Regulatory affairs (RA) professionals play critical roles in the pharmaceutical industry because they are concerned about the healthcare product lifecycle Direction and support for working within rules to speed up the development and delivery of safe and effective healthcare goods to people worldwide, including strategic, tactical, and operational guidance. In pharmaceutical companies, regulatory affairs is a respected, intellectually stimulating, and challenging career.
CTD, ECTD, NDA Application, Regulatory Affairs, Regulatory Bodies.
Regulatory Affairs, also known as government Affairs, is a profession in regulated industries, including pharmaceuticals, clinical gadgets and so on. The Regulatory Affairs profession at its heart is all about gathering, studying and speaking about the danger & amp; advantages of healthcare products to regulatory organizations and the public all globally. Drug Regulatory Affairs is a brand new carrier that has been initiated by governments to defend public health with the say of controlling the production efficacy of products in the area together with prescribed drugs, veterinary drugs treatment, medical devices, insecticides, agrochemicals, cosmetics & amp; complementary medicines. success of regulatory strategies is less dependent on the regulations than on how they are interpreted, applied and communicated within companies and outside constituent. (1)
Importance of Regulatory Affairs
The first point of contact between the corporation and the government agency is the regulatory affairs division. Aids in coordinating research efforts with regulatory requirements. Maximize resource efficiency for the business. Inform the company of the perspective of the government. Impact their companies, strategic decisions. Assist in getting the medication onto the market on time, as even a small delay could negatively impact the company's financial standing. Notify higher authorities with the specific marketing information. .(2)
Need of Regulatory Affairs
Ensuring that their companies namely with all of the system policies and laws pertaining to their business. collaborating with national, state, and local regulatory organizations, including the Food and Drug Administration or the European Medicines Agency. advising their businesses on the regulatory landscapes and issues that could impact suggested initiatives like the support of prescription medication .(3)
Drug regulatory Affair professional
The duty of the Regulatory Affairs expert is to keep up with the continuously changing rules in all of the countries where The business wants to to sell its products. Experts in regulatory affairs are in charge of submitting registration documents to regulatory bodies and conducting all required negotiations to maintain the products' availability on the market. They provide technological and strategic support at the highest levels of their businesses from the outset of a product's development, making a significant financial and clinical contribution to the development effort's advancement and the company's overall success.
The Regulatory Affairs Department's duties are listed below:
- Stay up to date on international regulations, rules, and consumer behavior.
- Keep abreast of a company's product offerings.
- Verify if a company's products comply with current laws.
- The Regulatory Affairs professional's role is to stay on top of the constantly evolving regulations in all of the countries where the organization wants to sell its goods. They will acquire, compile, and evaluate the scientific data produced by their colleagues in research and development, in addition to offering legal and technical advice.
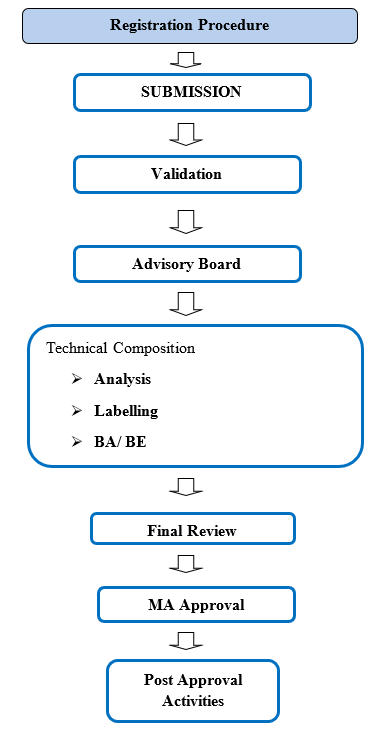
- Create a regulatory plan and include all required submissions for contract, international, and domestic projects. Coordination, planning, and review of all pertinent paperwork, including dossiers, should be done in conjunction with the agency. All paperwork should be sent to regulatory authorities on time. Create and research SOPs pertaining to RA Records linked to BMR, MFR, shift control, and other topics are examined.
- Monitor the status of each and every registration. Observe approved applications and registration costs paid for DMFs and other documentation. Give R&D, the Pilot Plant, ADL, and RA training. members of the team regarding current legal obligations.. (4).
COMMON TECHNICAL DOCUMENT (CTD):
A standardized set of standards for the application dossier used to register medicines is known as the Common Technical Document (CTD).It is intended for use in the United States, Europe, and Japan. For the goal of getting ready to submit new medication applications to regional authorities in these countries, the CD format is widely recognized. The Food and Drug Administration (FDA) in the US, the Ministry of Health, Labor, and Welfare in Japan, and the European Medicines Agency (EMA) in Europe worked together to generate this paper. The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) is in charge of maintaining the CTD. (5)
Objectives of CTD
Goals of CTD:
- The principal aim is to minimize the amount of time and resources needed for application compilation.
- It will aid in the preparation of electronic application submissions.
- To enable concurrent submission in three areas.
- It will facilitate the exchanging regulatory data, resulting in expedited access to novel medications. (7)
It can be organized into 5 Modules:
- Module 1: administrative and prescribing information
- Module 2: common technical documents CTD summaries
- Module 3: Quality data
- Module 4: Nonclinical study reports
- Module 5: Clinical study reports
Significance
- It offers data in an appropriate manner that is simple to comprehend and aids in data evaluation.
- CTD is is relevant to every all kinds of goods. This additional format makes the reviewer's job easier as well.
- In addition, CTD facilitates the exchange of regulatory data and the simultaneous filing of archives for approval in three locations. Additionally, it facilitates electronic submissions and expedites the provision of novel pharmaceutical treatments to patient cohorts. (8)
Benefits of CTD
1.Establishing a standard submission format is primarily intended to streamline the application review process and prevent the omission of important information or analysis. If these particulars are omitted, approvals may be unduly delayed.
2. the amount of effort and materials required to to put together applications in order to register human medicines will be significantly reduced by the adoption of a standard layout for technical material. It will also make the process of preparing electronic submissions easier.
3. The utilization of a standardized document containing recurring components will enhance regulatory examinations as well as facilitate communication between the regulatory authorities and the applicant.
4. It is projected that the industry will save a great deal of time and money by using the Common Technical Document (CTD) instead of assembling applications for worldwide registration.
5. Furthermore to raising the bar for Indian norms, the CTD gives the application process broad a methodical framework. The CTD's adoption will also facilitate the ability of regulatory bodies to to communicate information about regulations with one another. (9)
eCTD: Electronic Common Technical Document
The electronic Common Technical Document (eCTD) is used by the pharmaceutical industry to send regulatory data to regulatory agencies. It is based on the Multidisciplinary Group 2 Expert Working Group of the International Conference on Harmonization (ICH) (ICH M2 EWG), which created the Common Technical Document (CTD) format. The eCTD's transport format, which is designed to be easily transferred into the review setting of an agency, environment, makes electronic submissions viable. It facilitates the agency's receipt of regulatory data from the industry and eases the creation, evaluation, life-cycle management, and archiving of electronic submissions. The requirements for guaranteeing the technical correctness of electronic submissions are outlined in the eCTD specifications. This progress in information submission is very helpful to the new drug approval procedure. With a single keystroke in the future, businesses might even be able to submit their applications to several regulatory bodies at once (10)
- The benefits of eCTD
Better storing and handling of submissions advantages of computerized prescription medication
- Improved data arrangement
- Support for life cycle management
- Quick access to up-to-date and thorough information
- Reviewers' improved tracking abilities and search tools
- A more efficient evaluation procedure and enhanced process transparency
- Minimal effort and information use for assessment reports
- Managed communication with other experts
- Optimal utilization of accessible resources
- Better communication with the industry.
- Optimized business processes. (11)
Requirements to submission of eCTD Documents:
- Transmitting A Submission:
There are two ways For Electronically submission to CDER:
- ESG, or Electronic Submissions Gateway, is the recommended approach. Physical media, which could be a DVD/CD-ROM or USB drive
- The FDA Electronic Submissions Gateway is the mandatory mode of transmission for eCTD 10GB or less and is an agency-wide system for receiving electronic regulatory submissions. (12)
- Clinical Study Report:
The overview, report body, and individual appendices are its constituent sections.
A top-level folder hierarchy is necessary.
A "backbone" file in Extensible Markup Language (XML) that gives the receiving system lifecycle instructions and metadata about content files.(13)
- Hyperlink, Bookmark, Numbering :
Hypertext links and bookmarks are techniques used to improve navigation through PDF files. Hypertext links can be identified by blue text or by rectangles with thin lines around them.. The bookmark hierarchy must match the contents table exactly, with no further bookmark levels added on top of what is already there. It is advised to employ no more than four tiers in the hierarchy. When When establishing bookmarks and hyperlinks, make sure to use the Inherit Zoom magnification setting, which will ensure that the destination page appears at the same magnification level as the rest of the content. (14)
- File Naming:
File names, including the extension, must not exceed 64 characters. Also, folder names must not exceed 64 characters and the total file folder path length must not exceed 180 characters.
- PDF Size:
FDA page size for US Letters
All four sides of pages should have a one-inch margin, plus extra space for the binding edge (to prevent problems if documents need to be printed).
Either Times New Roman or Ariel fonts must be used for the content, with body text set at 12 points and tables no less than 10 points.
- Version:
Agencies should be capable of use Acrobat Reader version 4.0 or higher to read all PDF files. It should not be required for agencies to use any extra software to be able to view and navigate PDF files.
- Harmonize documents, data and submission Standard:
Reusing transferring content from one area to another is made possible by harmonizing standards and all content related to submissions in one location.This is among the greatest ways to accomplish this. This would expedite the submission process by drastically lowering the quantity of repetitive work needed to submit an applicationmany nations or regions. (15)
- Quality Control reviewing at the right point:
The publishing workflow shouldn't begin until all source documents have undergone quality control checks.
It is recommended to review all published PDF files on a screen.
Verify the links and bookmarks in published PDF files.
eCTD submissions should always be validated and conformity-checked before being submitted
- Submitting of eCTD Sample :
To submit a sample eCTD, you must first obtain an application number from the Electronic Submissions Team at esub-testing@fda.hhs.gov; this number should only be used for the sample and not for the real eCTD submission. Following submission of our request and contact information, a member of the Electronic Submissions Capability Team will be in contact. and include a Sample Application Number along with additional instructions..
- Requesting an application number that was pre-assigned::
We must obtain a priori issued application number submitting an application in in eCTD configuration to CDER. Pre-assigned application numbers are distinct six-digit numbers, such as 012345, that are given to sponsors so they may be identified
usage. It is required by the FDA to use this number.
whenever you submit an application for eCTD
Advantage of eCTD :
1) advantage over paper submissions is that it is more efficient.
2] Increased Procedure Visibility.
3] Shorter Lead Time and Speedier Approvals.
4] A uniform submission procedure and format.
5] Growth within the marketplace and Revenue.
6] Enhanced Capability to Track.
Publishers may try to prevent validation problems if the annual report is written as a single document by organizing it into a single node that corresponds to one of the annual report sections (e.g., Summary for Nonclinical Studies). This strategy, nevertheless, runs the possibility of creating confusion throughout the review process because the content will not match the node description exactly(16)
NDA APPLICATION
An NDA application must be submitted to the FDA prior to a novel medicine being put on the market. In order to receive this approval, the sponsor provides the NDA A clinical and preclinical examination findings for the analysis of drug information along with a description of the production methods. After the agency receives the NDA, it goes through a technical screening and evaluation process. The evaluation and its supporting documentation ensure that sufficient information and data have been supplied in each area to back up the FDA's official review.
The sponsor may be advised of three possible courses of action once the FDA evaluates an NDA:
Not Approvable: This letter lists the shortcomings and provides an explanation of the decision.
2. Approvable: Thus, it follows that while the drug might be approved, there might be a few minor problems that must be resolved, like labeling changes or a possible need for a commitment to conduct post-approval study.
3. Approval: This signifies that the drug has been given the go-ahead.
Clinical studies are conducted in many phases:
Pre-clinical research: In this study, mice, rats, rabbits, and monkeys are used to evaluate the medication.
- Phase I: It's a human pharmacology trial that focuses on estimating the safety and tolerability of the drug.
- Phase II: This one's an exploratory trial designed to evaluate the drug's short-term side effects and effectiveness.
- Phase III - Confirmatory trials: These studies are carried out to verify the therapeutic advantages of a certain intervention
Phase IV - post-marketing trial: These studies were carried out after a medication's approval to gather further details.


 Ms. Sanskruti kishan Wagh*
Ms. Sanskruti kishan Wagh*
 Prof. Waghmare S. U.
Prof. Waghmare S. U.


 10.5281/zenodo.12205641
10.5281/zenodo.12205641