Benzimidazole, a bicyclic compound formed by the fusion of benzene and imidazole rings, is recognized for its profound biological activity and significant impact on medicinal chemistry. This review provides a comprehensive analysis of benzimidazole derivatives, highlighting their key pharmacological activities, including analgesic, anti-inflammatory, diuretic, antimicrobial, antiulcer, antioxidant, anti-asthmatic, anti-diabetic, anticancer, antiviral, antiarrhythmic, anticonvulsant, antiprotozoal, hypotensive, and neuroprotective effects. The robust affinity of benzimidazoles for a diverse array of enzymes and protein receptors underscores their status as privileged sub-structures in pharmacological design.. The integration of the benzimidazole nucleus in drug development continues to play a crucial role in contemporary therapeutic research, offering versatile and effective treatments for a wide range of diseases.
Obesity, Causes of Obesity, Treatment of obesity, Prevention of Obesity, Natural Product
Based on a review of body mass index data, the World Health Organization (WHO) declared obesity to be a global epidemic threat in 1997 (BMI). Since then, the prevalence of obesity has alarmingly increased and is now a significant public health concern. In fact, obesity not only contributes to the development of chronic diseases such as stroke, osteoarthritis, sleep apnea, malignancies, and inflammation-based pathologies, but also to metabolic disorders such as diabetes, hypertension, and cardiovascular diseases. Studies conducted in several nations have shown that the cost of health care for an obese person is at least 25% greater than that of a healthy person. When production losses are combined with health care expenses, obesity contributes significantly to the loss of GDP in the majority of countries (>1% in the US, >3.6% in China). Iatrogenic obesity refers to obesity brought on by medication side effects (such as antipsychotic, antidepressant, antiepileptic, steroids, and insulin) or medical conditions (such as Cushing syndrome, hypothyroidism, and hypothalamic abnormalities). As a primary condition, obesity is characterized by a positive energy balance. Finding the underlying reasons of this imbalance, which account for most instances found after secondary obesity-related factors are ruled out, is still difficult. Complex interactions between behavioral, genetic, and environmental factors that are correlated with lifestyle choices and social and economic position lead to this chronic illness. Actually, populations that experience a long-term energy positive imbalance—caused by a sedentary lifestyle, a low resting metabolic rate, or both—are more likely to be obese. Genes, metabolism, food, exercise, and the sociocultural milieu that defines the 21st-century lifestyle are all contributing factors to obesity. Finding plausible biological targets that can be altered by outside influences, such food and medications, may help people learn to regulate their hunger and prevent obesity. Nutritional genomics may be able to identify the precise nutrients that cause phenotypic changes that impact the likelihood of obesity and find the most significant relationships. Global approaches to slowing the development of obesity center on dietary and lifestyle changes, such as reducing caloric intake and increasing physical activity. Studies have shown that natural ingredients have the ability to combat obesity. In comparison to chemical treatments, combining numerous natural products may produce a synergistic activity that boosts their bioavailability and impact on multiple molecular targets. These substances have anti-obesity properties that are mediated via regulating many pathways, such as lipid absorption, energy intake and expenditure, lipogenesis, preadipocyte differentiation, and proliferation, and an increase in lipolysis.
Definition:
A genetic, psychological, and socio-environmental imbalance between calorie intake and energy expenditure in favor of the former is the primary cause of obesity, which is defined by an excessive buildup of body fat [1, 2, 3, 4].
2. Causes of Obesity:
Over nutrition and a sedentary lifestyle lead to an imbalance in energy intake and expenditure, which is the primary cause of obesity. Adipose tissue (AT) stores excess nutrients as triglycerides, which, in the event of a nutrient deficiency, other tissues will use as nutrients through lipolysis. There are two main kinds of AT: brown AT, which is a specific kind of fat depot that generates heat by lipid oxidation and takes part in non-shivering thermogenesis. White AT (WAT) is the other type of AT. (5,6) Insufficient sleep, endocrine disruptors, decreased variability in ambient temperature, decreased smoking rates—smoking suppresses appetite—increased use of medications that can cause weight gain (e.g., atypical antipsychotics), proportionate increases in ethnic and age groups that tend to be heavier, later pregnancy (which may increase a child's susceptibility to obesity), epigenetic risk factors passed down through generations, natural selection for higher BMI, and assortative mating leading to increased concentration of obesity risk factors are ten additional potential causes of the recent rise in obesity.23 4.1. Between 1971 and 2000, the US had a rise in diet-related obesity from 14.5% to 30.9%.24 The average amount of food consumed increased throughout the same period (average rise for women 335 and 168 cal./day). Rather than fat consumption, the increase in carbohydrates was mostly responsible for this increased food energy. (7)
Environmental Factor:
It is evident that the environment must support weight gain for obesity to emerge, regardless of the genetic component of the tendency to acquire weight. This has undoubtedly played a significant role in the recent and sharp rise in obesity rates over the past several years. Environmental variables that could have an impact include shifts in the population's level of physical activity and the volume or makeup of the foods consumed.
Diet:
Research on the population's overall calorie consumption indicates a paradoxical decline since the 1980s (in the UK) in the context of the fastest-rising and longest-lasting rise in the prevalence of obesity. Soft drinks, alcoholic beverages, and food that is bought and consumed outside the home are not included in this, though. Due to its high fat content, this kind of food is typically high in energy.8 Experiments indicate that eating such food promotes overindulgence and results in less satiety than eating less energy-dense foods, indicating that the hypothalamus and peripheral regulatory systems may be less sensitive to a high-fat diet. (8)
Physical Activity:
Over the past 30 to 50 years, there has been a sharp fall in levels of physical exercise. There is an increased dependence on automobiles, a decline in manual labor, and a rise in the use of labor-saving technology. Physical activity during leisure time has decreased as well, coinciding with a rise in television and computer game use. The latter is especially concerning for kids, as obesity rates are startlingly on the rise. (9)
Social Factor:
Although it is a characteristic of wealthy Western civilization, individuals from socioeconomic classes 4 and 5 are more likely to be obese. This is probably due to the fact that these populations typically eat a diet higher in fat and engage in less physical activity, particularly given the reduction in traditional manual jobs.
Polygenic Traits:
In an attempt to learn more about the underlying causes of obesity, a great deal of work has gone into identifying genes that may be more prevalent in obese people. Despite the discovery of at least 250 putative gene loci, (10) the search has yielded little results thus far. However, a polymorphism in the ?3-adrenoceptor appears to be linked to an increased risk of diabetes in obese individuals as well as increased weight gain in cases of morbid obesity, but not with obesity per se. Obesity candidate genes have typically included well-known targets, like those encoding proteins that govern energy expenditure (like UCP1 or the ?3-adrenoceptor) or regulate energy intake (like NPY or leptin). It is plausible, therefore, that genes linked to a variety of behaviors, such as a predilection for high-fat foods, a fidgeting tendency, or a high degree of physical activity, could be polygenic features that result in obesity.
3. Disorders Associated with Obesity
Hypertension:
A higher BMI is also associated with an increased risk of hypertension. The Nurses' Health Study assessed the risk of hypertension in 41,541 primarily white female nurses between the ages of 38 and 63 (11). Overweight and obese women had a higher risk of hypertension during a 4-year follow-up compared to those with a BMI of less than 23 kg/m2. For people with a BMI of 32 kg/m2 or greater, the relative risk was 4.8 percent. Weight loss decreased the risk of hypertension, while weight gain in each textile of starting BMI significantly raised the risk in addition to BMI (12).Obesity raises the risk of hypertension, according to NHANES III data (13). Comparing respondents with a BMI of 30 kg/m2 or more to those who were not fat, the likelihood of having hypertension was twice as high. In a similar vein, individuals who met the criteria for abdominal obesity—a waist circumference of at least 102 cm for males and 88 cm for women—had a twice-higher risk of hypertension. There was a clear correlation between BMI, abdominal obesity, and the odds ratio for hypertension in the Hispanic, African American, and white populations. Increased vascular volume is a hallmark of obesity-related hypertension, but peripheral resistance is typically borderline or very slightly raised (12). Increased renal salt and water absorption, changes in Na+/H+-ATPase activity, growth factor-mediated structural alterations to the arterial wall, and sympathetic nervous system activation are some of the factors that may contribute to the development of hypertension in obese patients. Hyperinsulinemia might have a role in each of these situations.
Dyslipidemia:
The elevated amounts of free fatty acids in obesity have more effects than just impaired glucose uptake and increased hepatic glucose production. By raising the amount of very-low-density lipoprotein produced by the liver, decreasing levels of high-density lipoprotein (HDL) cholesterol, and raising the quantity of small, dense low-density lipoprotein (LDL) particles, increased FFAs also have an impact on lipid metabolism (14). Compared to bigger, buoyant LDL particles, these smaller particles are more atherogenic, have a greater ability to pass through the artery wall, and are more prone to oxidation and glycation. Because of the smaller LDL particles, atherogenic risk may be increased even in cases where the LDL cholesterol level does not change significantly. When combined, these modifications to the lipoprotein profile are linked to a higher chance of coronary heart disease. A study of women with modest or high abdominal obesity (15) shows how obesity affects lipid metabolism. In these two groups, the area of abdominal fat was 107 and 187 cm2, respectively. An abdominal fat region of 50 cm2 was present in the non-obese control group of women. Compared to women with modest abdominal obesity, those with high abdominal obesity had abnormally high triglyceride and low HDL-cholesterol levels in relation to the non-obese group. The obese women had a slight rise in LDL cholesterol, but their LDL particles were denser and atherogenic.
CHD:
Insulin fasting levels and CHD mortality are directly correlated. An independent predictor of CHD death in the Paris Prospective Study (16) included fasting insulin levels, which assessed CHD risk variables in almost 7000 working males. Men with fasting insulin levels of 5 ?U/mL or less had a lower incidence of CHD mortality, but those in the highest quintile (>19 ?U/mL) had a 2.5-fold greater incidence. Similarly, in a case-control study of Canadian men, the fasting insulin level was discovered to be an independent risk factor for the development of CHD (17). After controlling for LDL, HDL, plasma triglycerides, Apo lipoprotein B, and cholesterol, the insulin level was still independently linked to the risk of coronary heart disease. In women who had never smoked, the Nurses' Health Study showed a link between obesity and CHD mortality (18). With rising BMI, there was a substantial increase in the relative risk of dying from CHD (p < 0>
Gallbladder Disease:
A larger waist-to-hip ratio and an elevated BMI in women were associated with an increased likelihood of hospitalization for gallbladder illness. Compared to women with a BMI < 25>
Cancer:
A 12-year follow-up research involving 750,000 men and women assessed prospectively the effect of obesity on cancer mortality (19). Cancer deaths were 33% and 55% more common in men and women who were at least 40% overweight, respectively, then in medium weight individuals. More specifically, those who were at least 40% overweight had the greatest death ratios for colorectal and prostate cancer in males and endometrial, uterine, cervical, ovarian, gallbladder, and breast cancer in women. The cancers of the uterus and endometrium have the highest death rates. In a similar vein, cancer mortality rose as BMI increased in the Nurses' Health Study (18). Women with a BMI of at least 32 kg/m2 had a cancer death risk that was twice as high as that of women with a BMI of less than 19 kg/m2. The increased mortality from endometrial, breast, and colon cancers was the main cause of the higher rate.
4. Prevention of obesity:
Initiatives to improve nutrition and prevent obesity and its complications in different countries:
Several tactics have been tried with this method in various nations. Financial disincentives are used to discourage unhealthy behaviors, such as quotas for each employee who exceeds the accept - tabular limit of waist circumference (Japan) and incentives to promote healthy behaviors, such as tax breaks for families who play sports (Canada). There have also been initiatives to improve the variety and quality of the food supply, such as the provision of fruits and vegetables in remote or economically disadvantaged areas (UK, USA), restrictions on the availability of foods and beverages high in fat, sugar, or salt in schools (France, Spain, USA, etc.), measures to control and regulate food and beverage advertising through self-regulatory codes and other measures (Spain, UK, etc.); creating communities that support healthy eating and physical activity through initiatives like the EPODE program in France and other countries; Shape-Ups Somerville in the U.S. and other community interventions implemented in the U.S. and Australia; and changes in food labeling as the multiple traffic light (UK) or the Healthy Choices symbol (The Netherlands).(19, 21) In other situations, laws dictating the required minimum amount of time spent on physical education in schools have been proposed in a few U.S. states. Other measures have controlled the treatment of overweight individuals or those with eating disorders. They have even regulated the systematic gathering of data on height and weight under specific circumstances for monitoring reasons, such as with the French school population. Table II lists the various countries' adopted obesity prevention policy initiatives. The overall goal of policies is to create the conditions necessary to create areas (both temporal and spatial), welcoming and safe pathways to promote physical activity, and easy access to food and drink that support the adoption of healthy eating practices. Certain actions have resulted in modifications to the menus offered in school canteens or to the selection of food and drinks available at establishments within school buildings, sports facilities, or youth recreation centers. Other initiatives, such as quantitative advice and graphics like the multiple traffic light or symbols like the healthy choices logo or the green lock, have regulated the nutrition information on food labels to make it easier for consumers to understand and support them in making informed food and beverage choices in line with healthy eating patterns (22). Fiscal policies in various nations have advocated taxing foods and beverages that are high in energy density or lowering the price of foods and vegetables to encourage people to eat them more. (23). Furthermore, regulations ought to encourage people to modify their own behavior in order to adopt and practice better food and exercise habits influenced by their local surroundings.(20, 21 and 22) To reduce the burden of obesity and chronic diseases through dietary changes, the Global Strategy on Diet, Physical Activity and Health (DPAS) and PAHO list the following as important interventions: encouraging and protecting exclusive breastfeeding until the child reaches six months of age; increasing the availability, accessibility, and consumption of plain water; reducing the amount of added sugar in drinks; reducing the intake of saturated fat; eliminating the consumption and production of industrial trans fats; increasing the consumption of fruits and vegetables consumption of whole grains and fiber in the diet, cutting back on sodium intake, reducing portion sizes in eateries and stores that sell processed and prepared foods, raising nutrition awareness for health literacy, and enhancing the population's ability to make responsible dietary decisions.
Strategy for Nutrition, Physical Activity and Obesity Prevention (EsNAOS):
Spain adopted the Strategy for Nutrition, Physical Activity, and Obesity Prevention (EsNAOS) in 2005 in response to the commitments made with the approval of the DPAS. This ambitious strategic plan set the lines of action for the following years and considered the implementation of measures like nutrition education programs, improving the food supply in school canteens, limiting the availability of energy-dense products in vending machines located in the school environment, promoting school sport activities, controlling food and beverage advertising aimed at children, and ensuring that products meet adequate nutritional composition, primarily in terms of salt and trans fat content. Additionally, the strategy focused on primary care protocols were developed. Three primary locations are the focus of all these action lines. in the home and community through information campaigns, the formation of regional and local working groups, and partnerships with advertisers, toy companies, and entertainment businesses. At the school level, the NAOS Strategy consists of three main lines of action: regulation and supervision of food supply and operation of school canteens; regulation of the supply of food and beverages through school vending machines; and nutrition education through the curriculum, specialized workshops, and teacher training in this area. It also emphasizes classroom physical education. Meeting places are deliberately excluded from the approach in all lines of activity. During negotiations with important parties, including the pertinent scientific and professional societies, the Federation of Parents societies, and business associations of the impacted industries, including food and beverage makers, catering, vending, and commercial distribution. Through the creation of agreements with the Spanish Federation of Food and Drink Industries (FIAB) and commercial distribution companies, such as ANGED or ASEDAS, the NAOS approach specifically takes into account the industrial sectors involved. The adoption and implementation of the code of self-regulation of food and beverage advertising directed towards children PAOS, which supported the strategy's design and implementation, was one of the outcomes of these collaboration agreements. Other outcomes included trade policies pertaining to "own brand" products or the development of food advertising and promotion under the NAOS Strategy. The Food Security and Nutrition Law 17/2011, of July 5, 2011, Was enacted by the Parliament in July 2011. A few months later, a con-sensus paper regarding food in schools was produced.
5. Natural products:
Natural substances that inhibit lipase. After being exposed to pancreatic lipases, dietary fat is absorbed by the gut. An essential enzyme in the absorption of dietary triacylglycerols is pancreatic lipase, which hydrolyzes triacylglycerols into monoacylglycerols and fatty acids.

Figure: 1
Few drugs such as orlistat directly interact with the lipases. It is a byproduct of Streptomyces toxytricini, a naturally occurring lipase inhibitor. (26) Orlistat inhibits by attaching itself covalently to the serine active site of lipase.(27) It has some unpleasant gastrointestinal side effects even though it is clinically licensed to treat obesity.(28) A large variety of pancreatic lipase inhibitors can be found in natural items.(29) A wide range of plant materials have the ability to inhibit the activity of lipase, including flavonoids, saponins, polyphenols, and caffeine.(30) A number of carbs also block pancreatic lipase, (31) for example. For instance, chitosan/chitin (32). A wide range of microbial metabolites, such asPolyphenols Basic phenolic acids Stilbenes Curcuminoids Chalcones Lignans phenols Isoflavones Fruits, coffee beans, and soy beans all contain caffeine. Ferulic Acid in Vegetables and Soybeans Fruits, coffee beans, and soy beans contain chlorogenic acid. Resveratrol (Grapes, Red wine) Curcumin (Tumeric, Curry, Mustard) Phlorizin (Tea) Naringenin (Tomatoes) Matairesinol (whole grains, legumes, fruits) Secoisolariciresinol (whole grains, legumes, fruits) Flavonol Flavanols Anthocyanins Flavones Flavanones Flavanonols Kaempferol (Fruits, vegetables) Quercetin (Fruits, vegetables) Proanthocyanidins (apple, Cocoa, grape seeds) Catechins (Apple, red wine) Cyanidin (Black berries) Luteolin (Tea, fruits, vegetables) Naringenin (Citrus fruit, tomatoes) Taxifolin (Red onion) Genistein (Soy beans) Daidzein (Soy beans) Terpenoids Organosulfurs Phytosterols Carotene Sesquiterpenes Lycopene from tomatoes Dark green veggies, or lutein Carotene (vegetables, orange to yellow) (Veggies and fruits) Absisic acid Allyl sulfide (onion, garlic) Allicin (Onion, Garlic) Allixin (Onion, Garlic) Diabetoids (Caltrop) Diosgenin (wild yam, fenugreek) Guggul plant, or Guggulterone Alkaloids Xanthine Phenylalkylamine Ephedra sp., or ephedrine Citrus aurantium synthepherine Caffeine(Thea sinensis) Alkylamide Capsaicin (Chilli pepper) Pyridine Nicotine (Nicotinea tabacum) Figure 2 Classification of common dietary phytochemicals. 276 G.A. Mohamed et al. ing lipstatin from S. toxytricini and panclicins from Streptomyces sp. also possess pancreatic lipase inhibitory activity. (33) Different types of tea (e.g., green, oolong, and black tea) are among the most widely-studied materials for lipase inhibitors. Various polyphenols (e.g., L-epicatechin, epicatechin gallate (ECG), epigallocatechin (EGC) and epigallocatechin gallate (EGCG), which were extracted from tea leaves, demonstrated potent anti-pancreatic lipase activity (Fig. 3). (34) For increased pancreatic lipase inhibition, these polyphenols either polymerize their flavan-3-ols or add galloyl moieties to their chemical structures.(35)
Natural appetite suppressants.
The control of hunger and body weight is a complex process that arises from the interaction of hormones and nervous systems. Evidence suggests that the regulation of satiety is tightly linked to serotonin, histamine, dopamine, and their corresponding receptor activity. These receptors might make it possible for medications that reduce energy intake to treat obesity to have better targets.(36) The different levels of hypothalamic neuropeptides are changed by substances that act through peripheral satiety peptide systems. Also, they change the levels of important appetite-related monoamine neurotransmitters in the central nervous system and could be good candidates for appetite suppressants. (37) The brain's hunger centers are regulated by appetite suppressants, which makes one feel fuller. But when one consumes less food, the stomach's production of the hormone ghrelin may rise, encouraging consumption of more food. Thus, ghrelin antagonism may be a possible supplementary treatment for obesity since it may lessen the appetite that may result from reduced food. Hoodia gordonii is one example of a naturally occurring appetite suppressor. It controls hunger, drastically lowers caloric intake, and facilitates weight loss. (38) Garcinia cambogia's natural hydroxycitric acid (HCA) has the potential to act as a natural hunger suppressor. Super CitriMax and HCA-SX are the brands under which it is sold. (39) Hypericum perforatum suppresses the appetite and lowers food intake by preventing synaptosomal uptake of serotonin, which raises the amount of serotonin present within synaptosomes. Therefore, the connection between H. perforatum's antidepressant and anti-obesity properties may be enhanced serotonergic transmission. (40) Table 3 includes some natural appetite suppressants.
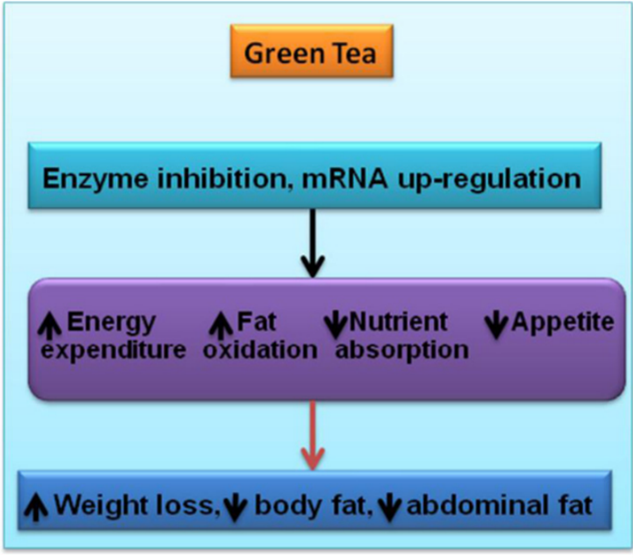
Natural energy expenditure stimulants.
When the effects of consuming too much food are not counterbalanced by rising energy expenditure, an energy imbalance occurs, leading to excessive adiposity. Physical activity, mandatory energy expenditure, and adaptive thermogenesis are the three main categories of energy expenditure. Mammalian brown adipose tissue (BAT) creates non-shivering thermogenesis by releasing excess energy as heat in order to control body weight and energy expenditure. Through the regulation of energy balance by UCP1 (Uncoupling protein), BAT plays a significant role in the management of obesity. The cause of oxidative phosphorylation is UCP1. Therefore, looking for drugs that raise the expression of the UCP1 gene may be a good way to treat obesity by increasing energy expenditure. For instance, the ethanolic extract of Solanum tuberosum dramatically decreased fat weight while activating UCP expression in the liver and BAT.(47) A number of naturally occurring substances, such as caffeine, capsaicin, and green tea extract, have been suggested as therapies for obesity through increased energy expenditure. (48)
Natural lipid metabolism regulators (increased lipolysis):
To address obesity, triglyceride hydrolysis can be stimulated to reduce fat deposits, which can lead to the pharmaceutical targeting of lipolysis. Activation of the b-adrenergic receptor is facilitated by natural compounds such as the flavonoids found in the leaves of Nelumbo nucifera.(49) Examples of natural compounds that support lipid metabolism are included in (Figure 3).


 Yashraj Narake* 1
Yashraj Narake* 1



 10.5281/zenodo.12673334
10.5281/zenodo.12673334