaceutical analysis. It ensures the integrity and reliability of analytical results, offering valuable contributions to pharmaceutical research and development.
Keywords
Diloxanide Furoate, DSC, FT-IR, UV-Visible, HPLC AND HPTLC, HPTLC etc
Introduction
Diloxanide furoate is used in the treatment of various forms of Amoebiasis 1, 6. It is an excellent luminal amebicide and is indicated after treatment with the 5-nitroimidazole compounds, which have relatively weak activity on the cyst stage. Diloxanide furoate is an antiprotozoal medication primarily used in the treatment of Amoebiasis, an infection caused by the parasite Entamoeba histolytica. Here's a drug profile of Diloxanide furoate:
Drug Class: Antiprotozoal:
- Molecular Formula: C14H11Cl2NO4
- Molecular weight: 328.1 g/mol
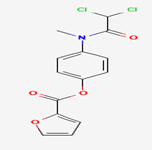
Figure 01: Structure and IUPAC name of Diloxanide furoate: [4-[(2, 2-dichloroacetyl)-methylamino] phenyl] furan-2-carboxylate.
Diloxanide is an antiprotozoal medication used primarily to treat infections caused by protozoa, particularly Amoebiasis, which is a condition caused by the parasite Entamoeba histolytica. It belongs to the nitroimidazole class of drugs and works by interfering with the DNA synthesis of the parasites, leading to their death. Diloxanide is typically administered orally and is well-absorbed from the gastrointestinal tract. Once absorbed, it undergoes metabolism in the liver, forming its active metabolite, Diloxanide furoate. This active metabolite is responsible for its therapeutic effects against protozoal infections. The medication is commonly used in combination with other drugs, such as Metronidazole, to ensure complete eradication of the parasites and prevent recurrence of the infection. It is considered a safe and effective treatment option for Amoebiasis when used as directed by a healthcare professional. In addition to Amoebiasis, Diloxanide may also be used in the treatment of certain other protozoal infections, although its primary indication remains the management of Amoebiasis. As with any medication, Diloxanide may cause side effects in some individuals, although they are usually mild and transient. It is important for patients to follow their healthcare provider's instructions carefully and report any adverse reactions experienced during treatment. Quantitative chemical analysis is an important tool that assures the use of crude substances and the intermediates to meet the requisite stipulations. Drug analysis is base for the determination of the product1. Every year numbers of drugs are brought in to market. Quality is most important in every an each product but it is essential in medicines as it involves with life. Generally, there is some delay in inclusion of a drug in pharmacopoeias from the date of its introduction to the market. This is so due to some probable changeableness in the prolong and broad use of these drugs, information of advanced toxicity, improvement of patient resistance and introduction more excellent drugs by rivalar. For such case, in Pharmacopoeias the remay unavailability of standard analytical methods for these drugs.2 Quality control is a concept, which strives to produce a perfect product by series of measures designed to prevent and eliminate errors at different stage of production. The decision to release or reject a product is based on one or more type of control action. With a increased growth of pharmaceutical industry during last several years, there has been rapid progress in the field of pharmaceutical analysis involving complex instrumentation. Providing a simple analytical procedure for complex formulation is a matter of most importance.3 Analytical chemistry is dealt in ascertaining the materials composition as elements (or compounds) contained in them.4 Analytical chemistry is a measurement science consisting of a group of effective ideas and the methods which are valuable in science and medicine. The ability to give up to date, correct and stable data is key to the duty of analytical chemists that particularly genuine in discovery4, development and manufacture of pharmaceuticals. Analytical data are advantageous for screening of potential drug candidates, in drug synthesis development, formulation studies, monitoring stability of bulk pharmaceuticals and formulated products and test final products for release.5 Thus most manufacturing industries entrust the qualitative, and quantitative chemical analysis to assure that raw material used meet absolute specifications and to verify the quality of final product6. Selection of better effective method is a bigger decision that is to be made by an analyst for a given analysis, in order to reach at the right decision, he must also be cognizant with the conditions under which each method is reliable, aware of possible interferences which may arise and competent to prevent such problems. Therefore, it is essential to develop new analytical methods for these drugs. Concisely the main reasons to develop newer methods of drugs analysis are: 6-7 Any Pharmacopoeias may not include the drug or with any other:
Appropriate analytical procedure for drug may unavailable in history due to the patent regulations may unavailable for drug as formulation excipients. Analytical method for any drug with combination may unavailable to quantify the drug in biological fluids. It may require expensive reagents & solvents for existing analytical procedures. The procedures require cumbersome extraction and also separation procedure and these may unsuitable. Analytical techniques are generally employed for drug analysis are spectral methods, chromatographic methods, electro analytical techniques, biological method and microbiological methods, physical methods, radioactive methods and other techniques like conventional titrimetric method, gravimetric and polarimetric methods. Diloxanide is an antiprotozoal agent primarily used in the treatment of Amoebiasis, an infectious disease caused by the protozoan parasite Entamoeba histolytica. Its pharmacology involves several key aspects:
Mechanism of Action:
Diloxanide works by inhibiting the growth and reproduction of protozoa, particularly Entamoeba histolytica. It interferes with the DNA synthesis of the parasite, leading to its death.
Metabolism:
After oral administration, Diloxanide is well-absorbed from the gastrointestinal tract. It undergoes extensive metabolism in the liver, primarily by hydrolysis, to form its active metabolite, Diloxanide furoate. This active metabolite is responsible for the therapeutic effects against protozoal infections.
Excretion:
Diloxanide and its metabolites are excreted primarily via the urine. The elimination half-life of Diloxanide is relatively short, typically ranging from a few hours to about 12 hours, depending on individual factors such as renal function and dosage regimen.
Antimicrobial Spectrum:
Diloxanide is specifically effective against Entamoeba histolytica, the causative agent of Amoebiasis. It may not be effective against other types of protozoa or bacteria.
Therapeutic Uses:
The primary indication for Diloxanide is the treatment of intestinal Amoebiasis, including both asymptomatic cyst passers and symptomatic amoebic infections. It is often used in combination with other antiprotozoal agents, such as Metronidazole, to achieve complete eradication of the parasite and prevent recurrence of the infection.
Adverse Effects:
Diloxanide is generally well-tolerated, with few reported adverse effects. Common side effects may include gastrointestinal disturbances such as nausea, vomiting, diarrhea, and abdominal discomfort. Allergic reactions and hypersensitivity reactions are rare but possible.
Drug Interactions:
Diloxanide may interact with other medications, particularly those that affect liver enzymes involved in its metabolism. Concurrent use with drugs that induce or inhibit these enzymes may affect the efficacy or toxicity of Diloxanide.
Experimental:
Chemicals and reagents:
HPLC and HPTLC grade Acetonitrile, Methanol, and glacial acetic acid were obtained from Rankem HPLC and HPTLC grade Acetonitrile, Methanol, and glacial acetic acid were obtained from Rankem (Mumbai, India). Pure sample of Diloxanide Furoate drug was obtained from Unichem Remedies, Mumbai, India. Ultra pure water obtained from Milli-Q academic system (Millipore Pvt. Ltd., Bangalore, India) was used to prepare all solutions for the method. The procured samples were tested to confirm their identity and this included UV-visible wavelength scan, and recording of FT-IR spectra. FT-IR spectra were recorded at ARACOP Dhule. The sample was prepared as a KBr pellet for recording the spectra. The UV-Visible spectra of Diloxanide Furoate were recorded using methanol as solvent was recorded using water as solvent on SICAN 2301 Instrument.
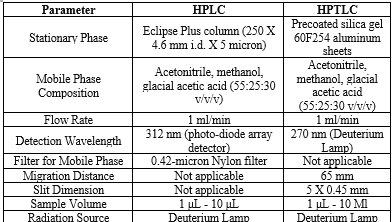
Chromatographic Conditions:
Table no 01: Chromatographic Conditions for HPLC and HPTLC:
Preparation of Standard Solutions:
- Prepare stock solutions of Diloxanide Furoate of known concentrations to be used for method calibration and validation.
- Ensure the accuracy and precision of the stock solutions through appropriate dilutions and analytical techniques.
Validation of the method:
The proposed analytical method was validated as per the International Conference on Harmonization (ICH) guidelines Q2 (R1)25: Linearity Precision Robustness Specificity Accuracy Linearity Precision Robustness. Validation of the proposed method Validation of the proposed methods was performed in accordance with the International Conference on Harmonization (ICH) guidelines (2005).
System Suitability Testing:
Perform system suitability tests for the HPLC and HPTLC method to ensure adequate chromatographic performance and resolution. Evaluate parameters such as retention time, peak symmetry, resolution, and column efficiency to ensure the reliability of the method.
Resolution:
The ability of the chromatographic system to separate adjacent peaks. Resolution is determined by the peak width and baseline separation between peaks.
Retention Time:
The time taken for a compound to travel through the chromatographic column and reach the detector. Retention time should be consistent and reproducible for each analyte.
Peak Symmetry:
The shape of chromatographic peaks should be symmetrical, indicating efficient and uniform elution of analytes. Peak asymmetry can indicate issues such as overloading, column degradation, or improper mobile phase composition.
Peak Area:
The area under each peak corresponds to the amount of analyte present in the sample. Peak area should be consistent and proportional to the concentration of analyte injected.
Selectivity:
The ability of the chromatographic method to separate analytes of interest from potential interferences or impurities. Selectivity ensures accurate quantification and identification of target compounds.
Method Validation:
Conduct validation studies according to the developed protocol.
Linearity:
Calibration curves were constructed using three series of standard Diloxanide Furoate solutions in the range of 1.0 - 40.0 ?g ml-1. The equation of linear regression and statistical data are shown in the table. The linearity of the calibration curve was validated by the high value of the correlation coefficient.
Limit of detection (LOD) and limit of quantification (LOQ):
The limit of detection and the limit of quantification are defined as LOD and LOQ respectively, where ? denotes standard deviation of y-intercepts of regression lines and s denotes slope of the corresponding calibration curve.17
Precision:
The assay was investigated with respect to system suitability test, method precision and intermediate precision. The system suitability test and method precision were carried out to monitor repeatability and reproducibility. In order to measure repeatability of the system (system suitability test), five consecutive injections were made and the results were evaluated by considering peak area values of Diloxanide Furoate. The precision values with their R.S.D. are shown in Table 7.2. The results in Table 2 indicate that the R.S.D. (%) is less than 2%. Three different concentrations of Diloxanide Furoate were analyzed in three independent series in the same day (intra-day precision) and three consecutive days (inter-day precision), within each series every sample was injected six times. The R.S.D. values of intra- and inter-day studies varied from 0.17 to 0.57 % showing that the intermediate precision of the method was satisfactory.
Accuracy and recovery studies:
The accuracy of a method is expressed as the closeness of agreement between the value found and the value that is accepted as a reference value. It is determined by calculating the percent difference (bias %) between the measured mean contents and the corresponding nominal contents.18 Shown in the table results obtained for intra- and inter-day accuracy. The accuracy of the proposed method was also tested by recovery experiments. Recovery experiments were performed by taking different sample concentrations and spiking with Diloxanide Furoate at two different concentration levels (50% and 100% Diloxanide Furoate). Six samples were prepared for each recovery level. Samples were treated as described in the procedure for sample preparations. The results obtained are shown in Table from which it is clear that both the recoveries and repeatabilities are excellent.
Robustness:
Robustness relates to the capacity of the method to remain unaffected by small but deliberate variations introduced into the method parameters. Influences of small changes in the mobile phase composition (±10%) and flow rate (±10%) were studied to determine robustness of the method. Peak areas and retention time changes were observed. Peak area values and retention time values varied by less than 2 %. Despite the changes in retention time there was no problem for quantification.
Assay method:
- API
- Pharmaceutical formulation.
Twenty tablets were weighed, crushed and an amount of powder equivalent to100 mg of Diloxanide Furoate was accurately weighed, transferred to a 100 ml volumetric flask, made up to volume with water and placed in an ultrasonic bath for 20 min. After filtration through a 0.45?m membrane filter, the solution was suitably diluted with mobile phase to obtain the required concentration. 20?L of solution was injected into the HPLC and HPTLC system to obtain the chromatograms for the standard drug solution and the sample solution. A steady baseline was recorded with the optimized chromatographic conditions. The standard solution of Diloxanide furoate was injected and the chromatogram recorded. The retention time of Diloxanide furoate was found to be 3.56 min. The sample solution prepared from the tablets was then injected and the amount of drug present was calculated from the calibration curve.
RESULTS AND DISCUSSION:
Confirmation of Identity of Diloxanide Furoate:
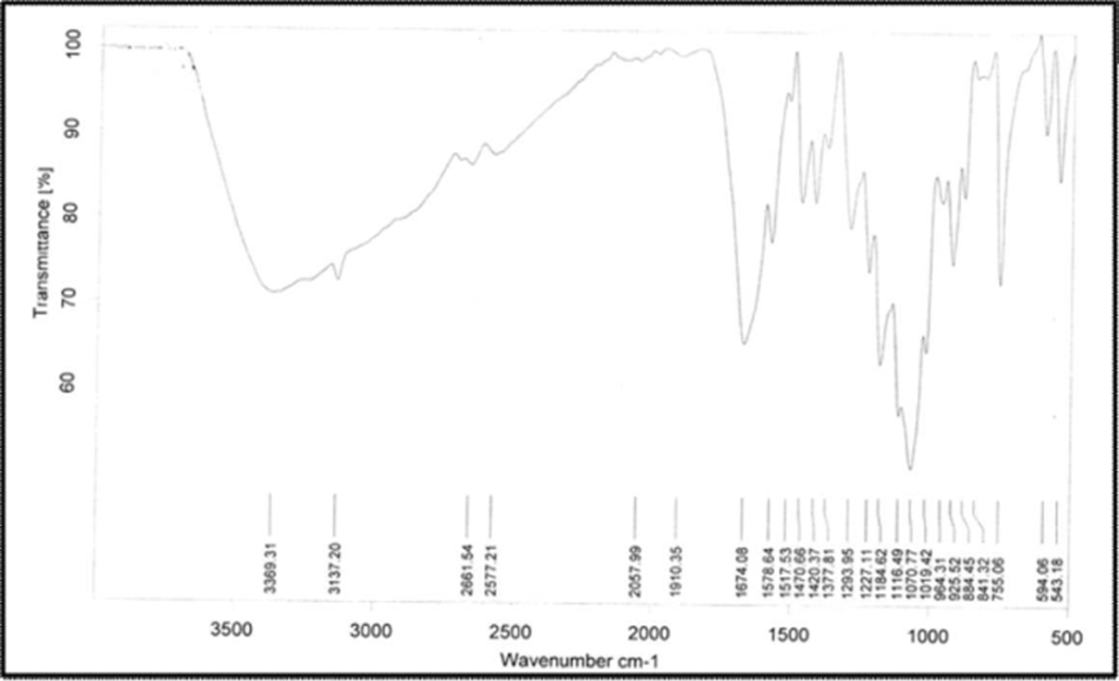
Figure 02: FT-IR spectra of Diloxanide Furoate.
Diloxanide Furoate is an antiviral drug used in the treatment of herpes simplex virus infections. Its chemical structure contains several functional groups that would produce characteristic peaks in the FT-IR spectra above table and figure. These peaks correspond to the functional groups present in Diloxanide Furoate, including the ester carbonyl group, aromatic rings, hydroxyl groups, and C-H stretching in both aliphatic and aromatic environments. The UV-Visible spectra of Diloxanide Furoate were recorded using methanol as solvent was recorded using water as solvent on SICAN 1900 Instrument.
Average of six determinations
Table 02: FT-IR spectra values of Diloxanide Furoate:
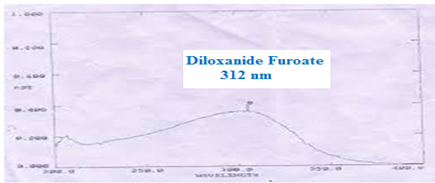
Figure no. 03: Uv-Visible spectra of Diloxanide Furoate at 312 nm.
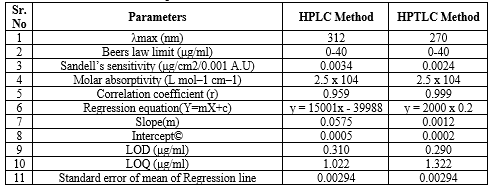
The UV-Visible spectra of Diloxanide Furoate were recorded using methanol as solvent was recorded using water as solvent on SICAN 2301 Instrument lambda max shows at 312 nm shown in figure 03 .
Table 03: Calibration curve for
Diloxanide Furoate:
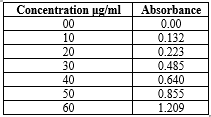
Average of six determinations
Figure 04: Std. Calibration curve of Diloxanide Furoate r2 =0.999
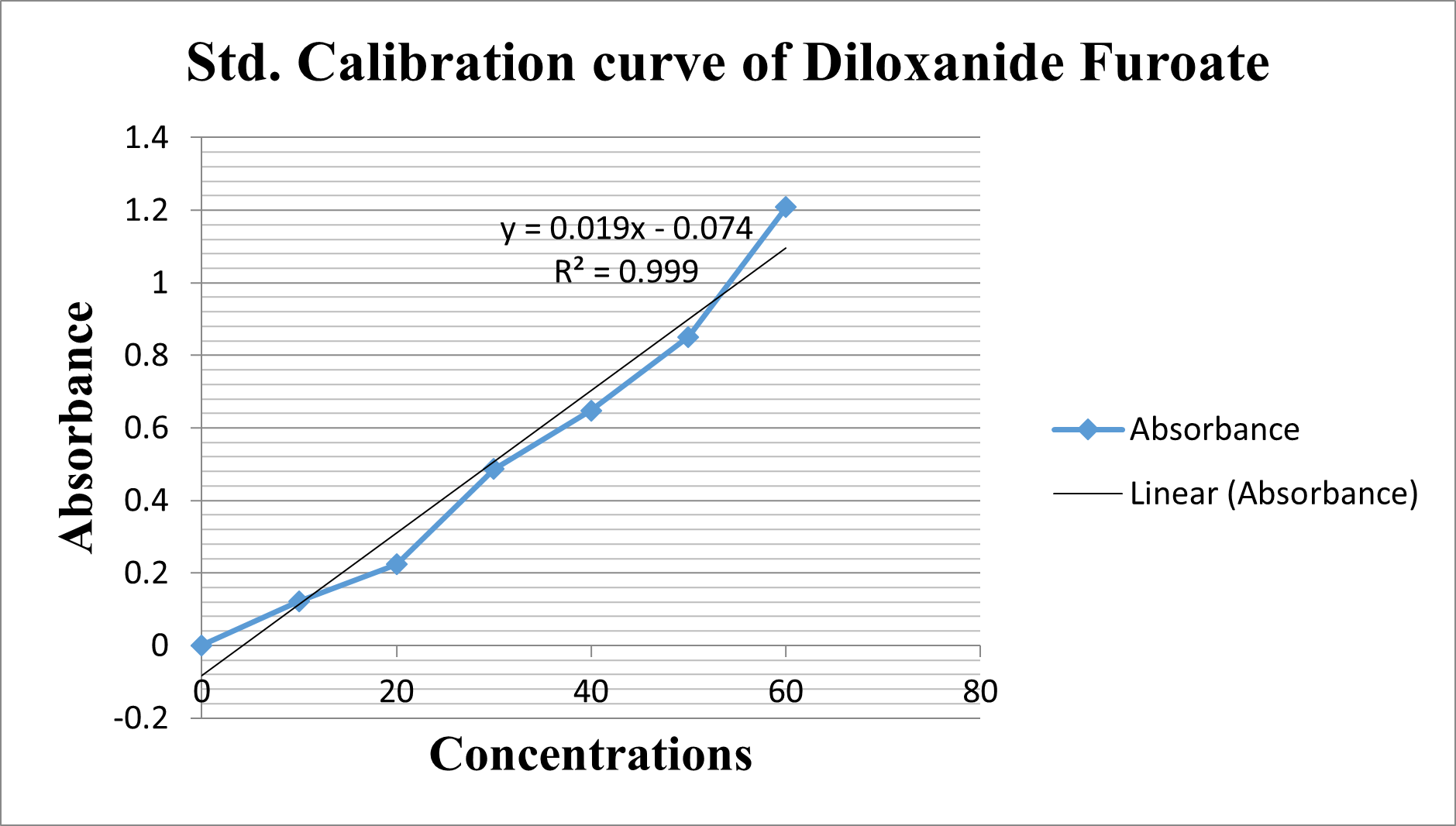
UV-Visible spectra of Diloxanide Furoate exhibit absorption at 312 nm. The standard calibration curve for Diloxanide Furoate demonstrates strong linearity with an R?2; value of 0.999, indicating reliable quantitative analysis capability.
Method Development:
The process was carried out on C18 column (5?m, 250 x 4.6mm, i.d) using the mobile phase consisting of Acetonitrile, methanol, and glacial acetic acid in the ratio 55:15:30 v/v/v respectively at a flow rate of 1 ml min-1. Wavelength was fixed at 312 nm. The mobile phase was filtered through 0.45?m membrane filter and degassed.
Table 04: Different composition of mobile phase:
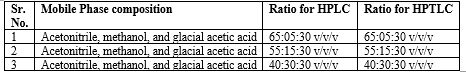
Mobile phase:
Acetonitrile, methanol, and glacial acetic acid in the ratio 55:15:30 v/v/v for Diloxanide Furoate determination peak shows HPLC 312 nm with RT 2.5 min and HPTLC 270 nm with RT 3.51 min shown in figure 05, 06 and table 05, 06.
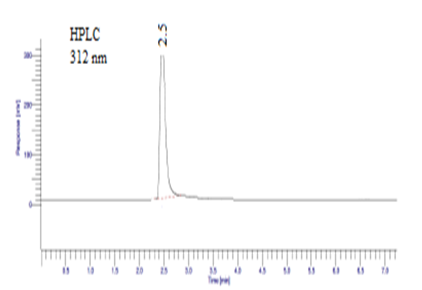
Figure 05: Mobile phase: Acetonitrile, methanol, and glacial acetic acid in the ratio 55:15:30 v/v/v for Diloxanide Furoate determination by HPLC.
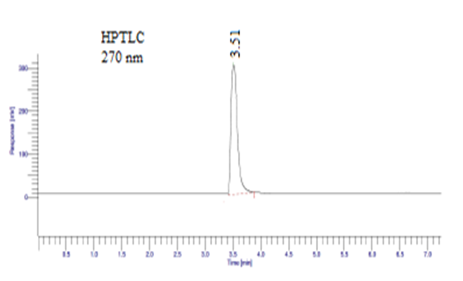
Figure 06: Mobile phase: Acetonitrile, methanol, and glacial acetic acid in the ratio 55:15:30 v/v/v for Diloxanide Furoate determination by HPTLC.
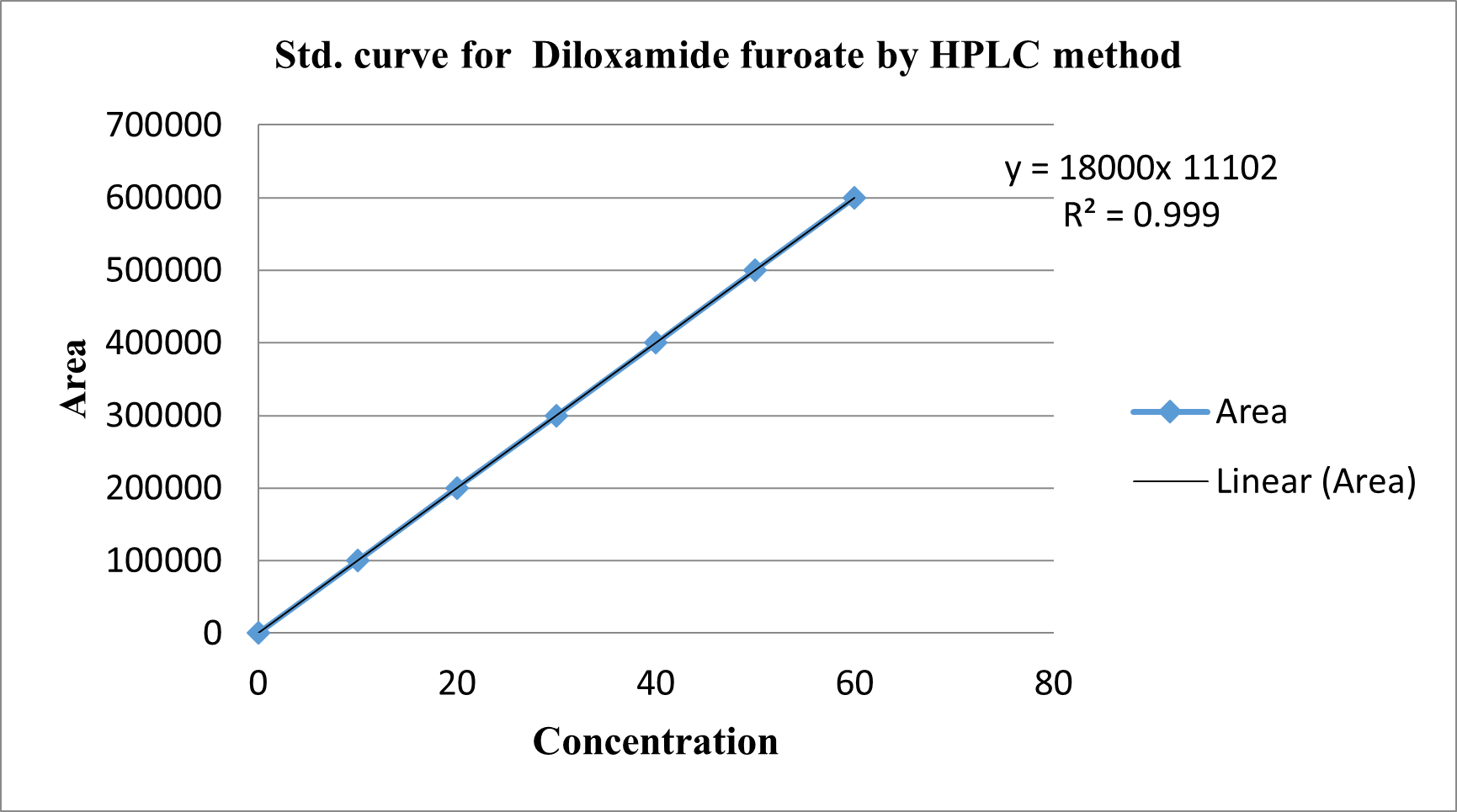

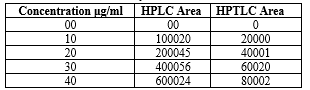
Figure no. 07: Std. curve for Diloxanide Furoate by HPLC and HPTLC method
Table 05 Std. curve for Diloxanide Furoate by HPLC method:
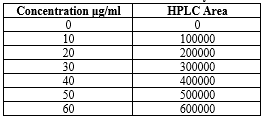
Table 06: Std. curve for Diloxanide Furoate by HPTLC method:
Method Validation:
Linearity:
Calibration curves were constructed using three series of standard Diloxanide Furoate solutions in the range of 0.0 - 40.0 ?g ml-1. The equation of linear regression and statistical data are presented in Table 07 and figure 08. The linearity of the calibration curve was validated by the high value of the correlation coefficient.
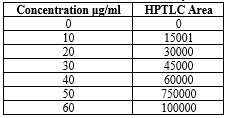
Table 07: Linearity of Diloxanide Furoate:
Average of six determinations
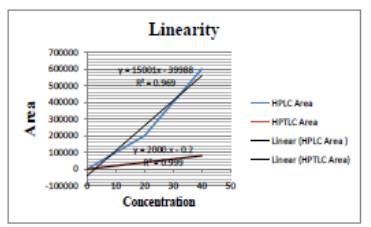
Figure 08: Linearity of Diloxanide Furoate.
Limit of detection (LOD) and limit of quantification (LOQ):
The limit of detection and the limit of quantification are defined as LOD and LOQ respectively, where ? denotes standard deviation of y-intercepts of regression lines and s denotes slope of the corresponding calibration curve.17
HPLC = LOD =3.3 ?/s = 0.310 and HPTLC = LOD =3.3 ?/s = 0.290
HPLC = LOQ=10 ?/s = 1.022 and HPTLC = LOQ=10 ?/s = 1.322
Table 08: Optical Characteristics of Diloxanide Furoate:
Precision:
The assay was evaluated for system suitability, method precision, and intermediate precision. To assess system repeatability, five consecutive injections were made, and the peak areas of Diloxanide Furoate were analyzed. The results, shown in Tables 09 and 10, indicate R.S.D. values below 2%, demonstrating good repeatability and reproducibility. Three different concentrations of Diloxanide Furoate were tested in three independent series on the same day (intra-day precision) and over three consecutive days (inter-day precision), with each sample injected six times. The R.S.D. values for intra- and inter-day studies ranged from 0.16 to 0.50%, confirming satisfactory intermediate precision.
Table 09 Precision: Intra- and inter-day precision of Diloxanide Furoate for HPLC:
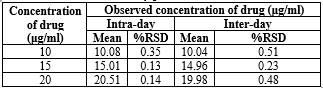
*Average of six determinations. R.S.D. (%): relative standard deviation; bias (%): [(found – taken)/taken] x 100.
Table 10 Precision: Intra- and inter-day precision of Diloxanide Furoate for HPTLC:

*Average of six determinations. R.S.D. (%): relative standard deviation; bias (%): [(found – taken)/taken] x 100.
Accuracy and recovery studies:
The accuracy of the method is measured by the agreement between the found value and the reference value, calculated as the percent difference between the measured mean contents and the nominal contents. Tables 11, 12, 13, and 14 present intra- and inter-day accuracy results. The method's accuracy was further verified through recovery experiments using different sample concentrations, spiked with 50% and 100% of Diloxanide Furoate. Six samples were prepared for each recovery level and treated according to the sample preparation procedure. The results in Tables 11 and 14 demonstrate excellent recoveries and repeatabilities.
Table 11: Precision: Intra- and inter-day precision of Diloxanide Furoate for HPLC:
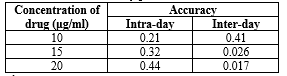
*Average of six determinations.
Table 12: Recovery Data for the Proposed HPLC method. Studies of Diloxanide Furoate:
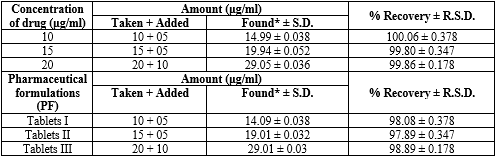
*Average of six determinations. R.S.D. (%): relative standard deviation; bias (%): [(found – taken)/taken] x 100
Table 13: Precision: Intra- and inter-day precision of Diloxanide Furoate for HPTLC:
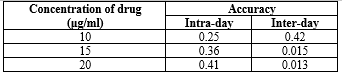
*Average of six determinations.
Table 14: Recovery Data for the Proposed HPTLC method. Studies of Diloxanide Furoate:
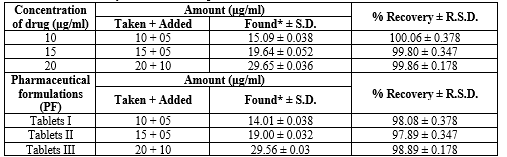
Average of six determinations. R.S.D. (%): relative standard deviation; bias (%): [(found – taken)/taken] x 100.
Robustness:
The method's robustness was confirmed, showing less than 2% variation in peak areas and retention times under slight changes in mobile phase composition and flow rate, ensuring accurate quantification of both drugs shown in table no 15 and 16.
Table 15: Diloxanide Furoate content and their respective RSD values following each HPLC (Chromatographic condition variation):
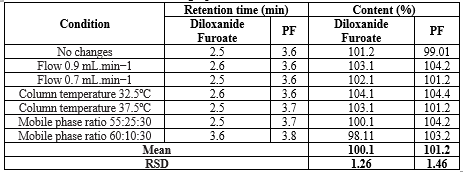
*Average of six determinations. R.S.D. (%): relative standard deviation; bias (%): [(found – taken)/taken] x 100.
Table 16: Diloxanide Furoate content and their respective RSD values following each HPTLC (Chromatographic condition variation):

*Average of six determinations. R.S.D. (%): relative standard deviation; bias (%): [(found – taken)/taken] x 100.
System Suitability Testing:
system suitability tests for the HPLC and HPTLC method to ensure adequate chromatographic performance and resolution such as retention time, peak symmetry, resolution, and column efficiency to ensure the reliability of the method shown in table 17 and 18.
Table 17: System Suitability Test for HPLC Parameters for Diloxanide Furoate:
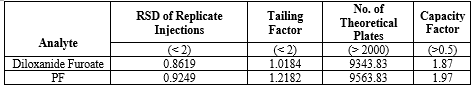
Table 18: System Suitability Test for HPTLC Parameters for Diloxanide Furoate:

Assay method:
For the assay method, 20?L of solution was injected into the HPLC system. Chromatograms for standard and sample solutions were recorded under optimized conditions. The retention time of Diloxanide Furoate was 2.5 minutes. The amount of drug in the tablet sample was calculated using the calibration curve shown in figure 09, 10, and table 19 .

Figure 09: Result of Assay method API for Diloxanide Furoate.

Figure 10: Result of Assay method pharmaceutical formulation for Diloxanide Furoate
Table 19: HPLC Analysis of Diloxanide Furoate from API and pharmaceutical formulations by proposed method.

*Average of six determinations. R.S.D. (%): relative standard deviation; bias (%): [(found – taken)/taken] x 100.
These results illustrate the findings from the analysis conducted on the consequential three days. The amounts found closely align with the reference method, indicating high accuracy, while the percentage recoveries demonstrate the method's reliability with low relative standard deviation (R.S.D.) values.
Forced Degradation Studies:
Monitor the degradation products using HPLC and HPTLC methods to ensure that the method can accurately quantify Diloxanide Furoate in the presence of degradation products.
Figure 11: Result of normal condition of Diloxanide Furoate.
Acid degradation studies:
To 10 mL of Diloxanide Furoate solution (2mg/mL in distilled water) added 10 mL of 1N HCL and was kept at room temperature for 3 hour. The solution was neutralized. Further dilutions were made with mobile phase to obtain concentration 20g/mL for HPLC analysis.

Figure 12: Result of Acid hydrolysis (1M HCL, 3 hr., 2.45 RT) of Diloxanide Furoate.
Alkali degradation studies:
To 10 mL of Diloxanide Furoate solution (2mg/mL in distilled water) added 10 mL of 0.1M NaOH and was kept at room temperature for 3 hour. The solution was neutralized and diluted with mobile phase for further study.

Figure 13: Result of ne hydrolysis (0.1 M NaOH, 3hr., RT) of Diloxanide Furoate.
xidation studies:
To 10 mL of Diloxanide Furoate solution (2mg/mL in distilled water) added 10 mL of 30% H2O2 at room temperature for 48 hour.
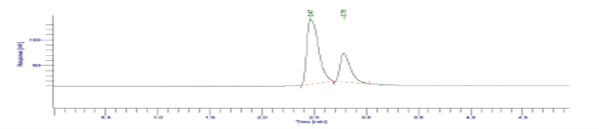
Figure 14: Result of Oxidation studies (30 % H2O2, 48 hr., RT) of Diloxanide Furoate.
Thermal studies:
The pure solid drug substance was spread to about 1mm thickness in petri dish exposed to dry heat at 50 0C for 48 hour in hot air oven. Then, powder equivalent to 25 mg dissolved in 25.0 mL of distilled water. Further dilutions were made in mobile phase to obtain appropriate concentration of Diloxanide Furoate.
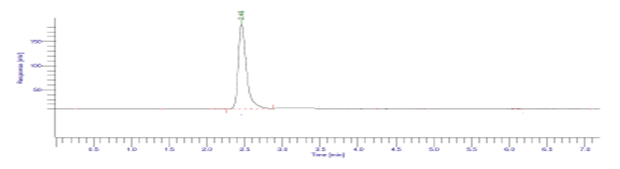
Figure 15: Result of Dry heat (500 C, 24hr.) of Diloxanide Furoate.
Photo degradation studies:
The pure solid drug substance was spread to about 1mm thickness in petri dish exposed to sunlight for 12 hours and then powder equivalent to 25 mg dissolved in 25.0 mL of distilled water. Further dilutions were made in mobile phase to obtain appropriate concentration of Diloxanide Furoate.
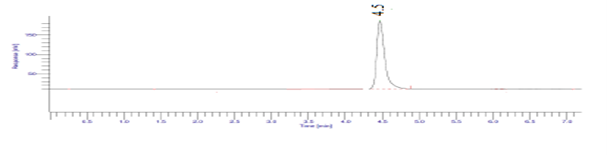
Figure 16: Result of Photo degradation (Sunlight exposure, 12 hr) of Diloxanide Furoate.
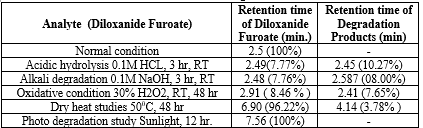
The results of the stress studies indicate the specificity and stability indicating ability of the method developed. Diloxanide Furoate was depredated in 1M HCl and 0.1M NaOH when kept at room temperature for 3 hr. The neutralization of degraded sample is important step in analysis. The degraded products have the good resolution from the Diloxanide Furoate main peak. The results of forced degradation studies are given in Table no.7.15.
Table 20: Results of forced degradation studies:
DISCUSSIONS:
The study developed and validated stability assays for Diloxanide Furoate using HPLC and HPTLC methods. Detailed specifications for each instrument were provided, and chromatographic conditions were optimized for best separation and detection. Pure samples of Diloxanide Furoate were confirmed using UV-visible wavelength scans and FT-IR spectra. Calibration curves showed strong linearity, and method validation assessed specificity, linearity, accuracy, precision, and robustness. System suitability testing ensured consistent chromatographic performance. The methods were successfully applied to analyze Diloxanide Furoate in API and pharmaceutical formulations with high accuracy. Forced degradation studies confirmed the stability-indicating capability of the methods.
CONCLUSION:
The study developed and validated HPLC and HPTLC stability assays for Diloxanide Furoate. Instrument specifications and chromatographic conditions were optimized. Pure samples were confirmed using UV-visible and FT-IR analysis. Calibration curves showed strong linearity. Methods were designed and validated for specificity, linearity, accuracy, precision, and robustness. System suitability tests ensured consistent performance. The assays accurately analyzed Diloxanide Furoate in API and pharmaceutical formulations. Forced degradation studies confirmed the methods' stability-indicating capability. Overall, the study demonstrated the methods' suitability for pharmaceutical analysis.
CONFLICT OF INTEREST:
The authors declare no conflict of interest.
ACKNOWLEDGEMENT:
I express my heartfelt gratitude to ARACOP, including all teaching and non-teaching staff, and Principal Dr. R.D. Wagh, for their support throughout this research. Special thanks to my research guide, Dr. Shailesh B. Patil, for his invaluable guidance and encouragement. Without their collective assistance, this work would not have been possible.
REFERENCES:
- K. Basavaiah, H. C. Prameela, U. Chandrashekar, Simple high-performance liquid chromatographic methof for the determination of acyclovir in pharmaceuticals, IL Farm., 2003; 58: 1301–1306.
- Indian Pharmacopoeia, The Indian Pharmacopoeia Commission, Ghaziabad, 2010, p. 775.
- K. Basavaiah, H. C. Prameela, Simple spectrophotometric determination of acyclovir in bulk drug and formulations, IL Farm., 2002; 57: 443-449.
- P. D. Tzanavaras, D. G. Themelis, High-throughput HPLC assay of acyclovir and its major impurity guanine using a monolithic column and a flow gradient approach, J. Pharm. Biomed. Anal., 2007; 43: 1526-1530.
- Candaele M., Candaele D., on behalf of the Famciclovir Herpes Zoster Clinical Study Group. Famciclovir: confirmed efficacy of 250 mg tid for the treatment of herpes zoster infection. Seventh International Conference on Antiviral Research (Abstract 118), Charlesston, South Carolina, 27 Feb 4 March 1994.
- Tyring S., Engst R., Corriveau C., Robillard N., Trottire S., Van Slycken S., Crann R. A., Locke L. A., Saltzman R., Palestine A. G., Br. J. Ophthalmol., 85, 576-581 (2001).
- Perry C. M., Wagstaff A. J., Drugs, 50, 396-415 (1995).
- Vere Hodge R. A., Cheng Y.-C., Antiviral Chem. Chemother., 4 (Suppl. 1), 13-24 (1993).
- Arabian F. A., Sacks S. L., Drugs, 52, 17-32 (1996).
- Pue M. A., Benet L. Z., Antiviral Chem. Chemother., 4 (Suppl. 1), 47—55 (1993).
- Murray A. B., Antiviral Chem. Chemother., 6, 34-38 (1995).
- Vere Hodge R. A., Sutton D., Boyd M. R., Harnden M. R., Jarvest R. L., Antimicrob. Agents Chemother., 33, 1765-1773 (1989).
- Schenkel F., Rudaz S., Daali Y., Kondo Oestreicher M., Veuthey L., Dayer P., J. Chromatogr. B, 826, 1-7 (2005).
- Brand B., Reese C. B., Song Q., Visintin C., Tetrahedron, 55, 5239— 5252 (1999).
- Zhang Y. S., Wang L. Q., Guo Y. J., Cui J. G., Li J., Zhongguo Yiyao Gongye Zazhi, 33, 454-457 (2002).
- Validation of analytical procedures, Proceedings of the International Conference on Harmonization (ICH). Commission of the Japan (1997).
- A. Loregian, R. Gaatti, G. Palu and Elico F. De Palo, Biomed. Sci. Application, 764, 289-311 (2001).
- S. Nizamuddian, D. Goli, Y. N. Manohara and M. C. Ravi, Asian J. Chem., 19(5), 3617-3620 (2007).
- K. V. Subramanyam, P. Mohanraj, V. S. Saravanan and N. Gopal, Asian J. Chem., 19(6), 4911-4913 (2007).
- D. G. Sankar, N. Sujatha, B. A. Kumar and P. V. M. Latha, Asian J. Chem., 19(2), 1602-1604 (2007).
- J. Zhang, West China J. Pharmaceut. Sci., 21, 302-303 (2006).
- S. Nizamuddin, B. M. Gurupadayya, M. C. Ravi, Y. N. Manohara and S. Appala Raju, Indian J. Pharmaceut. Sci., 69(3), 451-453 (2007).
- Jin O. Hung and Z. Y., Chinese J. Biochem. Pharmaceut., 22(4), 1891-1896 (2001).
- H. Zongyu and J. Ou, Chinese J. Biochem. Pharmaceut., 20(2), 111-113 (2000).
- V. Srinivas, M. Narasimha Rao, A. Appa Rao and G. Srinubabu, E-J. Chem., 5(1), 58-67 (2008).


 Kalyani Ashok Patil* 1
Kalyani Ashok Patil* 1
































 10.5281/zenodo.11543189
10.5281/zenodo.11543189