Abstract
Acute lymphoblastic leukemia (ALL) can affect both children and adults, with a peak incidence between ages one and four. Most cases occur in otherwise healthy individuals, with few having identifiable risk factors. The disease is characterized by chromosome abnormalities and genetic changes that affect lymphoid precursor cells.Outcomes have improved significantly for children and young adults due to tailored treatment strategies, but older adults and those with relapsed or refractory ALL still face poor prognosis. New immunotherapy options, like CAR T-cell therapy and monoclonal antibodies, are being developed to enhance treatment.ALL is part of a broader group of lymphoid cancers, and distinguishing it from other cancers involves analyzing its specific morphological, immunophenotypic, and genetic traits. Current aggressive chemotherapy regimens cure about 85-90% of children and 40-50% of adults, but results can vary based on the disease's genetic subtype and clinical features at diagnosis. Monitoring minimal residual disease is essential for assessing prognosis and optimizing treatment
Keywords
Acute Lymphoblastic Leukemia, Genome-Wide Analysis, Hematopoietic Stem Cell Transplantation,Treatment Resistance
Introduction
OVERVIEW OF TRAMADOL
A brief history of development: Grünenthal GmbH, a German pharmaceutical company, synthesised tramadol for the first time in 1962. Originally created as a less dangerous substitute for other opioids, Tramadol was released onto the market in the latter part of the 1970s. Because of its special pharmacological characteristics, which combine inhibition of monoamine reuptake with opioid receptor activation, it became well-known as a useful tool for treating moderate to severe pain. Tramadol has become widely accepted over the years in a number of medical specialities, from the treatment of neuropathic pain to general pain management. The pharmaceutical industry relies heavily on the development and validation of analytical methods to ensure the high quality, safety, and efficacy of the pharmaceuticals produced. Since tramadol is a commonly used opioid analgesic, precise quantification and qualification of its presence in different formulations necessitates the employment of stringent analytical procedures. The process of creating an analytical method for Tramadol entails choosing a suitable methodology, such as gas chromatography (GC) or high-performance liquid chromatography (HPLC), among others. Contrarily, method validation guarantees that the created procedure is dependable, repeatable, and appropriate for the goal for which it was designed. Parameters including accuracy, precision, specificity, linearity, and robustness are evaluated during this process. To achieve consistent production of Tramadol with optimal therapeutic efficacy and to comply with regulatory criteria, method development and validation must be integrated.
PHARMACOLOGY OF TRAMADOL :
1. MECHANISM OF ACTION :
Dual Action:
The two primary ways that tramadol works make it special.
Opioid Agonist:
Tramadol's main mechanism of action is its binding to the central nervous system's ?-opioid receptors, which produces analgesic effects that are comparable to those of other opioids but less strong than morphine.
Monoamine Reuptake Inhibition:
Tramadol differs from conventional opioids by inhibiting the reuptake of serotonin and norepinephrine, which contributes to its analgesic effects. The management of mild to moderately severe pain is especially well-suited for this dual mechanism.
2. PHARMACOKINETICS :
Absorption:
Tramadol has a bioavailability of approximately 75% when taken orally. It is quickly absorbed and goes through first-pass metabolism when taken orally.
Metabolism:
O-desmethyltramadol (M1), an active metabolite with a greater affinity for the ?-opioid receptor, is principally produced in the liver by the cytochrome P450 enzyme CYP2D6.
Elimination:
The kidneys are the main organ through which the medication and its metabolites are eliminated. The parent chemical has a half-life of roughly 6 hours, while the active metabolite has a half-life of 7.5 hours.
3. THERAPEUTIC USES :
Pain management:
Tramadol is frequently used to treat moderate-to-severe pain, which includes neuropathic pain, chronic pain syndromes, and post-operative pain.
For instance:
It is frequently used when non-opioid analgesics are ineffective for treating musculoskeletal pain, such as osteoarthritis.
4. SIDE EFFECTS AND SAFETY PROFILE :
Frequent adverse effects include headache, nausea, constipation, and dizziness. Although it is less likely than with other opioids, the risk of dependency and misuse is still present because of its opioid composition.
Serious Side Effects:
Patients who have a history of epilepsy or who are taking other drugs that reduce the seizure threshold may experience seizures while using tramadol. A rare but significant risk is serotonin syndrome, especially when combined with other serotonergic medications.
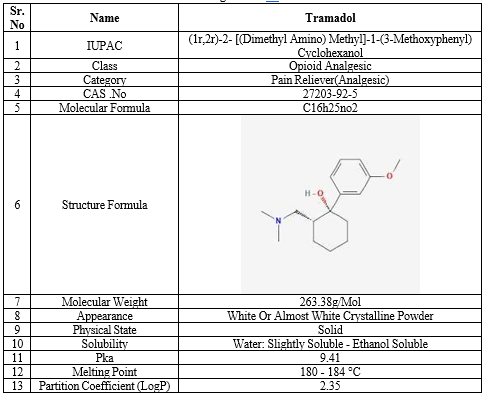
Table 1: Drug Profile Of Tramadol
VARIOUS ANALYTICAL ESTIMATION METHODS :
HPLC METHODS :
While tramadol is dosed at 1 mg/kg to treat children's acute pain, simultaneously administered drugs are frequently overlooked during the test for tramadol in plasma samples for pharmacokinetic and toxicological research. In order to measure tramadol and its primary metabolite, O-desmethyl tramadol, in human plasma in the presence of seven potentially interfering medications, we devised and validated an HPLC-UV approach in this study. A method of preparing samples that combined protein precipitation and liquid-liquid extraction was created. Using a double gradient approach, chromatographic separation was obtained on a BDSHypersil-C18 column (5µm, 250cm, 4.6µm). The tramadol and ODT limit of quantitation was 6.7 ng/ml. The accuracy and precision complied with ICH regulations. 137 blood samples were successfully analysed using this technology. A centrally acting analgesic, tramadol is used to prevent and treat moderate to severe pain. It is predicted that 0.1% of the amount given to breastfeeding newborns enters their breast milk and may have unintended effects. From a pharmacokinetic perspective, breast milk is thought to function as a distinct compartment where drugs are eliminated, primarily by passive diffusion. Breast milk has a complicated composition, therefore in order to determine the drug, an appropriate sample preparation method and subsequent chromatographic analysis are required. After testing a number of sample cleansing techniques, we decided that the liquid-liquid extraction method, which uses n-hexane as an organic phase and reverse extraction into an aqueous phase, was the most appropriate and compatible with the ensuing HPLC analysis. The accuracy and repeatability of the Using metoprolol as an internal standard increased the method's precision and reproducibility by about two times, increasing its robustness against variations in the composition of milk samples. These attributes, along with a low detection limit and a quick analysis time, demonstrated that the developed approach is appropriate for tracking tramadol levels in human breast milk.
LCMS TECHNIQUES :
The aim of the research was to evaluate the benefits of various mass spectrometric devices and commercially accessible metabolite identification software for the purpose of metabolite profiling. Because tramadol has a well-known and extensive metabolism, its hydrochloride metabolism and the excretion of both the drug and its metabolites into human urine were utilised as test cases. Using a quadrupole time-of-flight mass spectrometer (Q-TOF) fitted with a LockSpray dual-electrospray ionisation source, precise mass measurements were made. For full scan, product ion, precursor ion, and neutral loss scan measurements, a triple quadrupole mass spectrometer (QqQ) was utilised, and for full scan and product ion measurements, an ion trap apparatus was used. Two metabolite identification systems were examined for performance. The findings demonstrated that while metabolite programs are useful time-savers, fully automated metabolite profiling is still not possible with them. It is still nearly hard to detect unexpected metabolites, particularly at low concentrations in a complex matrix. Urine samples found difficult to analyse with low-resolution equipment, even when looking for predicted compounds. The automatic searching produced a large number of false-positive finds, necessitating the manual examination of the collected data. By employing the improved mass accuracy Q-TOF, false positives were prevented. While automated systems proved helpful in building product ion techniques, the laborious task of interpreting mass spectra was done by hand. The tramadol metabolites were confirmed using high-quality MS/MS spectra obtained with the QqQ instrument. Tramadol metabolite identification was challenging due to the low mass cutoff of the ion trap instrument, despite the equipment's undeniable value in MS(n). The tramadol urine sample contained some previously unreported metabolites, which were identified using only LC/MS and LC/MS/MS measurements.
UV ESTIMATION :
For the simultaneous quantitative evaluation of tramadol and paracetamol in paracetamol-tramadol pills, UV spectrophotometric methods were devised. In order to ascertain the amount of active ingredients in the tablets, the spectrophotometric data were processed using partial least squares (PLS) and genetic algorithms combined with PLS (GA-PLS) techniques. A statistical comparison was made between the outcomes of the chemometric processing of the spectroscopic data and the results acquired using an ultra-high performance liquid chromatographic method that has been validated. By evaluating the synthesised drug combination and computing recovery and relative standard error (RSE), the generated chemometric models' accuracy and precision were confirmed. The levels of paracetamol identified by this study and those obtained using the PLS and GA-PLS algorithms showed statistically good agreement. However, for tramadol, the GA-PLS results were found to be more reliable than the PLS ones. The PLS approach for paracetamol (mean recovery 99.5%, RSE 0.89%) and the GA-PLS method for tramadol (mean recovery 99.4%, RSE 1.69%) yielded the most straightforward, accurate, and exact models.
Table 2 : UV Spectrophotometric Method Of Tramadol
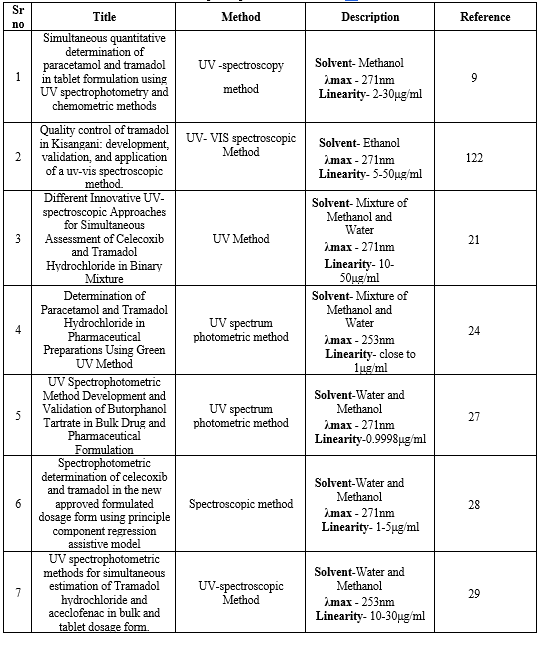
TABLE 3 : HPLC METHOD OF TRAMADOL

Table 4 : LC-MS Method For Tramadol
PHARMACOLOGICAL CLASSIFICATION
( OPIOID ANALGESIC ) :
Tramadol is classified as a centrally acting opioid analgesic. Unlike more potent opioids like morphine or fentanyl, Tramadol is considered a weak opioid, primarily due to its lower affinity for the µ-opioid receptor. This classification is crucial in understanding Tramadol’s therapeutic profile, as it offers pain relief while posing a lower risk of addiction and respiratory depression compared to stronger opioids. Tramadol's classification also reflects its dual mechanism of action, which provides a broader therapeutic
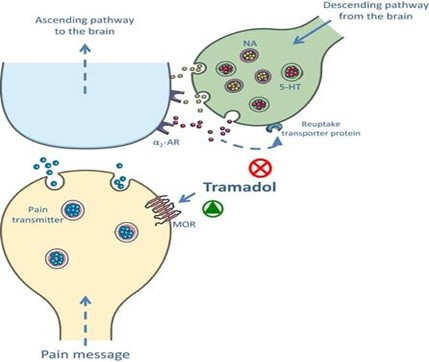
Figure 2. PHARMACOLOGY STRUCTURE OF TRAMADOL
PHARNACOKINETICS AND PHARMACODINEMICS
PHARMACOKINETICS
Absorption:
Due to first-pass metabolism, tramadol is virtually entirely and quickly absorbed when taken orally. Its bioavailability is roughly 70%. Usually occurring two hours after injection, the peak plasma concentration (C_max) can differ based on the formulation (immediate-release vs. extended-release).
Distribution:
Tramadol has a volume of distribution ranging from 2.6 to 2.9 L/kg and is broadly disseminated throughout the body. Its ability to effectively relieve central pain requires that it easily pass through the blood-brain barrier. Additionally, tramadol passes through the placenta and is present at trace levels in breast milk.
Metabolism:
The liver is the main site of tramadol metabolism. CYP2D6 and CYP3A4 in particular are involved in this process. O-desmethyltramadol (M1), the main active metabolite, is generated by CYP2D6 and has around six times the potency of the parent drug at the µ-opioid receptor. This metabolite has a major role in Tramadol's analgesic properties.
Excretion:
60% of tramadol's metabolites and 30% of the drug itself are eliminated as unaltered compounds through the kidneys. Tramadol has an elimination half-life of roughly 6 to 7 hours, though it can be longer in individuals with renal impairment.
PHARMACODINEMICS
Opioid Receptor Agonism: Tramadol is categorised as a weak opioid due to its moderate affinity for the µ-opioid receptor. Nevertheless, the central nervous system's activation of ?-opioid receptors efficiently regulates pain signals, producing analgesic effects that are comparable to those of stronger opioids like morphine, albeit less potent.
Norepinephrine and Serotonin Reuptake Inhibition:
Tramadol not only activates opioid receptors but also prevents norepinephrine and serotonin from being reabsorbed, which intensifies its analgesic effects. Compared to other opioids, this dual action is especially helpful in treating neuropathic pain and may also be a factor in the decreased prevalence of tolerance and dependency.
ANALAYTICAL METHODS FOR TRAMADOL
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY ( HPLC )
Principle:
High-Performance Liquid Chromatography (HPLC) is a widely used analytical technique for the separation, identification, and quantification of Tramadol in various matrices, including pharmaceutical formulations and biological samples. The method relies on the differential partitioning of Tramadol and its metabolites between a mobile phase (typically a solvent or solvent mixture) and a stationary phase (usually a packed column). Tramadol is separated based on its interaction with the stationary phase and is detected using UV or fluorescence detectors.
Applications:
HPLC has been extensively applied in the quality control of Tramadol formulations, as well as in pharmacokinetic studies where accurate measurement of Tramadol and its metabolites in plasma or serum is required. It is valued for its precision, reproducibility, and ability to analyze complex mixtures with high sensitivity.
LIQUID CHROMATOGRAPHY – MASS SPECTROMETRY (LC-MS/MS)
Principle:
Liquid Chromatography-Mass Spectrometry (LC-MS/MS) combines the separation capabilities of liquid chromatography with the detection power of mass spectrometry. LC- MS/MS is particularly useful for the quantification of Tramadol in biological matrices, offering enhanced specificity and sensitivity. The technique involves ionizing Tramadol molecules in the mass spectrometer and measuring their mass-to-charge ratio, allowing for precise quantification even at low concentrations.
Applications: LC-MS/MS is frequently employed in pharmacokinetic and forensic toxicology studies due to its ability to detect and quantify Tramadol and its metabolites in complex
biological samples such as blood, urine, and tissue. Its high sensitivity makes it suitable for detecting trace levels of Tramadol, which is crucial in cases of drug overdose or compliance monitoring.
GAS CHROMATOGRAPHY – MASS SPECTROMETRY (GC-MS)
Principle:
Gas Chromatography-Mass Spectrometry (GC-MS) is another powerful technique used for the analysis of volatile compounds. Although less commonly used for Tramadol due to its relatively non-volatile nature, GC-MS can be employed after derivatization to enhance volatility. The method involves the separation of Tramadol and its derivatives based on their volatilities and their subsequent detection via mass spectrometry.
Applications:
GC-MS is particularly useful in forensic toxicology for the detection of Tramadol in biological samples, especially in post-mortem cases where confirmation of drug use is required. The method is also used for the analysis of Tramadol in hair, which can provide a history of drug exposure over a longer period.
Method Validation
OVER VIEW OF METHOD VALIDATATION
Definition and Importance: Method validation is the process of proving that an analytical method is acceptable for its intended purpose. Validation is crucial in pharmaceutical analysis to ensure that the results obtained are accurate, reliable, and reproducible. It involves a series of tests to evaluate various parameters such as accuracy, precision, specificity, linearity, and robustness.
KEY VALIDATATION PARAMETERS
ACCURACY AND PRECISION:
Accuracy refers to the closeness of the measured values to the true value, while precision indicates the repeatability of the measurements under the same conditions. These parameters are assessed through recovery studies and repeated analyses.
LINERITY:
Linearity is the method's ability to produce results that are directly proportional to the concentration of analyte within a given range. Linearity is usually tested by preparing calibration curves and calculating the correlation coefficient.
SPECIFICITY:
Specificity is the ability of the method to measure the analyte response in the presence of other components like impurities, degradation products, or matrix elements.
ROBUSTNESS:
Robustness refers to the method’s capacity to remain unaffected by small, deliberate variations in method parameters, providing an indication of its reliability during normal usage.
LIMIT OF DETECTION:
In accordance with International Conference on Harmonisation (ICH) guidelines, the drug's limit of detection (LOD) was determined using the following formulas: LOD is 3.3 X ?/S. where ? is the response's standard deviation. S is the calibration curve's slope.
LIMIT OF QUANTIFICATION:
In accordance with guidelines established by the International Conference on Harmonisation (ICH), the drug's limit of quantification (LOQ) was determined using the following equations: LOQ is equal to 10 ? ?/S. where ? is the response's standard deviations is the calibration curve's slope.
Enhanced Analytical Techniques:
Future research should focus on improving the sensitivity and specificity of analytical methods for Tramadol and its metabolites. This could involve the development of new chromatographic techniques or the integration of advanced technologies like tandem mass spectrometry.
Exploring New Therapeutic Uses:
There is potential for expanding the therapeutic applications of Tramadol beyond pain management. Research into its effects on mood andcognitive function could lead to new indications, particularly in treating conditions where pain is accompanied by mood disorders.
Regulatory Challenges:
As the use of Tramadol increases, especially in light of the opioid crisis, there will be ongoing challenges in balancing accessibility with the need for stringent regulatory controls. Research into the long-term safety and efficacy of Tramadol, as well as strategies to minimize misuse, will be crucial.
CONCLUSION
Summary of Key Findings: This review article has provided a comprehensive analysis of Tramadol, highlighting its pharmacological properties, therapeutic applications, and the importance of validated analytical methods in its analysis. Tramadol’s dual mechanism of action, involving both opioid receptor agonism and the inhibition of neurotransmitter reuptake, underpins its effectiveness in treating a variety of pain conditions, particularly neuropathic pain. Despite its classification as a weak opioid, Tramadol remains a vital option in pain management due to its favorable safety profile compared to stronger opioids. Clinical Relevance: In clinical practice, Tramadol continues to be a valuable analgesic, particularly for patients who require pain relief but are at risk of opioid dependence or have contraindications to stronger opioids. The drug’s efficacy is closely tied to its pharmacokinetic and pharmacodynamic properties, which vary among individuals, emphasizing the need for personalized dosing strategies. The review of analytical methods underscores the necessity of robust validation processes to ensure the accuracy and reliability of Tramadol measurements in both pharmaceutical formulations and biological matrices. In conclusion, Tramadol’s unique pharmacological profile, combined with the rigor of validated analytical methods, ensures its continued relevance in pain management. However, its use must be carefully managed to maximize therapeutic benefits while minimizing potential risks. The evolving landscape of pain management and opioid regulation will undoubtedly influence the future role of Tramadol in clinical practice.
REFERENCE
- Grond, S., & Sablotzki, A. (2004). Clinical pharmacology of tramadol. Clinical Pharmacokinetics, 43(13), 879-923.
- Minami, K., & Ogata, J. (2015). Pharmacokinetics and pharmacodynamics of tramadol. Pain Research and Treatment, 2015, Article ID 903231.
- Leppert, W. (2009). CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology, 83(3), 138-147.
- McQuay, H. J., Moore, R. A., & Justins, D. M. (1997). Tramadol for pain relief. Expert Opinion on Pharmacotherapy, 7(3), 303-318.
- Cossmann, M., & Ziegler, H. (1999). Clinical pharmacokinetics of tramadol. Therapeutic Drug Monitoring, 21(6), 626-630.
- Raffa, R. B., Friderichs, E., Reimann, W., Shank, R. P., Codd, E. E., & Vaught, J. L. (1992). Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an “atypical” opioid analgesic. 8)Journal of Pharmacology and Experimental Therapeutics, 260(1), 275-285.
- Dayer, P., Desmeules, J., & Collart, L. (1997). Pharmacology of tramadol. Drugs, 53(Suppl 2), 18-24.
- Grond, S., Meuser, T., Zech, D., & Lehmann, K. A. (1995). Analgesic efficacy and tolerability of tramadol in comparison to other opioids. Drug Research, 45(12), 1433-1436.
- Emami, J., Tavakoli, N., & Amini, M. (2006). Simultaneous determination of tramadol and its two main phase I metabolites in human plasma by high-performance liquid chromatography. Journal of Chromatography B, 830(2), 207-211.
- De Sousa Mendes, M., Feliu, C., & Picard, N. (2016). Development and validation of an LC-MS/MS method for quantification of tramadol and O-desmethyltramadol in human plasma. Journal of Pharmaceutical and Biomedical Analysis, 118, 289-297.
- Verplaetse, R., & Tytgat, J. (2012). Development and validation of a sensitive gas chromatography-mass spectrometry method for the determination of tramadol and O- desmethyltramadol in hair. Journal of Analytical Toxicology, 36(3), 157-165.
- International Council for Harmonisation (ICH). (2005). ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology Q2(R1). Geneva: ICH.
- U.S. Food and Drug Administration (FDA). (2018). Analytical Procedures and Methods Validation for Drugs and Biologics: Guidance for Industry. Silver Spring, MD: FDA.
- Grond, S., & Sablotzki, A. (2004). Clinical pharmacology of tramadol. Clinical Pharmacokinetics, 43(13), 879-923.
- Leppert, W. (2009). CYP2D6 in the metabolism of opioids for mild to moderate pain. Pharmacology, 83(3), 138-147.
- Ashour, S., & Al-Khalil, R. (2005). "Simple extractive colorimetric and UV-spectrophotometric methods for the determination of tramadol hydrochloride in pharmaceutical formulations." Analytical Letters, 38(4), 639-651.
- Shafiee, M., Shamsipur, M., & Jalali, F. (2013). Simple and fast spectrophotometric determination of tramadol hydrochloride in pharmaceutical formulations. Asian Journal of Chemistry, 25(16), 9179-9182.
- Pawar, P., Chopade, V. V., & Chaudhari, S. R. (2012). Development and Validation of UV Spectrophotometric Method for Simultaneous Estimation of Aceclofenac and Tramadol Hydrochloride in Bulk and Tablet Dosage Form. International Journal of Pharmaceutical Sciences and Research, 3(3), 837-841
- Reddy, P., et al. (2024). "Green UV-Vis spectrophotometry for the quantitative analysis of Paracetamol and Tramadol Hydrochloride in pharmaceutical formulations." Analytical Methods, 16(4), 776-784.
- Published in International Journal of Pharmaceutical Sciences and Research (2023). This article covers the methodology, including optimal solvent and wavelength for the analysis.
- Bhinge, J. R.; Kumar, R. V; Sinha, V. R. A Simple and Sensitive Stability Indicating RP-HPLC Assay Method for the Determination of Aceclofenac. J. Chromatogr. Sci. 2008, 46, 440–444.
- Indian pharmacopoeia. Delhi: Govt. of India. Ministry of health & family welfare, the controller & publication. Vol. III; 2010.p. 2245-7.
- British Pharmacopoeia. London: The Stationery Office on behalf of the Medicines and Healthcare products Regulatory Agency (MHRA); 2003. p. 1868-9.
- United States Pharmacopoeia and National Formulary. 36thAsian Edition USA: The United States Pharmacopoeia Convention Inc. p. 5435-6.
- Atto RA. New Method for Determination of Diclofenac Sodium by High Performance Liquid Chromatography. Tikrit J of PharmSci 2012; 8(1):60-7.
- Choudhary B, Goyal A, Khokra S L, Kaushik D. Simultaneous Estimation of Diclofenac Sodium and Rabiprazole by Hplc Method in Combined Dosage Form. Int J of Pharm Sci and Drug Res 2009; 1(1): 43-5.
- Sinde VM, Desai BS, Tendolkar NM. Simultaneous determination of paracetamol, diclofenac Na and chlorzoxazone by HPLC from tablet. Indian J Pharm Sci 1995;57(1): 35-7.
- Abdelaleem EA, Abdelwahab NS. Stability-indicating TLC –densitometric Method for Simultaneous Determination of Paracetamol and Chlorzoxazone and their Toxic Impurities. J of Chromatographic Sci 2012; 1–5.
- ICH Harmonized Tripartite Guidelines. Validation of analytical procedures: Text and Methodology Q2 (R1) Geneva; 2005.
- Yoo, E.K.Y. Tang, M.N. Nguyen, S. Salman, A.J. Hua, B.S. von Ungern Sternberg, L.Y. Lim, HPLC-UV assay of tramadol and O-desmethyltramadol in human plasma containing other drugs potentially co-administered to participants in a paediatric population pharmacokinetic study, Journal of Chromatography B, Volume 1184, 2021, 122971, https://doi.org/10.1016/j.jchromb.2021.122971.
- Vojko Kmetec, Robert Rokar, HPLC determination of tramadol in human breast milk, Journal of Pharmaceutical and Biomedical Analysis, 32, 4, 2003, 1061-1066, https://doi.org/10.1016/S0731-7085(03)00209-7.


 Zakira Chaudhary*
Zakira Chaudhary*
 Aarti Yadav 2
Aarti Yadav 2





 10.5281/zenodo.13987731
10.5281/zenodo.13987731