Abstract
Chemistry laboratory is the home for synthesis of various organic and inorganic compounds. Synthesis of one chemical compound can lead to production of multiple by-product. Reactions like nitration are often characterized by progressive evolution of reddish-brown vapors. These sorts of reactions are advised to be performed under fume-hood, so as to limit the inhalation of such vapor. Among the various oxides of nitrogen, this pungent smelling vapor is of nitrogen dioxide (NO2). Electronic distribution of nitrogen dioxide causes it to be called as a ‘free radical’. This free radical when inhaled in excessive amount can lead to toxic conditions called ‘oxidative stress’ followed by cellular damage. Additionally, cascade of inflammatory mediators are activated due to response of the oxidative stress. Increased production of peroxynitrile causes nitrosative stress too. This paper explores the acute and chronic health effects of NO2, its properties, and pharmacokinetics properties. The toxicity caused by NO2 gas with view of mechanistic approach is also discussed here.
Keywords
Nitrogen dioxide, paramagnetic, reddish-brown vapor, nitration reaction, free radical.
Introduction
A chemical laboratory is the birthplace to several chemicals. Many of the chemicals that are synthesized in the laboratory are known and has been recorded [1]. Sometimes, few chemicals are synthesized with novel structure, which is a result of several research and hard works [1]. Multiple unknown chemicals which might be of great use to mankind have been accidentally discovered as a result of chemical reaction. Such discoveries are known as serendipity [2]. On the contrary, several chemical moieties are also developed which have known to be very toxic or life threatening to human beings. Routes of synthesis, where such hazardous chemical moieties are synthesized are generally avoided to reduce the formation of those chemicals [1,3]. Some chemical moieties, inspite being known to be hazardous and toxic to human beings are synthesized to synthesize other products. This is the case of byproduct [4]. Byproducts are defined as the outcome product of chemical reaction synthesized along the targeted product. The aimed product should be separated from the by-product after successful completion of the chemical reaction. Nitrogen is the most abundant gas in the atmosphere [5]. There are several oxides of nitrogen which are commonly found. Nitric oxide, nitrous oxide and nitrogen dioxide are three of the most common oxides that are found in nature. Nitrogen dioxide is a common air pollutant [6]. It is an chemical moiety which is though toxic in nature, is inevitably an important byproduct of chemical laboratories [6]. There are several categories of experiments, which involves release of Nitrogen dioxide as its by-product. This review article emphasizes on ‘nitration’ reaction as a chemical reaction where a major byproduct is nitrogen dioxide [7]. This paper also encompasses the toxicodynamics and toxicokinetics of Nitrogen dioxide when inhaled and enters human body. This review will give ideas to researchers, and clinicians about environmental health and mechanism of action of nitrogen dioxide toxicity.
CHEMICAL PROPERTIES
Nitrogen dioxide occurs as a reddish-brown gas, with pungent odour [8]. This gas due to its high oxidizing property is highly combustible and easily used as fuel. Nitrogen dioxide has a bent chemical structure, where two oxygen atoms are covalently bound to a nitrogen atom, resulting in one unshared electron on the Nitrogen. This hence, forms a radical [9]. Thus, Nitrogen dioxide occurs as a free radical. This is illustrated in Figure 1.
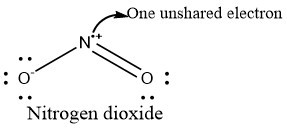
Figure 1: Structure of NO2.
It occurs as gas in normal room temperature, indicating its low boiling point [8, 10]. The boiling point is about 21.2°C (70.2°F). It exists as liquid below this temperature. The melting point of nitrogen dioxide is about -11.2°C (11.8°F). The density of nitrogen dioxide is about 1.88 kg/m3 at 20°C. The Chemical Abstract Identification Number is 10102-44-0. The resonance structure of Nitrogen dioxide is represented in Figure 2(A) Nitrogen dioxide is paramagnetic in nature [11]. This is because of the unpaired electron on the nitrogen atom. Materials with unpaired electron is paramagnetic. Unpaired electrons have a magnetic dipole moment and act like tiny magnets. Nitrogen dioxide when cooled, looses the paramagnetic and exhibits diamagnetic characteristics. Nitrogen dioxide, due to a positive charge on the Nitrogen atom, exist as dimer [12]. Thus, the dimerization form of the compound has molecular formula N2O4. The unpaired electron on the Nitrogen makes NO2 unstable and paramagnetic. To become more stable, two NO2 molecules pair up to form a dimer, thereby stabilizing themselves [Figure 2(B)]
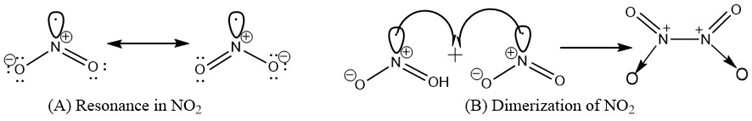
Figure 2: Resonance and dimerization of nitrogen dioxide
FORMATION OF NITROGEN DIOXIDE AS BY-PRODUCT
Several Chemical reactions lead to formation of Nitrogen dioxide. In laboratory environment, nitrogen dioxide is mainly evolved as by-product in nitration reaction [13]. Nitration is defined as a type of chemical reaction, where nitrate or (NO2-) group is added to the reactant by electrophilic substitution reaction. This reaction occurs by formation of Nitrogen dioxide as reddish brown gas, which until evolves completely, product is not formed. Nitration occurs mainly by using mixed acid medium [14], like nitric acid and sulphuric acid where nitric acid is the nitrating agent and sulphuric acid is the proton donor to complete the reaction. The role of the proton is mainly to generate the electrophile called the nitronium ion. The mechanism of nitration reaction by mixed acid is illustrated in Figure 3.
Few examples of nitration reaction include:
- Naphthalene to 5-Nitronaphthalene by nitric acid and sulphuric acid [15]
- Benzene to Nitrobenzene. The mechanism for the same is illustrated in Figure 3.
- Nitrobenzene to meta-dinitrobenzene
- Acetanilide to Nitro-acetanilide by mixed acid and acetic acid medium
- Formation of picric acid from phenol [16]
- Guanidine to nitroguanidine formation
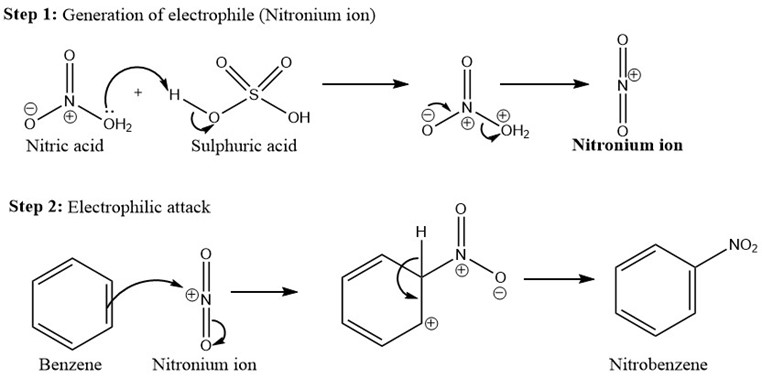
Figure 3: Mechanism of aromatic nitration reaction
The photographic representation of evolution of nitrogen dioxide while nitration of nitrobenzene and phenol with mixed acid under heat is depicted in Figure 4A and Figure 4B respectively.
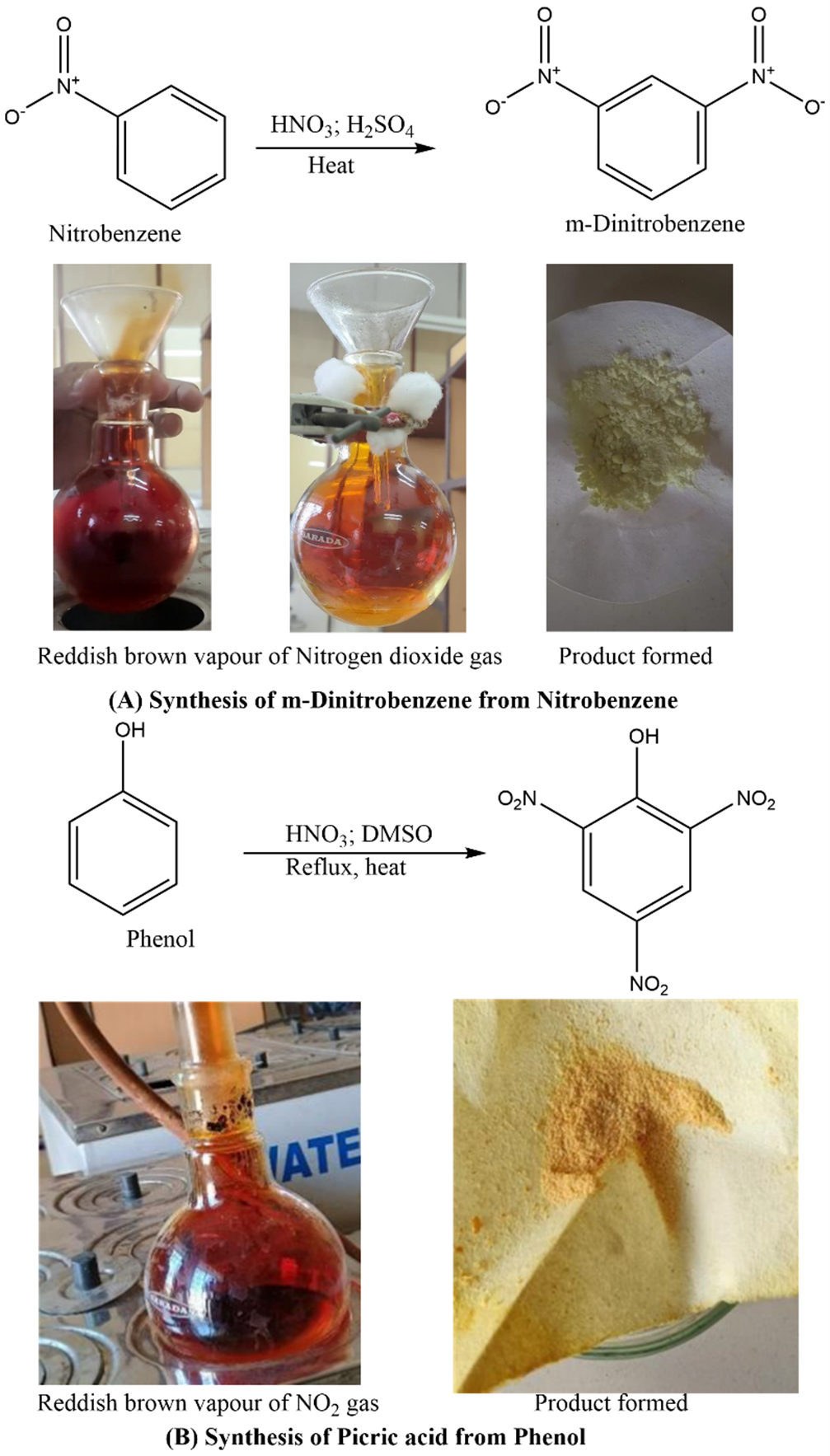
Figure 4: Evolution of Nitrogen dioxideas by-product in laboratory environment
Since evolution of nitrogen dioxide has multiple hazardous health effect, proper precautionary measures should be employed while handling the evolution of nitrogen dioxide. Use of masks and completing the reaction in fumehood [17] are some of the precautions, one must employ while conducting such reaction.
FATE OF NITROGEN DIOXIDE
Pharmacokinetics can be defined as how the drug or drug metabolite changes over time inside our body. In simple terms, Pharmacokinetics is what body does to the drug [18]. The ADME are the four basic steps of Pharmacokinetics. The process of Absorption, Distribution, Metabolism and Elimination of Nitrogen dioxide in our body is represented in Figure 5 given.
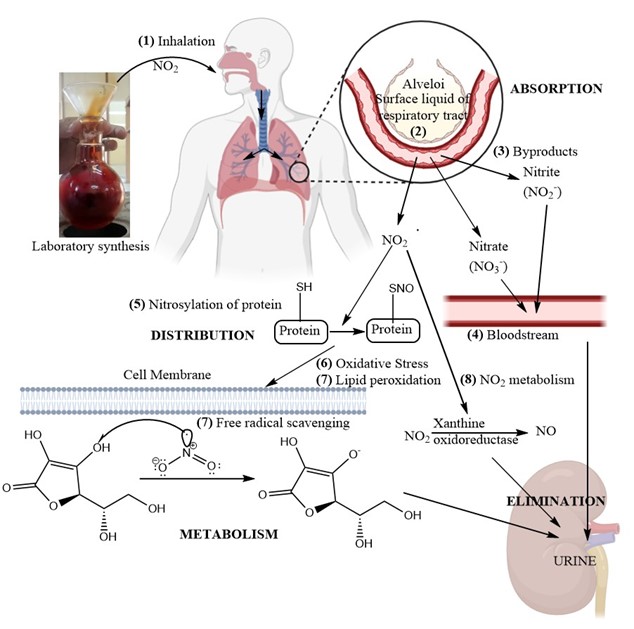
Figure 5: Pharmacokinetics of Nitrogen dioxide
Absorption
Nitrogen dioxide is a reddish brown gas. Being gaseous in nature, the major route of absorption is through respiratory tract. Thus, NO2 enters our body through inhalation. It reaches the upper respiratory tract, moves to lower respiratory tract and then moves to lungs and alveoli [19]. In each of these three stages, the action of nitrogen dioxide is quite significant. The main site of absorption of nitrogen dioxide is from the nasopharyngeal region to the alveoli.
- In upper respiratory tract, nitrogen dioxide gas mixes with the water and surface liquid. This in turns forms nitric acid and nitrate ion which in turn causes local irritation in the respiratory tract immediately when inhaled. This is the mucus layer responsible for formation of nitric and nitrous acids. [19, 20]
2NO2 + H2O ? HNO2 + HNO3
- More than 70% of the inhaled nitrogen dioxide is absorbed through the upper respiratory tract. The rest portion reaches the lower respiratory tract and alveoli. There, in alveoli the gas gets dissolved in the epithelial lining fluid (ELF). Nitrogen dioxide has unpaired electron and hence is a free radical. In the ELF, it reacts with the dissolved anti-oxidants like ascorbic acid where some of the radicals are scavenged. The rest portion is finally diffused across the alveolar-capillary membrane and get absorbed in the bloodstream [21, 22].
The amount of Nitrogen dioxide gas being absorbed from the respiratory tract to the blood stream depends on several factors [23]:
- The concentration of mucus and surface liquid on the respiratory tract
- The concentration of nitrogen dioxide gas being inhaled
- Rate of inhalation in the toxic environment
- Concentration of anti-oxidants present in the ELF
- Rate of NO2 scavenging by anti-oxidants.
A study was conducted to demonstrate the factors that affect the absorption of nitrogen dioxide from the respiratory tract. In-vitro models were used to describe how the composition of the airway surface liquid and the antioxidants present in the surface liquid of the respiratory tract controls the concentration of nitrogen dioxide being absorbed to the blood stream [24].
- Nitrite and nitrate are produced when NO2 reacts with antioxidants in the liquid on the surface of the airway.
- In the airway surface fluids, ascorbate (vitamin C) plays a crucial role in scavenging NO2 and mitigating its deleterious effects.
- Nitrogen dioxide causes nitration of tyrosine residues in proteins in an un-discovered and unknown mechanism, which might be due to peroxynitrile ion.
Distribution
Distribution is defined as the phenomenon how the component/ drug/ chemical moiety moves and circulates in the body and their interaction with protein [18].
Once nitrogen dioxide is absorbed, it occurs as either nitrate or nitrite ions or as nitrogen dioxide. [25]
HNO2 ? H+ + NO2- (nitrite)
HNO3 ? H+ + NO3- (nitrate)
In blood stream, these components get distributed to entire body un-uniformly, predominantly found in liver, lungs and kidney.
- Nitrogen dioxide can potentially cross blood brain barrier and enter the central nervous system [25]
- Nitrogen dioxide reacts with haemoglobin present in blood to form methemoglobin and nitric oxide, while nitrate in case of oxyhemoglobin, thereby reducing the oxygen carrying capacity of the haemoglobin present in the Red blood cells. The interaction of nitrogen dioxide and its metabolites [26-28] in blood is represented in Figure 6.
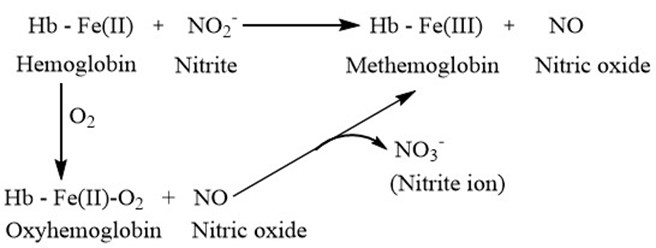
Figure 6: Interaction of NO2 with haemoglobin in blood
- Lungs is the major site of absorption of nitrogen dioxide, so has highest concentration of NO2 and its metabolites [29, 30].
- Nitrogen dioxide and its metabolites are responsible for evoking cascade of inflammatory events, which are discussed later in this review.
- Nitrosylation of proteins are defined as a post-translational modification that occurs when a nitric oxide group attaches to a cysteine thiol in a protein. Excessive nitrogen dioxide inhalation shows to increase the nitrosylated protein [31].
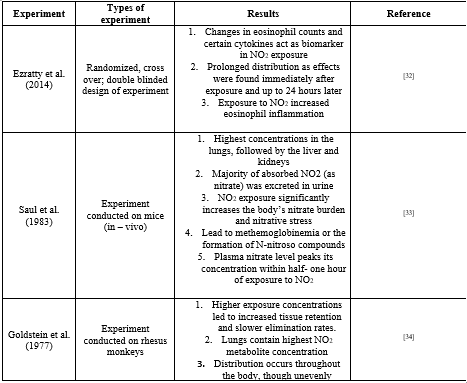
Several experimental proofs of the distribution of nitrogen dioxide and its metabolites are represented in
Table 01 given below
Table 01: Different scientific interventions to find distribution pattern of NO2 and its metabolites in body
Metabolism
Metabolism is also called biotransformation [18, 35]. It is the process, how a chemical moiety (like a drug) is converted to inactive form, so as to ease its elimination from our body. The basic aim of metabolism is to convert the chemical compound to a water soluble derivative, which can be removed from our body through urination. These derivatives are called metabolites [35, 36]. Sometimes, these metabolites has other profound effect in our body, different from its parent chemical compound. Such derivatives are re-metabolized to form inactive water soluble metabolites. The main site of drug metabolism in human body is the liver. There are two basic phases of metabolism – Phase I and Phase II. Phase I reaction, primarily carried out by the cytochrome P450 (CYP) enzyme system [37]. CYP enzymes can lead to various outcomes such as hydroxylation, N-dealkylation, O-dealkylation, S-oxidation, or deamination. Phase II biotransformation is the phenomenon where the Phase I metabolites are further converted to water soluble derivatives [38].
Nitrogen dioxide is metabolized by the following processes:
- Metabolism of nitrogen dioxide right at absorption site: The metabolism of NO2 primarily occurs through its reaction with water in the respiratory tract and blood, forming nitrous acid (HNO2) and nitric acid (HNO3). These acids quickly dissociate into their respective ions: nitrite (NO2-) and nitrate (NO3-) [39].
- Reaction with anti-oxidants: Anti-oxidants are defined as chemical moiety, which are used to stabilize reactive oxygen species or reactive nitrogen species. These ROS and RNS are highly reactive free radical, with unpaired electron. They lead to detrimental effects with rapid cellular damage. Nitrogen dioxide is a free radical. Antioxidants like Vitamin C and glutathione are responsible for stabilizing NO2 [40, 41].
- Vitamin C or ascorbic acid, as represented in Figure 5, forms dehydroascorbic acid by oxidation. Thus, it reduces the reactive NO2 by donating one electron the Nitrogen and stabilizing it [40].
- Glutathione (GSH) is the reduced form. Basically, it is a tripeptide containing cysteine, glutamate and glycine. In contact with free radical, it itself get oxidized to Glutathione disulphide by coupling reaction and thereby stabilizing the free radical [41]. The process is illustrated in Figure 7 given below.
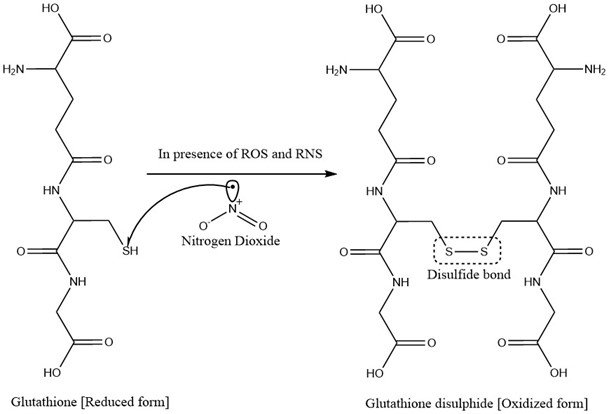
Figure 7: Stabilization of Nitrogen dioxide by Glutathione
- Phase II biotransformation: It was found that NO2-derived molecules in the liver coupled with glutathione to generate S-nitrosoglutathione (GSNO). It is believed that this procedure serves as a detoxifying agent [42, 43].
- Nitric oxide formation: Under acidic conditions, nitrite can be reduced to nitric oxide (NO). The process of conversion of nitrite to nitric oxide involves Xanthine oxidoreductase, Deoxyhemoglobin and Cytochrome C oxidase. The Nitric Oxide (NO) formation sites includes vascular endothelium, red blood cell and skeletal muscle cells [44, 45].
- NO2- + e- + H+ ? NO + OH-
- Bile excretes a significant quantity of NO2 metabolites, especially nitrate. This implies that these metabolites are circulated enterohepatically [46].
Elimination
Elimination of drug is defined as a Pharmacokinetic event by which body removes the drug. Drug elimination refers to the irreversible removal of drug from the body by all routes of elimination. Decrease in plasma level represents distribution as well as elimination of drug [47-50]. Removal of occurs through
- through renal filtration and urine (Small, polar, soluble, non-volatile drug) [47]
- through bile, sweat, lactating milk etc. [51]
- through lungs (volatile drug – general anesthetics)
Clearance can be defined as the hypothetical volume of blood, from which drug is completely and irreversibly removed per unit time [49]. According to Linear Pharmacokinetics, clearance is constant but rate of drug elimination is variable. Elimination is a first order process. Renal clearance is the ratio of rate of elimination of drug through urine and plasma concentration of drug [51].
- The volume of blood filtered by kidney per minute is called the glomerular filtrate. Nitrate is readily water soluble. Hence, nitrate ions formed from nitrogen dioxide are eliminated through renal excretion by glomerular filtration [44, 52].
- Bacteria in the gastrointestinal system and oral cavity have the ability to convert a tiny amount of nitrate back to nitrite, which can either be reabsorbed or excreted through in feces [44, 53].
- The reversible binding of NO2 to respiratory tract fluids and tissues, allows elimination through pulmonary route or exhalation [54]. NO is a major metabolite of nitrogen dioxide as discussed earlier. It is often eliminated through pulmonary system by exhaled breath. The concentration of NO in exhaled breath act as a biomarker of inflammation [55].
- Significant amount of nitrate is eliminated through bile [52].
Research conducted gave the following key finding regarding elimination of Nitrogen dioxide and its metabolites [56]:
- The renal route was confirmed as the predominant elimination route by the researchers, due to a considerable rise in urinary nitrate excretion within 4–8 hours of NO2 exposure.
- Urinary nitrate excretion increased with higher NO2 exposure levels, indicating a distinct dose-response relationship.
- Urinary nitrate levels peaked 2-4 hours after exposure, and levels returned to baseline within 24 hours.
TOXICOLOGY STUDIES OF NITROGEN DIOXIDE GAS
As discussed earlier, Nitrogen dioxide is a free radical because of the presence of an unshared electron in the Nitrogen atom. This leads to cascade of events and various cellular damages by oxidative stress. The different toxicity reported due to Nitrogen dioxide gas and their mechanism of action are:
Oxidative Stress
Stress is defined as the process where supplied component is not sufficient to meet the demand. When the body produces too many free radicals, which are unstable molecules, and not enough antioxidants to counteract them, the condition known as oxidative stress results. Damage to cells and tissue may result from this [57, 58].
Reactive oxygen species and reactive nitrogen species like NO2 lead to cellular damage by increasing oxidative stress. NO2 is a strong oxidizing agent and can directly oxidize cellular components, including lipids, proteins, and DNA. This oxidative damage triggers several cellular responses [59, 60]
- Lipid peroxidation refers to an oxidative chain reaction wherein successive lipid molecules undergo maximal oxidation, resulting in the formation of lipid peroxide, which is a lipid molecule that contains one or more O-O links. Lipid peroxidation in cell membranes can be triggered by NO2, which results in the production of reactive aldehydes such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA). These compounds can intensify the oxidative stress by causing further harm to DNA and proteins [61, 62].
- Direct oxidation of proteins by NO2 can lead to conformational changes, loss of function, and the formation of protein carbonyls. This can disrupt cellular processes and trigger stress responses. The conformational change to protein reduces or looses their specificity and selectivity towards substrate or can induce cellular damage too [63].
- Nitrogen dioxide being potent oxidizing agent can generate reactive oxygen species (ROS), such as superoxide anions (O?•?), hydroxyl radicals (•OH), and peroxynitrite (ONOO?). This can cause [64]:
- Oxidation of guanine to 8-oxoguanine (8-oxoG), a mutagenic lesion that can result in G-to-T transversions during DNA replication.
- ROS-induced damage can lead to improper base pairing.
- ROS can induce breaks in the phosphodiester backbone of the DNA molecule.
- Oxidative DNA damage activates the tumor suppressor protein p53, which can induce cell cycle arrest by upregulating cyclin-dependent kinase (CDK) inhibitors like p21. This prevents the transition from G1 to S phase, ensuring that damaged DNA is not replicated.
- The oxidative attack by NO2 gradually consumes antioxidants found in cells, such as glutathione (GSH), vitamin C, and vitamin E. This makes the cell's resistance to oxidative stress even more vulnerable [65].
Inflammation and associated events
Inflammation is a part of defence mechanism of the body. This is a cascade of event evoked by the immune system of our body in response to external stimuli, pathogen or any irritant. It aims at removing the harmful stimuli to imitate healing process [66].
The basic cascade of responses which causes inflammation are [8, 67-69]:
- NO2 increases oxidative stress and ROS
- This activates transcription factor:
- Nuclear Factor-?B (NF-?B) [in normal conditions remain inactive due to inhibitory proteins called I?Bs]
- Oxidative stress, activates I?Bs kinase [IKK] which phosphorylates I?B. This causes degradation of I?B. Hence, activated NF-?B translocates to nucelus
- NF-?B bind to specific protein sequence
- Promotes translation of numerous pro-inflammatory genes: Cytokines: IL-1?, TNF-?, IL-6; Chemokines: IL-8, MCP-1; Adhesion molecules: ICAM-1, VCAM-1 and Enzymes: COX-2, iNOS
- AP-1 activation occurs:
- AP-1 is composed of proteins from the c-Fos and c-Jun families.
- NO2 oxidative stress activates mitogen-activated protein kinases (MAPKs); c-Jun N-terminal kinase (JNK) and p38 MAPK
- These phosphorylates AP-1 components and activate it
- It also promotes translation of various pro-inflammatory genes
- Damage-associated molecular patterns (DAMPs) are released from the cells
- Pattern recognition receptors (PRRs) and Toll-like receptors (TLRs) recognizes DAMP
- This activates NLRP3 inflammasome.
- This promote activation of TNF -?; IL-1? and IL-18, potent pro-inflammatory cytokines
- IL-1?: promotes leukocyte recruitment
- IL-6: promotes B cell differentiation, and has both pro- and anti-inflammatory effects
- IL-8: Recruits neutrophil to site of inflammation
- MCP-1: Recruits monocytes, memory T cells
- 7. This induces inflammation, as represented in Figure 8
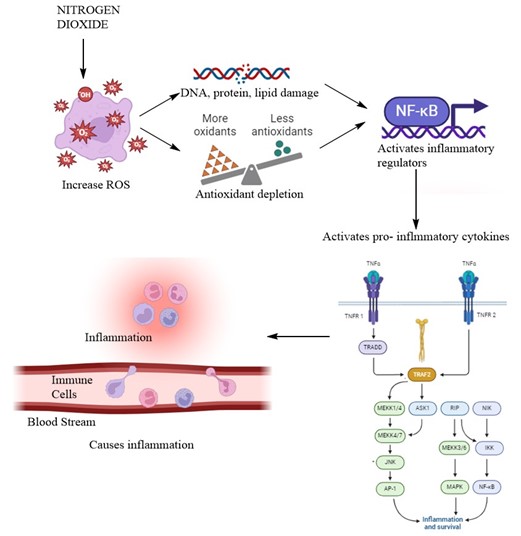
Figure 8: Nitrogen dioxide mediated inflammation
Other effects:
The different other effects caused by NO2 are:
- Nitrosylation of proteins, S-nitrosylation of proteins, alters the function and disrupts the cellular signaling cascades. [69]
- Exposure to NO2 modifies the activity of ion channels in airway smooth muscle cells, potentially contributing to airway hyperresponsiveness [70].
- By altering the cellular redox state, NO2 can interfere with redox-sensitive signalling pathways [71].
- NO2 causes nitrosative stress. It leads to formation of peroxynitrite (ONOO-), a highly reactive species that can cause nitration of proteins, particularly tyrosine residues [72].
- Increased production of pro-inflammatory mediators that sensitize nerves, causing airway hypersensitiveness and exaggerated bronchoconstriction [73].
CONCLUSION
Nitrogen dioxide is a very common by-product in both industrial and academic laboratories and cannot be avoided. But, continuous exposure and inhalation of nitrogen dioxide can cause respiratory problem, inflammation, irritation and cellular damage by oxidative stress, which is undesirable. So, taking proper precaution while handling and dealing with NO2 gas should be of utmost priority. Using fume hood for working and covering the respiratory organs by masks should be encouraged to prevent further complications.
ACKNOWLEDGEMENT
The author thanks Guru Nanak Institute of Pharmaceutical Science and Technology for giving the opportunity to write the review work.
Conflict of interest: None
Funding: None
REFERENCES
- Johnson A, Smith B. The role of chemical laboratories in modern synthesis. J Chem Res. 2022;45(3):210-225.
- Lee C, Wang D. Serendipity in chemical discovery: a historical perspective. Chem Rev. 2021;121(8):4567-4589.
- Brown E, Taylor F. Hazardous chemical moieties: synthesis and safety protocols. Safety Sci. 2023;87:102356.
- Wilson G, et al. Byproducts in chemical reactions: identification and management. Org Process Res Dev. 2022;26(5):1234-1250.
- Anderson H, Park J. Atmospheric nitrogen and its oxides: environmental impact and health effects. Environ Sci Technol. 2021;55(12):7890-7905.
- Garcia M, Lopez N. Nitrogen dioxide: from laboratory byproduct to environmental concern. J Environ Chem. 2023;18(4):567-582.
- Nakamura S, Yamada T. Nitration reactions and nitrogen dioxide formation: a comprehensive review. Chem Soc Rev. 2022;51(15):6789-6810.
- Smith JA, Johnson BC. Physical and chemical properties of nitrogen dioxide. J Chem Phys. 2020;152(14):144305.
- Brown LM, Davis RK. Radical nature and resonance structures of nitrogen dioxide. Chem Rev. 2019;119(15):9456-9497.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 3032552, Nitrogen Dioxide. https://pubchem.ncbi.nlm.nih.gov/compound/Nitrogen-Dioxide. Accessed Sept. 14, 2024.
- Wilson PG, Thompson KL. Paramagnetic characteristics of nitrogen dioxide and its temperature-dependent behavior. J Magn Reson. 2018;292:44-54.
- Lee SH, Park YJ. Dimerization of nitrogen dioxide: thermodynamics and kinetics. J Phys Chem A. 2021;125(24):5217-5229.
- Anderson CM, Taylor RS. Nitration reactions in organic synthesis: mechanisms and applications. Chem Soc Rev. 2017;46(19):5881-5902.
- Garcia-Fernandez E, Lopez-Alonso M. Mixed acid nitration: a comprehensive review of reaction conditions and mechanisms. Org Process Res Dev. 2022;26(5):1234-1250.
- Nakamura T, Yamamoto K. Nitration of aromatic compounds: from naphthalene to benzene derivatives. Eur J Org Chem. 2020;2020(31):4980-4995.
- Chen X, Zhang Y. Synthesis and applications of nitroaromatic compounds: from pharmaceuticals to explosives. Molecules. 2019;24(18):3376.
- Fonseca AS, Kuijpers E, Kling KI, et al. Particle release and control of worker exposure during laboratory-scale synthesis, handling and simulated spills of manufactured nanomaterials in fume hoods. J Nanopart Res. 2018;20(2):48.
- Fan J, de Lannoy IA. Pharmacokinetics. Biochem Pharmacol. 2014;87(1):93-120.
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants. Geneva: World Health Organization; 2010.
- Morrow PE. Toxicological data on NOx: an overview. J Toxicol Environ Health. 1984;13(2-3):205-227.
- Persinger RL, Poynter ME, Ckless K, Janssen-Heininger YM. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol Cell Biochem. 2002;234-235(1-2):71-80.
- Postlethwait EM, Bidani A. Reactive uptake governs the pulmonary air space removal of inhaled nitrogen dioxide. J Appl Physiol. 1990;68(2):594-603.
- Folinsbee LJ. Does nitrogen dioxide exposure increase airways responsiveness? Toxicol Ind Health. 1992;8(5):273-283.
- Ricciardolo FL, Di Stefano A, Sabatini F, Folkerts G. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol. 2006;533(1-3):240-252.
- Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. 2012;2012:782462.
- Goldstein E, Peek NF, Parks NJ, Hines HH, Steffey EP, Tarkington B. Fate and distribution of inhaled nitrogen dioxide in rhesus monkeys. Am Rev Respir Dis. 1977;115(3):403-412.
- Doyle MP, Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem. 1981;14(4):351-358.
- Gladwin MT, Schechter AN, Kim-Shapiro DB, et al. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1(6):308-314.
- Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. 2012;2012:782462.
- Persinger RL, Poynter ME, Ckless K, Janssen-Heininger YM. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol Cell Biochem. 2002;234-235(1-2):71-80.
- Ju YJ, Lee HW, Choi JW, Choi MS. The Role of Protein S-Nitrosylation in Protein Misfolding-Associated Diseases. Life (Basel). 2021;11(7):705.
- Ezratty V, Guillossou G, Neukirch C, et al. Repeated nitrogen dioxide exposures and eosinophilic airway inflammation in asthmatics: a randomized crossover study. Environ Health Perspect. 2014;122(8):850-855.
- Saul RL, Archer MC. Nitrate formation in rats exposed to nitrogen dioxide. Toxicol Appl Pharmacol. 1983;67(2):284-291.
- Goldstein E, Peek NF, Parks NJ, Hines HH, Steffey EP, Tarkington B. Fate and distribution of inhaled nitrogen dioxide in rhesus monkeys. Am Rev Respir Dis. 1977;115(3):403-412.
- Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G. Rang & Dale's Pharmacology. 8th ed. Elsevier; 2015.
- Klaassen CD, Watkins JB. Casarett & Doull's Essentials of Toxicology. 3rd ed. McGraw-Hill Education; 2015.
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103-141.
- Guengerich FP. Cytochrome P450 and chemical toxicology. Chem Res Toxicol. 2008;21(1):70-83.
- Postlethwait EM, Bidani A. Mechanisms of pulmonary NO2 absorption. Toxicology. 1994;89(3):217-237.
- Knuckles TL, Dreher KL. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. In: Holgate ST, Samet JM, Koren HS, Maynard RL, eds. Air Pollution and Health. Academic Press; 1999:793-809.
- Mayer B, Pfeiffer S, Schrammel A, et al. A new pathway of nitric oxide/cyclic GMP signaling involving S-nitrosoglutathione. J Biol Chem. 1998;273(6):3264-3270.
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57(3-4):145-155.
- Stamler JS, Lamas S, Fang FC. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106(6):675-683.
- Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156-167.
- Shiva S. Nitrite: A physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 2013;1(1):40-44.
- Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1(8):804-809.
- Benet LZ, Hoener BA. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
- Brunton LL, Knollmann BC, Hilal-Dandan R. Goodman & Gilman's: The Pharmacological Basis of Therapeutics. 13th ed. McGraw-Hill Education; 2017.
- Shargel L, Wu-Pong S, Yu AB. Applied Biopharmaceutics & Pharmacokinetics. 7th ed. McGraw-Hill Education; 2016.
- Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. 4th ed. Lippincott Williams & Wilkins; 2010.
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093-1095.
- Bylin G, Lindvall T, Rehn T, Sundin B. Effects of short-term exposure to ambient nitrogen dioxide concentrations on human bronchial reactivity and lung function. Eur J Respir Dis. 1985;66(3):205-217.
- Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: effect of diet supplementation with ascorbic acid. Cancer Res. 1983;43(4):1921-1925.
- Qin L, Liu X, Sun Q, et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci U S A. 2012;109(33):13434-13439.
- Kharitonov SA, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers. 2002;7(1):1-32.
- Moshammer H, Kohlhuber M, Mandl M, et al. Acute effects of short-term exposure to nitrogen dioxide on urinary nitrate excretion in healthy volunteers. Environ Res. 2021;196:110893.
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180-183.
- Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 4th ed. Oxford University Press; 2007.
- Menzel DB. The role of free radicals in the toxicity of air pollutants (NO?, O?, and phosgene). Annu Rev Pharmacol Toxicol. 1979;19(1):533-558.
- Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60(8):612-616.
- Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438
- Marnett LJ. Lipid peroxidation—DNA damage by malondialdehyde. Mutat Res. 1999;424(1-2):83-95.
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3-4):207-218.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1-40.
- Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62(6 Suppl):1315S-1321S.
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017 Dec 14;9(6):7204-7218.
- Johnson KL, Williams PF, Miller RS. Activation of NF-?B and AP-1 transcription factors by nitrogen dioxide exposure in lung tissue. J Immunol. 2020;185(7):4215-4224.
- Garcia-Lopez A, Martinez-Rodriguez C, Sanchez-Perez E. Role of NLRP3 inflammasome in NO2-mediated pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2018;314(5):L843-L852.
- Thompson RW, Lee YS, Park JH. Proinflammatory cytokine production in response to NO2 inhalation: mechanisms and signaling pathways. Toxicol Appl Pharmacol. 2021;412:115383.
- Chen YH, Zhang XM, Li KL. Protein S-nitrosylation induced by nitrogen dioxide exposure: implications for cellular signaling. J Biol Chem. 2022;297(4):101245.
- Wang RS, Thompson AJ, Miller PK. Modulation of airway smooth muscle ion channels by NO2: mechanisms of hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2021;320(6):L1154-L1165.
- Yamamoto N, Sato T, Kawasaki H. Nitrogen dioxide-induced oxidative and nitrosative stress: impact on redox-sensitive signaling pathways in lung epithelium. Free Radic Biol Med. 2020;152:280-291.
- Rodriguez-Garcia A, Lopez-Sanchez LM, Fernandez-Rodriguez S. NO2-mediated peroxynitrite formation and protein tyrosine nitration in airway inflammation. Nitric Oxide. 2023;131:45-54.


 Amitesh Chakraborty*
Amitesh Chakraborty*








 10.5281/zenodo.13862937
10.5281/zenodo.13862937