Abstract
Parkinson's disease (PD) is a complex neurodegenerative disease, manifested by the progressive functional impairment of the midbrain nigral dopaminergic neurons. Due to the unclear underlying pathogenesis, disease-modifying drugs for PD remain elusive. In Asia, such as in China and India, herbal medicines have been used in the treatment of neurodegenerative disease for thousands of years, which recently attracted considerable attention because of the development of curative drugs for PD. In this review, we first summarized the pathogenic factors of PD including protein aggregation, mitochondrial dysfunction, ion accumulation, neuroinflammation, and oxidative stress, and the related recent advances. Secondly, we summarized 32 Chinese herbal medicines (belonging to 24 genera, such as Acanthopanax, Alpinia, and Astragalus), 22 Chinese traditional herbal formulations, and 3 Indian herbal medicines, of which the ethanol/water extraction or main bioactive compounds have been extensively investigated on PD models both in vitro and in vivo. We elaborately provided pictures of the representative herbs and the structural formula of the bioactive components (such as leutheroside B and astragaloside IV) of the herbal medicines. Also, we specified the potential targets of the bioactive compounds or extractions of herbs in view of the signaling pathways such as PI3K, NF-?B, and AMPK which are implicated in oxidative and inflammatory stress in neurons. We consider that this knowledge of herbal medicines or their bioactive components can be favorable for the development of disease-modifying drugs for PD.
Keywords
Parkinson's disease (PD) is a complex neurodegenerative disease, manifested by the progressive functional impairment of the midbrain nigral dopaminergic neurons. Due to the unclear underlying pathogenesis, disease-modifying drugs for PD remain elusive. In Asia, such as in China and India, herbal medicines have been used in the treatment of neurodegenerative disease for thousands of years, which recently attracted considerable attention because of the development of curative drugs for PD. In this review, we first summarized the pathogenic factors of PD including protein aggregation, mitochondrial dysfunction, ion accumulation, neuroinflammation, and oxidative stress, and the related recent advances. Secondly, we summarized 32 Chinese herbal medicines (belonging to 24 genera, such as Acanthopanax, Alpinia, and Astragalus), 22 Chinese traditional herbal formulations, and 3 Indian herbal medicines, of which the ethanol/water extraction or main bioactive compounds have been extensively investigated on PD models both in vitro and in vivo. We elaborately provided pictures of the representative herbs and the structural formula of the bioactive components (such as leutheroside B and astragaloside IV) of the herbal medicines. Also, we specified the potential targets of the bioactive compounds or extractions of herbs in view of the signaling pathways such as PI3K, NF-?B, and AMPK which are implicated in oxidative and inflammatory stress in neurons. We consider that this knowledge of herbal medicines or their bioactive components can be favorable for the development of disease-modifying drugs for PD.
Introduction
Parkinson's disease (PD), a long-term neurodegenerative disorder of the central nervous system (CNS) that mainly affects the motor system, was first described in “Essay on the Shaking Palsy” by James Parkinson in 1817 [1, 2]. In epidemiology, PD incidences are estimated to range between 5 and 346/100,000 person-years in European countries, which also increases by 5- to 10-fold in populations from 60 to 90 years old [2, 3]. Patients with PD commonly manifest clinical symptoms including tremor, rigidity, slowness of movement, difficulty in walking, autonomic dysfunction, pain, and cognitive decline in the later stages [4–6]. In pathology, the brain tissues of PD patients mostly display the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the midbrain, the deposit of intraneuronal protein (called Lewy bodies), and aggregates of cytoplasmic inclusions containing insoluble ?-synuclein [2]. Over the past decades, it has been well documented that oxidative stress, impaired mitochondrial function, inflammation, apoptosis, dysfunction of proteolysis, and loss of neurotrophic factors are implicated in the pathogenesis of PD [7]. In treatment, dopamine replacement and levodopa, two prevalent medications for PD, only exhibit some effects of limited symptomatic relief but cause many severe adverse effects, such as hallucination and involuntary movement [8, 9]. Therefore, disease-modified therapy for PD is currently unavailable.
Herbal medicines, as the fundamental part of traditional medicine (such as in China and India), have been gradually accepted for use in the treatment of various diseases worldwide due to their multilevel function characteristics and remarkable efficacy (in some cases) with fewer adverse effects [10]. For example, natural products derived from Chinese herbal medicines, such as curcumin, epigallocatechin gallate, ginsenosides, berberine, artemisinins, emodin, ursolic acid, silibinin, triptolide, cucurbitacins, oridonin, tanshinone, artesunate, shikonin, ?-elemene, gambogic acid, cepharanthine, and wogonin, have been demonstrated with multiple bioactivities including proapoptotic, antiangiogenic, and antifibrotic effects, as well as immunity balance, autophagy regulation, and chemotherapy improvement both in vitro and in vivo [11, 12]. In ancient China, many herbal medicines listed in Shennong's Classic of Materia Medica, the earliest complete pharmacopeia of China, are still being practiced in the treatment of PD, such as Radix achyranthis bidentatae, Herba asari, Fructus viticis, and Fructus xanthii [13]. In India, there has also been a long history of using herbal medicines in the treatment of neurodegenerative diseases, such as Withania somnifera, Mucuna pruriens, and Tinospora cordifolia. These lines of evidence indicated that herbal medicines may be promising candidates to obtain disease-modifying drugs for PD. In modern pharmacological research, the ingredients or extracts of herbal medicines (such as Acanthopanax, Alpinia, and Astragalus) indeed have been demonstrated to exhibit continuous and considerable effects on the models of PD [14, 15]. Over the past decades, the potential molecular targets of herbal medicine extracts have been extensively discovered, which will facilitate the identification of the bioactive compounds of the pharmacodynamic mechanisms of these herbs [15]. In this review, we will summarize the recent updates in studies that (1) elevate the effects of herbal medicine extracts on PD models and (2) explore the potential working mechanisms or targets of herb extracts or bioactive ingredients. We also included the usage of some common Chinese herbal formulations with considerable anti-Parkinsonian activities. We hope the knowledge may facilitate the development of disease-modifying drugs for PD.
Pathogenesis of PD
Although the underlying mechanism remains elusive, protein misfolding and aggregation are the most common molecular phenomena and causative factors for the pathogenesis of PD. For example, the protein of SNCA, PARK2, PINK1, DJ-1, and LRRK2 frequently misfold in the SNpc of the midbrain due to the mutations in their gene [16–18]. Lewy bodies (LBs), a kind of neuronal inclusion, are the aggregation of abnormal proteins in the nerve cells of certain brain regions, which also serve as the major pathological hallmark of PD and dementia [19]. Although ?-synuclein is the main component of LBs, it also has been found to play critical roles in other Lewy pathologies, such as pale bodies and Lewy neurites [20, 21]. In physiological conditions, ?-synuclein is naturally present as an unfolded and structured protein, unlikely to transform into highly organized fibrils (Figure 1). However, in the presence of extreme stimuli such as acidic pH and high temperature, it exhibits a strong proneness to transform into a partially folded conformation or intermediate, which intensely promotes the formation of ?-synuclein fibrils [22]. Therefore, a model for the fibrillation of ?-synuclein was proposed, in which the first step is the conformational transformation of the natively unfolded protein into the aggregation-competent partially folded intermediate.
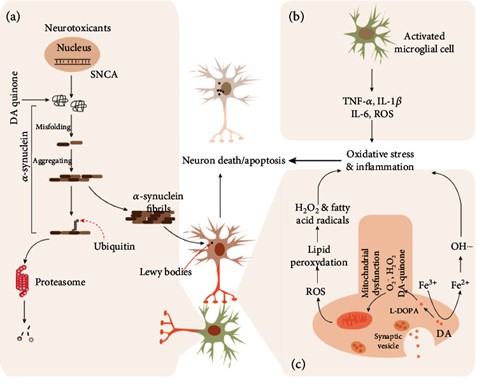
Figure 1
Major mechanisms involved in Parkinson's disease. In the dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the midbrain of patients with Parkinson's disease, mutations in SNCA (coding gene of ?-synuclein) or protein modification of ?-synuclein induced by neurotoxicants (or reactive oxygen species) (a) leads to the ?-synuclein misfolding. The misfolded ?-synuclein can further aggregate into ?-synuclein fibrils when the proteasome-mediated degradation system cannot fully clear the fibrils, and then contribute to the production of Lewy bodies in neurons. The inflammatory cytokines, such as TNF-?, IL-1?, and IL-6, secreted by activated microglial cells (b) also induce the death or apoptosis of neurons. Besides, the mitochondrial dysfunction induced by L-DOPA or Fe3+ induces the product of ROS, which enhances death or apoptosis via causing oxidative stress (c). L-DOPA: L-levodopa; ROS: reactive oxygen species.
Consistently, Uversky et al. observed several different aggregated ?-synuclein forms such as ring-like protofibrillar, amorphous, oligomeric intermediates, amyloid fibrils, and spherical-shaped [27]. In support of the environment-induced pathogenesis of PD, many exogenous chemical compounds such as pesticides, herbicides, and metal ions were demonstrated to accelerate the aggregation process of ?-synuclein [21]. In another line, multiple missense point mutations (such as A30P, G51D, E46K, A53T, and A30P) of the ?-synuclein coding gene have been identified in the familial PD cases from different populations including Spanish, Italian-American, and German [21] which aggravate the misfolding and aggregation of this protein in the SNpc of patients. Also, the increased accumulation of ?-synuclein protein was frequently observed in family members of PD patients, suggesting point mutations of ?-synuclein may be critical risks of its aggregation. Fujiwara et al. identified a posttranslational modification p-Ser129 of ?-synuclein, and also found that Ser129 of ?-synuclein is extensively phosphorylated in synucleinopathy lesions . In vitro data by Fujiwara et al. showed that p-Ser129 of ?-synuclein promotes ?-synuclein fibril formation . In 2019, Hu et al. found that adenosine triphosphate- (ATP-) dependent Clp protease (ClpP), a mitochondrial matrix protease, suppresses the phosphorylation of ?-synuclein Ser129 to promote neuronal morphology of neurons derived from PD patients carrying the ?-synuclein A53T mutant [10]. This finding suggests that ClpP might be a useful therapeutic target for ?-synuclein-induced neuronal pathologies, such as PD and other synucleinopathies.Although age is considered the greatest risk factor for ?-synuclein formation, the underlying details are still exclusive. Based on the evidence that misfolded ?-synuclein protein is found in both the brain and periphery system of PD patients, Braak et al. have carried out animal experiments to prove that the initial misfolded ?-synuclein may be formed from nonnerve tissues and then spread to the brain via peripheral autonomic nerves [21]. They found a robust age-dependent gut-to-brain and brain-to-gut spread of ?-synuclein pathology along the sympathetic and parasympathetic nerves of rats, and ?-synuclein pathology is more densely packed and resistant to enzymatic digestion in old rats. Their observations indicate that age is a crucial factor for ?-synuclein aggregation. Mitochondrial Dysfunction: Mitochondria are the most critical energy-producing center by generating ATP in almost all eukaryotic cells [21]. Over the past several decades, mitochondrial dysfunction (particularly oxidative stress) has been demonstrated to contribute to the pathogenesis of PD by multiple lines of evidence both in PD patients and related animal models). MPTP, a synthetic opioid drug produced during the manufacture of 1-methyl-4-phenyl-4-propionoxypiperidine (MPPP), interferes with the components of the mitochondria electron transport chain (ETC) to be transformed into a toxic cation named 1-methyl-4-phenylpyridinium (MPP+) via a monoamine oxidase B enzymatic action. In neurons, MPP+ efficiently induces oxidative stress (e.g., nitric oxide) and ATP production restrains, which further leads to an elevation of intracellular calcium concentration and excitotoxicity-mediated neuronal damage . Importantly, it was frequently observed that MPTP intake results in mitochondrial dysfunction, and causes permanent PD symptoms among different experimental models . In the substantia nigra region of PD patients, the elevation of MPTP metabolites also was frequently observed, which causes the inactivation of ETC components (i.e., complex I) . On the other hand, the aberrations of mitochondrial functions, such as rotenone-induced functional inhibition of complex I (rotenone, lipophilic pesticides) also cause PD-related anatomical, behavioral, neurochemical, and neuropathological abnormalities in human patients.Moreover, in patients from familial PD, the maternally inherited mutations in mitochondrial DNA (encoding proteins for the synthesis of ETC components) or 12S rRNA (influencing cytochrome c oxidase production) that lead to mitochondrial dysfunction are tightly associated with the pathogenesis of PD . Recently, many researchers tried to explain the pathogenesis of PD in the view of mitochondria-lysosome crosstalk. In 2021, Kim et al. observed that mitochondria-lysosome contacts were dynamic in the soma, axons, and dendrites of human neurons . Whereas, it exhibited a morphological contact prolongation in the neurons derived from PD patients that harbor mutant GBA1 . They also demonstrated that the prolongation was due to the decreased GBA1 lysosomal enzyme activity because the phenotype could be rescued by restoring enzyme activity with a GCase modulator. Furthermore, the contact prolongation resulted in the disruption of mitochondrial distribution and function. Therefore, all the observations definitely indicate the association between mitochondrial dysfunction and PD. More recently, a study by Matsui et al. showed that cytosolic double-strand DNA (dsDNA) of mitochondrial origin escaping from lysosomal degradation exhibits cytotoxicity in cultured cells and PD phenotypes in vivo .The cytotoxicity was largely neutralized by the overexpression of DNase II (a lysosomal DNase that degrades discarded mitochondrial DNA) or the depletion of IFI16 (a sensor for cytosolic dsDNA of mitochondrial origin). Moreover, reducing cytosolic dsDNA by overexpressing human DNase II ameliorates movement disorders and dopaminergic cell loss in GBA-mutated PD zebrafish models. These results support a common causative role for the cytosolic leakage of mitochondrial DNA in PD pathogenesis. Acanthopanax senticosus roots and stems (ASRS), also named Wujipi in Chinese, are widely used in traditional Chinese medicine. The pole-climbing test showed that the ethanol extracts (45.5?mg/kg daily) of Acanthopanax senticosus (Figure 2) roots possess neuroprotective effects on MPTP-induced PD mice [107]. In pathology, the number of dopamine receptor D1/2-positive cells and caspase-3 protein levels of substantia nigra were significantly reduced after the administration of the extract. Sesamin, a component of Acanthopanax senticosus roots, pharmacologically offers protective effects against PD-related depressive behaviors in rotenone-administered rats by enhancing tyrosine hydroxylase or glial cell line-derived neurotrophic factor- (GDNF-) positive neuron activity in the midbrain . Lahaie et al. observed that sesamin also elicits a strong elevation of SOD activity and decreases catalase activity and synthase protein level of nitric oxide (NO) in MPP+-induced neuronal PC12 cells [110] (Figure 3). Eleutheroside B (Figure 4), another main component of ASRS, can also relieve fatigue, enhance memory, and improve human cognition. In MPP+-induced PC12 cells, eleutheroside B effectively increases the phosphorylation of ERK1/2 (extracellular signal-regulated kinase 1/2) and reduces the expression level of c-Fos and c-Jun [12] (Figure 3). In 2016, Li et al. carried out lncRNA microarray analysis to systematically investigate the effects of ASRS on the CNS both in pathology and physiology [12]. However, they observed that ASRS fails to inhibit ?-synucleinopathies but produces some potential neurotoxicity to CNS under physiological conditions, indicated by no significant difference in the expression of lncRNA/mRNA that may cause potential neurotoxicity analogous to ?-synuclein that exists between ASRS-treated and -untreated ?-synuclein mice in physiological conditions [13]. These findings hint that, in different situations, the bioactivities of ASRS may be bidirectional for pathological and physiological CNS.

Inhibition of Proteasome-Mediated Degradation
The proteasome is an extremely vital molecular apparatus that ubiquitously locates in the nucleus and cytoplasm of eukaryotic cells, which degrades unwanted or misfolding proteins with ploy-ubiquitin modifications via its protease activity . It is well accepted that an abnormal ubiquitin-proteasome system (UPS) is tightly associated with PD symptoms (Figure 1). Previously, the upstreams of UPS, BDNF (brain-derived neurotrophic factor), and its receptor TRKB (tyrosine kinase B) were demonstrated to regulate the expression of key synaptic proteins in response to neuronal activity, which is also considered to play vital roles in the pathogenesis of PD [21]. PARK2, a gene coding the essential ubiquitin ligase enzyme of UPS, has been found with several types of mutations including missense, frameshift, nonsense, point mutations, exon deletions, and duplications in PD patients. PARK7, encoding a protein that inhibits ?-synuclein aggregation, also was reported that its mutations increase the susceptibility to proteasome inhibition and enhance oxidative stress in neurons [19]. FBXO7 is a clinically relevant F-box protein linked to early-onset PD, in which mutations near the F-box domain and substrate recruiting domains were reported to influence SCFFBXO7/PARK15 ubiquitin ligase activity. In 2016, Teixeira et al. conducted a high-throughput screen to identify the ubiquitinated substrates of SCFFBXO7 that may be directly involved in PD etiology [22]. They validated GSK3? (glycogen synthase kinase 3?, a kinase of ?-synuclein) and TOMM20 (translocase of outer mitochondrial membrane 20, a mitochondrial translocase) as SCFFBXO7 substrates both in vitro and in vivo. Although it promoted K63 ubiquitination of GSK3?, it was found that FBXO7 failed to affect the protein level and localization of endogenous GSK3?. Besides, they reported that ectopic FBXO7 with mutants associated with early-onset PD could not alter the ubiquitination level of TOMM2. Therefore, whether GSK3?/TOMM2 involves the pathological processes of PD remains ambiguous.
Neuroinflammation Both innate and adaptive immune responses have been demonstrated to involve the pathophysiology of PD (Figure 1). For example, the expression level of nuclearly translocated NF-?B (nuclear factor kappa-light-chain enhancer of activated B cells) was reported to be increased in the dopaminergic neurons of PD patients. In the cerebrospinal fluid and striatum of PD patients, the increment of cytokine levels, such as T-cell activation-associated cytokine (IL-2), proinflammatory cytokines (TNF-?, IL-1?, and IL-6), anti-inflammatory cytokine (IL-4), and several growth factors (EGF and TGF-?1), is the main feature of inflammation-induced processes. In MPTP-induced PD rats, mice, and monkeys, the increased astroglial reaction and microglial activation also were observed in both the SNpc and the striatum. Recently, an in vivo study in Tlr4-knockout mice by Perez et al. showed that Tlr4-mediated inflammation plays an important role in intestinal and/or brain inflammation, which may be one of the key factors leading to neurodegeneration in PD. Overall, these findings support the hypothesis that inflammatory cytokines are produced in the dopaminergic neurons that play potentially vital roles in the pathogenesis of PD. On the other hand, Brochard et al. found the increased amounts of CD8+ T-cytotoxic and CD4+ T-helper cell infiltration in the nigrostriatal system of MPTP-injected mice. In MPTP-exposed PD patients, there is an elevated expression of Fas ligand, a cell-surface ligand of the TNF-? family that triggers the Fas receptor and induces apoptosis, within the striatum and SNpc. Another neuroinflammatory modification in PD is the increased expression of major histocompatibility complex (MHC), the molecules that bind to the pathogen-derived peptide fragments exposed on the cell surface. Initially, McGeer et al. observed that the number of HLA-DR-positive microglial cells (MHC-II) is significantly increased in the SNpc of PD patients. Similarly, the increased level of light chain MHC-I also was observed in the striatum of PD patients compared with normal controls. Besides, Bokor et al. found that killer cells induced by antibody-dependent cell-mediated cytotoxicity reaction also play roles in the pathogenesis of PD. Recently, Sulzer et al. showed that a defined set of peptides that are derived from ?-synuclein act as antigenic epitopes displayed by these alleles and drive helper and cytotoxic T-cell responses in PD patients. Previously, circulating CD4+ and CD8+ T-cells derived from PD patients have been demonstrated to produce Th1/Th2 cytokines in the presence of ?-synuclein, suggesting that chronic memory T cell response may exist in PD. In 2021, Williams et al. generated an ?-synuclein overexpression and T cell-deficient mouse model to elucidate whether ?-synuclein aggregation in the midbrain of mice can induce memory T cells to lead to PD. Indeed, they observed that ?-synuclein overexpression upregulates the MHC-II protein level in CNS myeloid cells and induces infiltration of IFN?-producing CD4+ and CD8+ T cells into the CNS. More importantly, loss of function of TCR? or CD4 using the immunosuppressive drug fingolimod could reduce the CNS myeloid MHC-II response to ?-synuclein. All the observations highlight the critical roles of inflammation in the pathogenesis of PD.
Oxidative Stress
In human bodies, oxidative stress occurs when the production of reactive oxygen species (ROS) cannot be neutralized by antioxidants, and often leads to the damage of cellular components including lipids, proteins, and DNA. Numerous experimental studies in dopamine metabolism, lipid peroxidation (LPO), and glutathione depletion have demonstrated that oxidative stress plays a critical role in the pathogenesis of PD (Figure 1). In dopaminergic neurons of the SNpc, DA metabolism generates various oxidative byproducts including O? (superoxide anion), H2O2 (hydrogen peroxide), and DA quinone species, which can modify cellular nucleophiles including low molecular weight sulfhydryls (e.g., GSH) and protein cysteinyl residues . It has been demonstrated that DA quinones are implicated in PD pathophysiology by modifying proteins including ?-synuclein, parkin, SOD2, DJ-1, and UCH-L1 [22]. Also, DA quinone species cause the dysfunction of brain mitochondrial respiration and lead to ROS production by altering the subunits of the ETC (complexes I and III) [19]. Lipid peroxide (LPO) in the plasma membrane is capable of removing hydrogen atoms from the methylene bridges (-C2H2-) to produce H2O2 and fatty acid radicals. In the substantia nigra of patients with Parkinson's disease, the level of basal malondialdehyde, an intermediate in the production of LPO, was previously reported to be increased significantly when compared with other brain regions, suggesting that LPO may participate in the development of PD. Also, the end products of LPO, such as 4-hydroxynonenal and thiobarbituric acid reactive substance, are increased in the substantia nigra and striatum of PD brains . Recently, Jiang et al. reported that Tianma-Gouteng granules significantly decrease the susceptibility of PD by inhibiting ALOX15-mediated lipid peroxidation, suggesting that intervention by targeting LPO production may be an effective therapy for PD [20]. Oxidative stress was previously reported to activate the integrated stress response, which further ignites activating ATF4 (transcription factor 4). In 2021, Demmings et al. explored the role of ATF4 in neuronal cell death in MPP+- and (6-hydroxydopamine-) 6-OHDA-induced PD mouse models and found that ?-synuclein aggregation could cause significant elevation of ATF4 expression in mouse cortical and mesencephalic dopaminergic neurons. Furthermore, they demonstrated that neuronal death induced by PD neurotoxin and ?-synuclein fibrils is attenuated in ATF4-deficient dopaminergic neurons, and ectopic expression of ATF4 restores sensitivity of ATF4-deficient neurons to PD neurotoxins. These results collectively indicate the key roles of oxidative stress in the pathogenesis of PD.Glutathione (GSH), a critical “scavenger” of ROS such as free radicals, peroxides, and LPO in cells, is expressed at a relatively low level in the substantia nigra when compared with other brain regions such as the cortex, hippocampus, and cerebellum. In early 1992, Sofic et al. reported that, compared with the control subjects, the level of GSH in the substantia nigra of PD patients is significantly decreased . Nandita et al. demonstrated that the early GSH losses in the substantia nigra may directly cause a reduction in the activity of ETC complex I, which results in dopaminergic cell death and eventually promotes the development of PD [104]. Furthermore, the depletion of GSH also causes the dysfunction of the UPS, and thereby deprives the 26S proteasome protein degradation system in neurons of PD [22]. Besides, GSH depletion induced inflammation stress in neuronal tissues of PD patients by modulating IL-1 signaling and JNK- (c-Jun N-terminal kinase-) activated inflammatory pathways [21]. Astragali Radix (Huangqi in Chinese), the dried root of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or Astragalus membranaceus (Fisch.) Bge. (Leguminosae), is a common and well-known drug in traditional Chinese medicine.
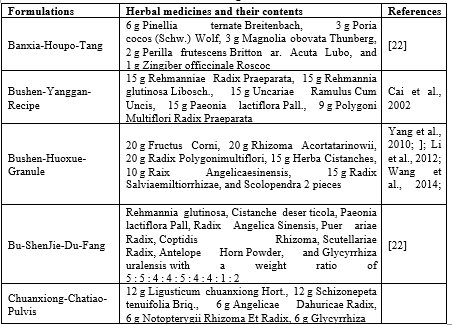
Table 1 Formulations with PD-alleviating effect in Chinese herbal medicines
LITERATURE RIVIEW
- Dr. Gilbert Hosts, with Dr. Britt Stone 2024-A movement disorders specialist with a particular interest in herbal remedies, medicinal plants, and supplements and their use in Parkinson’s disease (PD).
- Hyo Yu, et al 2021- The prevalence of Parkinson’s disease is on an upward trend along with an increase in the aging population but there is no available treatment that halts the progression of neurodegeneration. This study reports a numerical analysis on Donguibogam and suggests novel herbal drugs, which have never been researched before but found to be deemed effective in this study.
- Rong Yin, Chuantao Fang, Xinyu Mei, and Dashi Qi-2021- Performed particle tracking and analysis of experimental data. All contributed to the writing of the paper. All authors read and approved the final manuscript. Rong Yin and Jie Xue contributed equally.
- Saurabh Srivastav, Mahino Fatima et al 2021- The etiology of PD is not fully understood and a lot of research in this field has provided evidence that redox destabilization and mitochondrial dysfunctioning are the key players. Besides, the current therapeutic strategy against PD relies primarily on Levodopa treatment which is capable of slowing down disease progression to a limited extent with associated side effects
- Tae-Hun Kim, ki HO chu et al 2012- We conducted systematic review to evaluate current evidence of herbal medicines (HMs) for Parkinson's disease (PD).
- Ki HO Chu et al 2013- This study reports a numerical analysis on Donguibogam and suggests novel herbal drugs, which have never been researched before but found to be deemed effective in this study.
METHODS AND METHODOLOGY
Methods
This systematic review and meta-analysis is conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement (Moher et al., 2010b) and our previous study (Yang et al., 2017).
Search Strategy
Randomized double-blind placebo-controlled clinical trials of HM formulas for PD were searched in eight databases from their inception to February 2018. They are PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Web of science, Chinese National Knowledge Infrastructure (CNKI), Chinese VIP Information, Wanfang database and Chinese Biological Medical Literature Database (CBM). Moreover, we hand searched additional relevant studies using the reference list of previous reviews. The search strategy of PubMed was as follows, and was modified to suit other English or Chinese databases. The therapy of herbal medicine (HM) for PD is particularly common. In China, HM could be traced in the Huangdi Neijing (Inner Canon of Yellow Emperor) (Zheng, 2009), the earliest existing classics in Chinese herbal medicine (CHM). Up to now, HM is still very popular in the treatment of PD especially in Asian countries (Wang et al., 2011, 2013). Previous reviews (Wang et al., 2012; Zhang et al., 2015) found lack of evidence of supporting the use of HM for PD patients because of the generally low-quality studies included. Here, we performed a systematic review and meta-analysis of randomized double-blind placebo-controlled clinical trials of HM formulas for PD patients and further explored the mechanisms of high-frequently used herbs against PD.
Data Cleansing and Standardisation
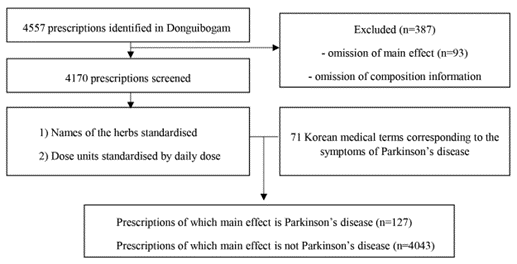
A database was built using the following three steps. First, a data cleansing process was performed for all 4557 prescriptions on Donguibogam, the classical literature of traditional Korean medicine. Ninety-three prescriptions were excluded due to the omission of the main effect. A total of 294 prescriptions were excluded due to the omission of composition information. With this process, 4170 prescriptions were organised in the database. Second, medicinal herbs with synonymous names and sobriquets were unified under one name that is the most widely used. Third, dose units were standardised by 1-day dose when dose units of prescriptions described in the literature were different (e.g., single-dose, 1-day dose, or 10-day dose). The authors then applied 71 traditional Korean medicine symptom terms identified above to the database and 127 prescriptions, in which the main effect was identified as PD symptoms (Figure 1).
Fig. 1 The flow chart of creating and standardising basic data for analysis
3. Chi-square test for selecting effective medicinal herbs
All prescriptions were grouped based on whether their main effect was for symptoms of PD, and the groups were named ‘a group of prescriptions for PD’ and ‘a group of prescriptions not for PD.’ The prescription frequency was identified by counting how often each of the 201 herbs appeared in 127 prescriptions for PD. And 40 herbs that appeared in more than 5% of the total prescriptions were selected. The 5% criterion is an arbitrary point to obtain sufficient prescription frequency for the following statistical analysis. Next, the prescription frequency of the candidate herbs not used for PD was identified. A chi-square test was performed to evaluate the statistically significant differences in the prescription frequency of the candidate herbs when used in the two groups (p < 0>4. T-test and Wilcoxon test for determining the dose of medicinal herbs
Selected herbs were statistically verified to determine whether there was a difference in the dose when used in the two groups. When testing the selected herbs in the two prescription groups, a t-test was performed if the data followed a normal distribution, and the sample size was large enough (n = 30), while the Wilcoxon test, a nonparametric statistical hypothesis test, was performed if the data or the sample size did not meet the conditions (p < 0>
RESULT
1. Medicinal Herbs Potentially Effective for PD
Among the total of 201 medicinal herbs used in 127 prescriptions for PD, 40 candidate herbs with a frequency of more than 5% of the total prescriptions were selected. And 17 herbs were identified to be potentially effective for PD treatment with statistical significance (p < 0>
Table 1The herbs considered to be potentially effective for Parkinson’s Disease. (n=17)
Table 2 The result of the chi-square test and Fisher’s exact test for the herbs considered to be potentially effective for Parkinson’s Disease.
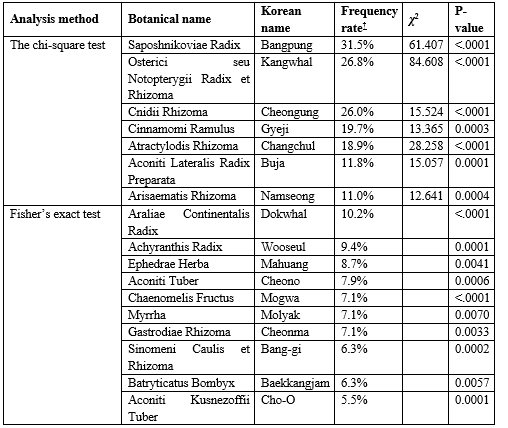
† Frequency rate: The rate of how often each herb appeared in the prescriptions to the total of 127 prescriptions that have been used for treating PD
Osterici seu Notopterygii Radix et Rhizoma(?2 = 84.608), Saposhnikoviae Radix (?2 = 61.407), Atractylodis Rhizoma(?2 = 28.258), Cnidii Rhizoma (?2 = 15.524), Araliae Continentalis Radix (p < 0>
2. Proper Dose for Medicinal Herbs
Among the 17 herbs selected above, Osterici seu Notopterygii Radix et Rhizoma, Aconiti Lateralis Radix Preparata, Arisaematis Rhizoma, Achyranthis Radix, Chaenomelis Fructus, Sinomeni Caulis et Rhizoma, and Batryticatus Bombyx showed a normal distribution on the Shapiro-Wilk test; hence, they were further analysed using the t-test. The Wilcoxon test was used for Saposhnikoviae Radix, Cnidii Rhizoma, Cinnamomi Ramulus, Araliae Continentalis Radix, Atractylodis Rhizoma, and Ephedrae Herba as these herbs did not follow a normal distribution. There was no significant difference in the dose of medicinal herbs used in the two groups (p < 0>
Table 3 Mean and median value of daily dose of 17 herbs deemed potentially effective for Parkinson’s Disease.
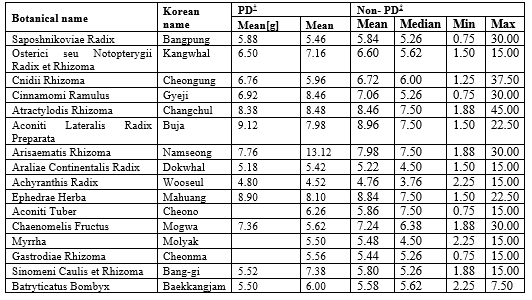
† PD: The group of prescriptions for Parkinson disease
‡ Non-PD: The group of prescriptions not for Parkinson disease
Aconite Tuber, Myrrha, and Gastrodiae Rhizoma were not calculated for the PD group as they were used in solid form, or the dosage were not clearly described. As for Aconiti Kusnezoffii Tuber, it was excluded from the analysis as every prescription containing the herb is solid form.
DISCUSSION
This research conducted a process of statistical analysis on Donguibogam, the traditional literature of Korean medicine for finding herbal candidates deemed effective for PD to identify a list of candidate herbs. Based on the idea that herbs prescribed with the highest frequency are more likely to be effective, 40 herbs that made up more than 5% of the total prescriptions used for treating PD were searched and selected for analysis. Glycyrrhiza uralensis, Saposhnikoviae Radix, Osterici seu Notopterygii Radix et Rhizoma, Cnidii Rhizoma, Angelicae Gigantis Radix, Paeoniae Radix, and Ginseng Radix were prescribed more than 25 prescriptions among the 127 total prescriptions, being the most frequently prescribed herbs in the list and they could be considered candidate substances. However, when it comes to experimental research trying to find candidate herbs more likely to be effective, additional information including statistical values would increase the success rate of the research. In addition, comparatively less frequently prescribed herbs still could be on the list of candidate substances if there is additional information. In the first analysis, the chi-square test was used to verify the difference of prescription frequency of herbs in the two groups and 17 herbs were selected for potentially treating PD. This was derived from the fact that those herbs were more statistically significant in the group of prescriptions for PD than in the prescription group not for PD. Among them, Saposhnikoviae Radix, Osterici seu Notopterygii Radix et Rhizoma, Cnidii Rhizoma, and Cinnamomi Ramulus were included in the top 10 frequently used herbs. The four herbs are more reliable to be candidate substances deemed effective for PD because we found out the fact through the statistical tests that the herbs have been more specifically used for PD symptoms. A notable part of this research is that Araliae Continentalis Radix, Achyranthis Radix, Chaenomelis Fructus, Sinomeni Caulis et Rhizoma, Aconiti Tuber, and Aconiti Kusnezoffii Tuber were found to be relatively infrequently prescribed herbs but eventually selected to be deemed effective herbs. If the prescription frequency was only considered, they would not have been suitable candidate substances. However, the herbs were tested using Fisher’s exact test and found to be more statistically significantly used in the group of prescriptions for PD than in the group of prescriptions not for PD, which means they have been more specifically used for PD symptoms. (p-value < 0>
CONCLUTION
Saposhnikoviae Radix, Osterici seu Notopterygii Radix et Rhizoma, Cnidii Rhizoma, Cinnamomi Ramulus, Atractylodis Rhizoma, Aconiti Lateralis Radix Preparata, Arisaematis Rhizoma, Araliae Continentalis Radix, Achyranthis Radix, Ephedrae Herba, Aconiti Tuber, Chaenomelis Fructus, Myrrha, Gastrodiae Rhizoma, Sinomeni Caulis et Rhizoma, Batryticatus Bombyx, and Aconiti Kusnezoffii Tuber were selected as herbs potentially effective for treating PD. Since there was no significant difference in the dose of individual herbs used in the two groups for a daily dose, we speculated that the average dose of the entire prescription would be appropriate for each herb. Furthermore, this study presented quantitative statistical evidence for obtaining a list of medicinal herbs and appropriate dose for each herb useful for treating PD by applying the chi-square test, t-test, and Wilcoxon test to Donguibogam, the original literature.
REFERENCES
- Lee A., Gilbert R. M. Epidemiology of Parkinson disease. Neurologic Clinics. 2016;34(4):955–965. doi: 10.1016/j.ncl.2016.06.012. [PubMed] [CrossRef] [Google Scholar]
- Simon D. K., Tanner C. M., Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clinics in Geriatric Medicine. 2020;36(1):1–12. doi: 10.1016/j.cger.2019.08.002. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Balestrino R., Schapira A. H. V. Parkinson disease. European Journal of Neurology. 2020;27(1):27–42. doi: 10.1111/ene.14108. [PubMed] [CrossRef] [Google Scholar]
- Cabreira V., Massano J. Parkinson’s disease: clinical review and update. Acta Médica Portuguesa. 2019;32(10):661–670. doi: 10.20344/amp.11978. [PubMed] [CrossRef] [Google Scholar]
- Hou Y., Dan X., Babbar M., et al. Ageing as a risk factor for neurodegenerative disease. Nature Reviews. Neurology. 2019;15(10):565–581. doi: 10.1038/s41582-019-0244-7. [PubMed] [CrossRef] [Google Scholar]
- Zeng B. Y. Effect and Mechanism of Chinese Herbal Medicine on Parkinson's Disease. International Review of Neurobiology. 2017;135:57–76. doi: 10.1016/bs.irn.2017.02.004. [PubMed] [CrossRef] [Google Scholar]
- Ascherio A., Schwarzschild M. A. The epidemiology of Parkinson's disease: risk factors and prevention. The Lancet Neurology. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [PubMed] [CrossRef] [Google Scholar]
- Connolly B. S., Lang A. E. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [PubMed] [CrossRef] [Google Scholar]
- Olanow C. W., Schapira A. H. Therapeutic prospects for Parkinson disease. Annals of Neurology. 2013;74(3):337–347. doi: 10.1002/ana.24011. [PubMed] [CrossRef] [Google Scholar]
- Hu Y., Wang J. Interactions between clopidogrel and traditional Chinese medicine. Journal of Thrombosis and Thrombolysis. 2019;48(3):491–499. doi: 10.1007/s11239-019-01945-3. [PubMed] [CrossRef] [Google Scholar]
- Luo H., Vong C. T., Chen H., et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chinese Medicine. 2019;14(1):p. ???. doi: 10.1186/s13020-019-0270-9. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Zhao Y., Ma X., Wang J., et al. A system review of anti-fibrogenesis effects of compounds derived from Chinese herbal medicine. Mini Reviews in Medicinal Chemistry. 2016;16(2):163–175. doi: 10.2174/1389557515666150709121908. [PubMed] [CrossRef] [Google Scholar]
- Zheng G. Q. Therapeutic history of Parkinson’s disease in Chinese medical treatises. Journal of Alternative and Complementary Medicine. 2009;15(11):1223–1230. doi: 10.1089/acm.2009.0101. [PubMed] [CrossRef] [Google Scholar]
- Li X. Z., Zhang S. N., Liu S. M., Lu F. Recent advances in herbal medicines treating Parkinson's disease. Fitoterapia. 2013;84:273–285. doi: 10.1016/j.fitote.2012.12.009. [PubMed] [CrossRef] [Google Scholar]
- Song J. X., Sze S. C., Ng T. B., et al. Anti-Parkinsonian drug discovery from herbal medicines: what have we got from neurotoxic models? Journal of Ethnopharmacology. 2012;139(3):698–711. doi: 10.1016/j.jep.2011.12.030. [PubMed] [CrossRef] [Google Scholar]
- Chiti F., Dobson C. M. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annual Review of Biochemistry. 2017;86(1):27–68. doi: 10.1146/annurev-biochem-061516-045115. [PubMed] [CrossRef] [Google Scholar]
- Dickson D. W., Braak H., Duda J. E., et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. The Lancet Neurology. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [PubMed] [CrossRef] [Google Scholar]
- Savitt J. M., Dawson V. L., Dawson T. M. Diagnosis and treatment of Parkinson disease: molecules to medicine. The Journal of Clinical Investigation. 2006;116(7):1744–1754. doi: 10.1172/JCI29178. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Jellinger K. A. Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. Journal of Neural Transmission (Vienna) 2018;125(4):615–650. doi: 10.1007/s00702-017-1821-9. [PubMed] [CrossRef] [Google Scholar]
- De Virgilio A., Greco A., Fabbrini G., et al. Parkinson's disease: Autoimmunity and neuroinflammation. Autoimmunity Reviews. 2016;15(10):1005–1011. doi: 10.1016/j.autrev.2016.07.022. [PubMed] [CrossRef] [Google Scholar]
- Shahmoradian S. H., Lewis A. J., Genoud C., et al. Lewy pathology in Parkinson's disease consists of crowded organelles and lipid membranes. Nature Neuroscience. 2019;22(7):1099–1109. doi: 10.1038/s41593-019-0423-2. [PubMed] [CrossRef] [Google Scholar]
- Fares M. B., Ait-Bouziad N., Dikiy I., et al. The novel Parkinson's disease linked mutation G51D attenuates in vitro aggregation and membrane binding of?-synuclein, and enhances its secretion and nuclear localization in cells. Human Molecular Genetics. 2014;23(17):4491–4509. doi: 10.1093/hmg/ddu165. [PMC free article] [PubMed] [CrossRef] [Google Scholar]


 Wasif Rao*
Wasif Rao*
 Bishal Singh
Bishal Singh
 Mohd Asad
Mohd Asad
 Vipul Gupta
Vipul Gupta
 Shivani Pegowal
Shivani Pegowal







 10.5281/zenodo.13272740
10.5281/zenodo.13272740