Abstract
Transdermal drug delivery (TDD) has emerged as an effective alternative to oral and parenteral administration due to its low invasiveness, low dilution, and ease of administration. A transdermal patch is a drug patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. The use of cross-linking agents can avoid many of the problems associated with oral drug delivery, such as first-passage of the liver, attack by enzymatic digestion, hydrolysis and degradation. of drugs in acidic environments, drug resistance and gastrointestinal irritation.
Keywords
Transdermal drug delivery, Through Patches, first passage of the liver.
Introduction
Transdermal drug delivery is an alternative method of drug delivery through the skin layer. This substance enters the bloodstream through the skin and is metabolized in the body before reaching its destination. The transdermal drug delivery method has several advantages over other methods of administration. Examples are the ability to release doses of the drug over a long period of time, the ability to avoid the digestive system, and the ability to avoid the first cycle in the liver. Other methods of administration, such as injections, can be painful. and increase the risk of infection. However, the oral route is ineffective, and in the inhalation method dose control is difficult. Because of its benefits in other ways, transplant delivery is used to deliver treatments for conditions such as smoking cessation, chronic pain and motion sickness, and hormone therapy. The advantages of transdermal drug delivery include improving the effectiveness of the drug, reducing the risk of primary liver disease, and maintaining drug concentrations in the blood. The first transdermal system was approved by the FDA in 1979 to prevent nausea and vomiting. Confirmation of the absorption of the drug can be confirmed by measuring blood levels, detection of the removal of the drug and its metabolites in the urine, observing the patient's clinical response to drug therapy. A transdermal patch is a medical tablet that can release medication from the skin layer into the bloodstream at a set rate. Generally, patches are the most convenient method of administration. There is no rejection, the treatment lasts for a few days and can be stopped at any time. They come in different sizes and contain many ingredients. When the patch comes into contact with the skin, it can release active ingredients into the systemic circulation through diffusion mechanisms. Transdermal patches can contain high doses of active ingredients that remain on the skin for a long time. One of the first transdermal patches developed in 1985 was the nitroglycerin patch. This patch, developed by Gill and Bergren, uses an ethylene vinyl acetate membrane. It can be applied to the side of the nitroglycerin box. The duration of the release of the drug varies, depending on the method of use, from 9 hours to 9 days.
-
- Advantages of TDDS1 :
- In order to avoid the initial transfer, transdermal delivery ensures continuous penetration and continuity of a drug over a long period of time.
- Increase patient compliance.
- Does not interfere with gastric and intestinal fluids.
- It maintains stable blood levels and provides long-term control.
- Reduce the plasma concentration of drugs.
- Minimize inter drug variations in plasma levels, use drugs with short half-lives and low therapeutic index.
- In case of poisoning, the release of the medicine is lost.
- Reduce the number of doses and increase the patient's requirements.
- A Regular medication plan will reduce the variability of drug response within and between patients.
- It increases the effectiveness of many drugs by avoiding some of the problems associated with drugs, such as poor absorption and abdominal pain.
- Disadvantages of TDDS :
- The drug must have excellent chemical properties
- To penetrate the stratum corneum.
- For daily doses, the amount of medicine should not exceed 5 mg per day. If it exceeds 10 to 25 mg per day, transdermal drug delivery becomes difficult.
- Food coating, including drugs, resins, and other additives, may cause local problems.
- A clear clinical need for the use of a transplantable delivery system must be established.
- Inability to achieve high blood/plasma drug levels.
- Medicines with large molecular sizes cannot be synthesized.
- The application area can be inflamed.
- Dressing is not appropriate.
- It may not be economical
- The skin barrier varies between people and can change over time.
2. Transdermal Patch Design:
The transport of drugs across the skin is affected by various factors such as skin penetration, site and duration of application, as well as the physical activity of the skin (i.e. first transition). In fact, each drug has its own characteristics that affect delivery through the skin. In order to achieve good skin absorption and penetration, the substance must be non-ionic and lipophilic to cross the skin barrier. Molecules larger than 500 daltons are difficult to pass through the stratum corneum, and the best thing is that the therapeutic dose of the drug should be less than 10 mg per day.
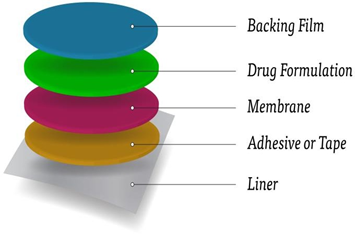
A transdermal patch is a drug patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. Here's an overview:
- Backing Layer: A waterproof layer to prevent moisture from penetrating the patch.
- Adhesive Layer: a hypoallergenic adhesive to attach the adhesive to the skin.
- Medication Reservoir: a container containing the drug, usually a gel or matrix.
- Rate-controlling Membrane: A membrane that controls drug delivery.
- Release Liner: A removable line to protect the patch before applying.
- Design considerations:-
- Patch size and shape: determined by drug dose and skin application area.
- Selection of materials: Materials should be compatible, non-irritating and compatible with the drug.
- Adhesion: strong to stay on the skin, but easy to remove. 4. Release profile: The controlled release of a drug over a specific period of time.
- Skin permeability: patch design should consider skin types and permeability.
- Storage and shelf life: Adhesives must be stored properly to ensure the durability of the fabric.
- Skin Structure:
Basically, the skin is the largest organ in the body, serving as a major protective barrier and protecting the body from a wide range of external factors and threats. Its large surface, about 1.7 square meters in the average person, means that the body is well protected against microorganisms, ultraviolet (UV) radiation, chemicals, allergies and dehydration. This maintenance is essential for maintaining health and well-being. In addition, the skin also plays a role in regulating body temperature, sensation and vitamin D synthesis through exposure to sunlight. Skin care is important to support its function and maintain health. The skin is usually divided into three primary layers: the outermost layer known as the epidermis;
(b) The middle layer called the dermis and (c) the inner layer called the hypodermis. Here is a brief overview of each layer.
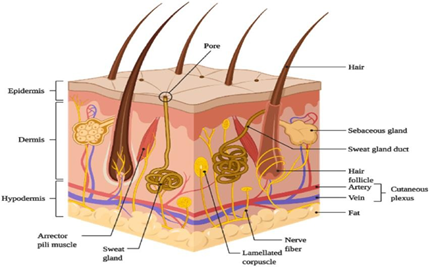
Epidermis
The epidermis is the outermost layer of the skin. A waterproof barrier is formed, without blood vessels. The cells of the epidermis contain keratinocytes, which produce the protein keratin and help harden and protect the skin. The epidermis contains melanocytes, responsible for skin color, and Langerhans cells, components of the immune system.
The epidermis has several lower layers, namely:
- stratum corneum
- Stratum granulosum
- Stratum spinosum
- Stratum basale
The thickness of the parts of our body is different, the palms and soles are 0.8 mm thick. 13 There is a layer of epidermis inside the skin. Most of these cells are called keratinocytes. It is present in 95% of epidermal cells. The epidermis contains a variety of cell types, including melanocytes, Langerhans cells, and Merkel cells.
Above the skin is a thin layer called the stratum corneum. The surface of our skin affects everything around us. This layer is special because it keeps our body safe. Thickness and fluidity are important. The stratum corneum is mainly hard proteins (70% keratin) and some lipids (20?t).
The water in this layer is associated with these proteins.
-
- Dermis:
The dermis lies below the epidermis and is 3 to 5 mm, thicker than the epidermis. The dermis is responsible for delivering nutrients to the epidermis and maintaining skin appendages and sensory receptors.
Different structures, for example:
- Blood vessels
- Hair follicles
- Sweat glands
- Sebaceous glands
- Nerve endings
- Collagen and elastin fibers.
Small blood vessels called capillaries are very close to the surface of the skin, about 0.2mm apart. These cells act as drainage pipes, drawing most of the substances they want out into the skin. This is necessary because it keeps the concentration of substances that penetrate the skin as low as possible. This difference in concentration inside and outside the skin helps things get inside. When it comes to drug delivery through the skin, this layer is like a gel that is mostly water. For most drugs that dissolve in water, this layer doesn't have much of a barrier, making it easier for the drugs to penetrate. However, if the medicine is oily (such as some ointments or creams), this layer can be more of a challenge.
-
- Subcutaneous Tissue (hypodermis):
The underlying tissue, known as the hypodermis, forms the deepest layer of the skin, containing fat cells (fat cells) and tissue connected. This layer acts as an insulator, helping to regulate body temperature and acting as a protective cushion, protecting the body's organs and bones from wear and tear. When medicines come into contact with the skin, such as creams or lotions, they must pass through these three layers in order to enter our bloodstream. For some drugs, they have to go deeper and reach the bloodstream of the body. But for most skin treatments, they only need to pass through the outer layer, the stratum corneum, and stay within the dermis layer to work.
5. Routes of drug penetration into the skin:
Drug penetration into the skin can be achieved by two routes: the epidermal route, which includes through the skin, and through the transdermal route, including penetration. through appendages such as hair follicles and sweat glands.
- The transdermal route: In this route, drugs penetrate the outermost layer of the skin, called the stratum corneum. Structurally, this layer is a complex, multi-layered, multi-wire barrier.
- Intracellular pathway: Some drugs can travel through specialized skin cells called corneocytes, which are specialized skin cells. This method is for substances that dissolve in water (aqueous or polar solutes).
- Intercellular pathway: Other drugs can move through the spaces between these skin cells. This way is for substances that dissolve in lipids (lipophilic or non-polar solutes). It moves through the continuous fat layer of the skin.
- Transcutaneous pathway: This pathway involves drugs that travel through the sweat glands and hair follicles in the skin.
(a) Sweat glands and hair follicles: These glands are tunnel-like or small openings in the skin through which certain substances enter.
- Penetration Enhancer:
Enhances penetration It is also known as a skin enhancer. Penetration enhancers are chemicals that increase the penetration of active compounds, such as drugs, across the skin. It works by temporarily changing the structure and properties of the stratum corneum, the outermost layer of the skin. This change allows active ingredients to better penetrate into the bloodstream or deeper layers of the skin and increase the effectiveness of medical treatment.
- Methods of Enhancing Transdermal Drug Delivery:
Skin penetration can be enhancing by following method:
-
- Drug/prodrug –
The prodrug approach has been used to improve the delivery of drugs to and from drugs with poor distribution coefficients. It is the main substance in the stratum corneum. Upon reaching the living epidermis, the esterase releases the main drug via hydrogen, thereby improving solubility in the aqueous epidermis. For example: the weak permeability of the highly polar 6-mercaptopurine was increased up to 240-fold by using S6-acyloxymethyl and 9- dialkylaminomethyl promoters. The prodrug approach has also been investigated to increase skin penetration of non-steroidal anti-inflammatory drugs, such as naltrexone nalbuphine, buprenorphine alpha blockers and other drugs .
7. 2. Eutectic system-
A eutectic system is a mixture of chemical compounds and elements that have a chemical composition that solidifies at a lower temperature than other compositions. According to common solution theory, the lower the melting point, the higher the solubility of a substance in a solvent, including the skin. The melting of the drug delivery system can reduce the EMLA cream, which is a eutectic mixture of lignocaine and prilocaine used under the occlusive layer, which provides local anesthesia for muscle contraction. pain free, etc.
-
- Liposomes and carriers –
Liposomes are colloidal particles designed as bilayers capable of entrapping drugs. There are many examples of cosmetic products that contain active ingredients in vesicles. These include humectants such as glycerol and urea, anti-aging and tanning agents, enzymes, and more.
-
- Solid Lipid Nanoparticles –
Solid lipid nanoparticles (SLN) have recently been studied as a carrier to increase the production of sunscreen, vitamins A and E, triptolide and glucocorticoids in the skin. It is believed that the increased skin penetration is due to the increased hydration of the skin due to the barrier film that appears on the skin surface. Cholesterol added to the compound stabilizes the structure, resulting in strong liposomes. The mechanism of drug absorption in the stratum corneum is not clear. Liposomes can penetrate the stratum corneum and then interact with the skin tubes to release the drug, only their parts penetrate the stratum corneum.
-
- Iontophoresis:
This technique involves the introduction of a local therapeutic agent by sending a low level electrical current directly to the skin or through a barrier in a medical fashion. Parameters affecting the design of iontophoretic skin delivery systems include electrode type, current strength, and system pH. The increase in drug absorption resulting from this method can be attributed to one or a combination of the following methods: electrolysis (for charged solutions), electroosmosis (for free solutes), and electrical breakdown (for both charged and free solutes.
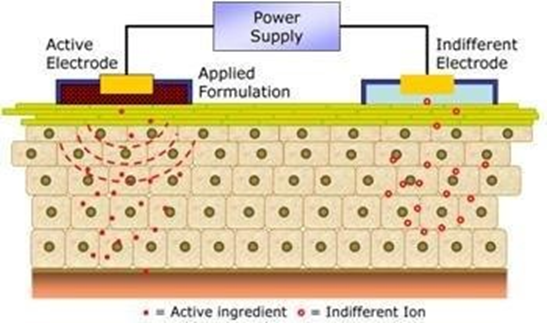
Fig. Basic principle of Iontophoresis
Electroporation -
It involves the application of high voltages to the skin, which is supposed to open the pores. High voltages (100 V) and short processing times (milliseconds) are used. The technology has been successfully used to increase the skin penetration of molecules of different lipophilicity and size (ie small molecules, proteins, peptides and oligonucleotides), including to biological agents weighing more than 7kDA6.

Fig. Basic Priciple of electroporation
Ultrasound (sonophoresis and phonophoresis) -
this method uses the power of ultrasound to increase the transfer of solutes at the same time, by or treatment. It uses low-frequency ultrasound waves (55 kHz) for a duration of 15 seconds to increase skin permeability. The face is rejuvenated. This method involves direct exposure and control of the laser to the skin, which leads to the erosion of the stratum corneum without significant damage to the underlying epidermis.
-
- Laser radiation and photomechanical waves –
lasers are often used to treat skin conditions such as acne and facial rejuvenation. This technique involves direct exposure and control of the laser to the skin, leading to the erosion of the stratum corneum without significant damage to the underlying epidermis..
-
- Radio frequency –
exposing the skin to high frequency alternating current, which leads to the formation of micro channels in the membrane. The speed of drug delivery is controlled by the number and depth of the microchannels created by the device. The treatment takes less than a second.
7.10. Magnetophoresis -
involves the use of a magnetic field that acts as an external driving force to increase the distribution of a diamagnetic solution across the skin. Dispersion of the skin to the magnetic field can also change the structure and increase permeability.
7.11.Microneedle-Based Devices -
The first drug delivery patent for transplant management based on this method These microneedles with a length of 50-110 ?m penetrate the SC and epidermis for drug delivery.
7.12.Skin abrasion -
abrasion means the removal or direct disruption of the upper layers of the skin. These tools are based on techniques used by dermatologists to rejuvenate the skin and are used to treat acne, scarring, hyperpigmentation and other skin blemishes.
7.13.Needle-free injection -
Transdermal delivery is performed by spraying liquid or solid particles at supersonic speed through the outer layers of the skin using a direct current source. This meansthat the force of the compressed air (helium) in the nozzleis said to move the drug particles through the jetat the speed they need to enter to the skin.This method avoids the issues of safety, pain and fear 10
7.14.Pressure application -
Application of mild pressure, i.e. 25 kPa is a very non-safe method and ease of skin absorption of molecules such as caffeine..
-
- Transdermal patch:
Transdermal patch, also known as a skin patch, is a device designed to administer a precise dose of medication by placing it on the skin. This allows the substance to penetrate the skin and enter the bloodstream. A special membrane controls the flow of water from the plastic bag into the skin and blood. 24 Many patients have difficulty swallowing tablets or injections, and tablets are effective longer than tablets, so the dose may be reduced. Tablets are used in a variety of treatments such as pain management, heart disease treatment, smoking cessation, and motion sickness control and hormone therapy.
-
- Components of transdermal patch:
- Polymer matrix
Polymers are the backbone of a transdermal drug delivery system. Translucent delivery systems are composed of multi-layered polymer materials containing a drug reservoir or drug polymer matrix between two polymer layers: an outer backing layer to prevent drug loss in substrate surface; and inner polymeric layer that functions as an adhesive and/or rate? controlling membrane. Polymer selection and design must be considered when striving to meet the diverse criteria for the fabrication of effective transdermal delivery systems. The main challenge is in the design of a polymer matrix, followed by optimization of the drug loaded matrix not only in terms of release properties, but also with respect to its adhesion cohesion balance, physicochemical properties, compatibility and stability with other components of the system as well as with skin. The polymers utilized for TDDS can be classified as (1) natural polymers includes cellulose derivatives, zein, gelatin, shellac, waxes, gums, natural rubber and chitosan etc, (2) synthetic elastomers includes polybutadiene, hydrin rubber, polyisobutylene, silicon rubber, nitrile, acrylonitrile, neoprene, butylrubber etc, (3) synthetic polymers includes polyvinyl alcohol, polyvinylchloride, polyethylene, polypropylene, polyacrylate, polyamide, polyurea, polyvinylpyrrolidone, polymethylmethacrylate etc. The polymers like cross linked polyethylene glycol, eudragits, ethyl cellulose, polyvinylpyrrolidone and hydroxypropylmethylcellulose are used as matrix formers for TDDS. Other polymers such as EVA, silicone rubber and polyurethane are used as coating membranes.
- Drug
The most important criterion for TDDS is that the drug must have physical and chemical properties and pharmacokinetics. Transdermal patches are highly recommended for first-pass transdermal drugs, drugs with a narrow therapeutic window, or drugs with a short half-life that cannot be achieved due to multiple injections. For example, drugs such as rivastigmine for Alzheimer's disease and Parkinson's dementia, rotigotine for Parkinson's, methylphenidate for attention deficit disorder and selegiline for depression have been approved as TDDS.
- Penetration Inhancer
To increase the penetration of the stratum corneum to reach higher clinical levels the synthesis of drug enhancers to the structural components of the stratum corneum such as proteins and lipids. The increased absorption of oil-based drugs due to the washing of epidermal lipids and chemical additives, improves the appearance of the skin for hydration and transepidermal and transfollicular absorption. . The surfactant and solvent properties of the excipients used may be responsible for increasing the skin absorption of water-soluble drugs..
- Pressure Adhesive (PSA)
PSA maintains close contact between the adhesive and the skin surface. Be sure to stick to no more than finger pressure, and keep a firm, firm grip. This includes polyacrylate, polyisobutylene and silicone-based adhesive The choice of adhesive depends on many factors, including patch design and drug pattern. PSA should be physiologically and biologically compatible and should not alter drug delivery. PSA can be placed in front of the device (like a tank system) or behind the device and extend to the side (like a matrix system).
- Backing Laminate
The main function of back concrete is support. The backing layer must be chemically resistant and compatible with solvents because prolonged contact between the backing layer and the solvents may result in additional release or release of solvents or drugs. , or reinforcement. The moisture vapor delivery rate should be low. It must have excellent elasticity, softness and tensile strength.
Some examples of underlay materials are: vapor coated aluminum foil, plastic film (polyethylene, polyvinyl chloride, polyester) and heat seal foil.
Rerelease liner
The release liner prevents the substance that has migrated to the contact layer from being lost and contaminated during storage. Therefore, it is considered to be part of the primary packaging material and not part of the pharmaceutical release dosage form. Release tape consists of a base layer that is non-absorbent (paper gel) or occlusive (polyethylene and polyvinyl chloride) and a release coating made of silicone or Teflon. Other materials used for TDDS sheets are polyester sheets and metal sheets.
Other Excipients
Various solvents such as chloroform, methanol, acetone, isopropanol and dichloromethane are used to prepare the drug source. In addition, surfactants such as dibutyl phthalate, triethyl citrate, polyethylene glycol and propylene glycol are added to the transdermal patch to make it easier..
-
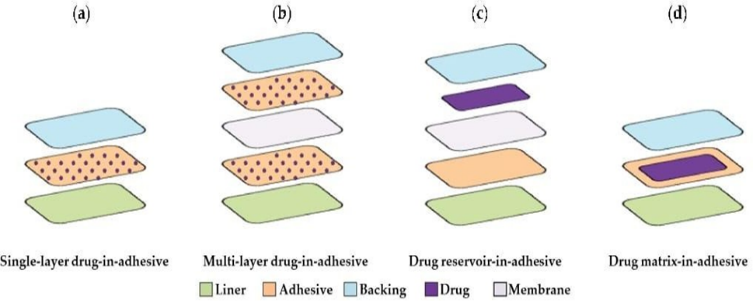
- Types of transdermal patches:
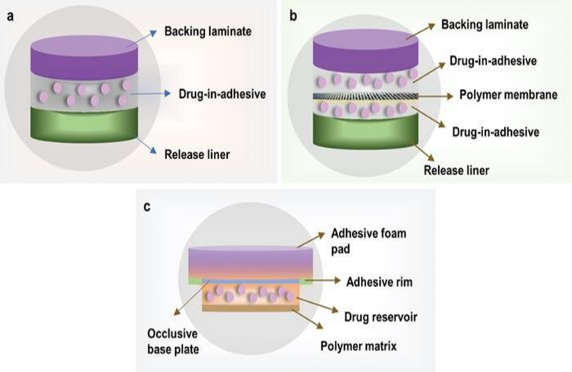
- Single layer drug-in-adhesive patches:
The source is used as a polymer layer and adhesives to disperse the drug. A backing layer is placed under this single layer. The drug, contained in the polymer layer and attached to it, is released from the backing layer that supports the drug reservoir. 29 Daytarana is a single layer formulation of methylphenidate for transdermal patch treatment.
- Multilayer drug-in-adhesive patches:
Drug release is controlled over a period of time consist of a drug reservoir layer and adhesive layer. 2,3 It contains a temporary protective layer and backing laminate multilayer systems. Multi-layer patches can be prolonged for up to seven days are used to deliver pain medication, smoking cessation treatments, and hormone therapy.
- Vapor Transdermal Patches:
These patches are adhesive polymer films designed with vapor release and diffusion properties. Different foams are used for different purposes. One example is Nicoderm CQ®, a patch with nicotine vapor that contains essential oils to help quit smoking. Introduced to the European market in 2007, this oil helps people quit smoking by releasing these oils. Another type is the Altacora steam bath, which contains essential oils and is used to relieve menstrual cramps. In addition, there are vaporizers on the market that are designed as antidepressants or tranquilizers.
- Membrane Moderated Transdermal Reservoir Patches:
A transdermal patch is a drug reservoir, an impermeable backing layer consisting of a metallized plastic shell and a porous polymer membrane to facilitate drug delivery. This membrane is made of polymers such as ethylene vinyl acetate copolymer and hypoallergenic self-adhesive polymer. The drug in the transfer patch is controlled by molecular dispersion of the drug in the polymer matrix as part of the manufacturing process.
- Micro reservoir transdermal Patches:
Micro reservoir transdermal adhesives the substance to disperse the matrix. Reservoir is created by suspending the drug in an aqueous solution of a hydrophilic polymer followed by uniform dispersion of the drug suspension on a lipophilic polymer. This dispersal process involves high mechanical shear and results in the formation of microscopic balls, which cannot be washed. Drug release from these fragments follows an order-free rate and stabilizes plasma drug levels. To maintain the stability of the drug, polymeric transfer agents are incorporated into drug dispersions.
- Matrix system:
- Drug in an adhesive system:
In this system, the drug source is made by mixing the drug with an adhesive polymer. Next, the healthy polymer paste is spread using a solvent or solvent. When using a metal mold, a non-stick adhesive polymer layer is placed over the baking layer.
- Matrix dispersion system:
In this system, the material is dispersed in a hydrophilic and lipophilic polymer matrix. This polymer disk containing the drug is attached to an occlusive plate in the space created by the protective backing layer. Instead of applying it directly to the medical source, the paste is spread around the perimeter to form the edge of the paste.
Evaluation:
- Physical appearance
- Interaction Studies
- Thickness
- Folding Endurance
- Weight uniformity
- Percentage Moisture Content
- Percentage Moisture Uptake
- Percentage Moisture Vapour Permeation
- Drug Content
- Flatness Test
- Tensile Strength
- Shear and Adhesion Test
- Quick stick (Peel tack) Test
- Rolling ball tack test
- Stability studies
- Physical Appearance:
Each of the panels was created under a visual inspection to evaluate characteristics such as color, lightness, opacity, lightness, softness and flexibility.
- Interaction studies:
In various dosage forms, not limited to transdermal drug delivery systems (TDDS), additives are used. These additives must be compatible with the drug to avoid loss of stability and availability. Interaction studies are usually performed using thermal analysis, Fourier transform infrared spectroscopy (FT-IR), ultraviolet (UV) spectroscopy, and chromatographic techniques. These studies include comparing the physicochemical properties of the substance and additives to ensure compatibility.
- Thickness:
The thickness of the transfer patch is measured using an instrument such as a caliper, dial gauge, caliper, or micrometer. Measurements are made at three different locations of the patch, and the average of these three measurements is called the thickness of the patch. A uniform thickness patch shows a constant thickness at each measurement point.
- Folding Endurance:
Fold Toughness is evaluated by repeatedly bending the patch or film in a specific area until it breaks or folds 300 times. The number of times a note can be folded without breaking gives the note strength to note. This measures the ease of the update.
- Weight Uniformity:
The clays are dried at 60°C before weighing. To evaluate the uniformity of weight, pieces of 1 cm2 were cut from three parts and weighed individually. The weight variance is calculated ensuring that the individual weights do not differ from the average weight. The average
weight of the three parts is called the weight of the note.
- Percentage Moisture Content:
Individual pieces are weighed and then placed in desiccators containing molten calcium chloride at a specified temperature for 24 hours. After this time, the pieces are weighed again and the percentage of moisture is calculated from the difference in weight before and after drying. This method helps to determine the amount of moisture in the sprouts.
Moisture content(%) = Initial mass - final mass
Initial mass× 100
- Percentage Moisture Uptake:
Weighed films are first placed a dryer for 24 hours and exposed to 84% relative humidity obtained with potassium chloride in another dryer.
The films are weighed periodically until they reach a weight of This process determines how the layers absorb and retain moisture under specific moisture conditions, and provides valuable information on stability and performance.
Moisture uptake (%) = Final mass - Initial mass
Initial mass× 100
- Percentage Water vapor permeation:
Use containers of the same diameter as inlet cells. These bottles are washed, dried and filled with 1 gram of molten calcium chloride. A patch with a surface of 1 square centimeter is measured and attached to the bottom of the container. The vials were carefully weighed and then placed in a desiccator containing sodium chloride solution to maintain a relative humidity of 63%. After 72 hours, the vials were removed and the weight was measured again. This process helps to evaluate the penetration characteristics of the patch under wet conditions.
- Drug content:
To measure the drug content of a transfer patch, a specific portion of the patch is dissolved in a specific volume of a specified solvent. The solution is continuously shaken for 24 hours, treated ultrasonically for several times and then filtered. The drug content in the filter is determined using an appropriate analytical method.
10.Flatness test:
Cut a transverse plate into three long pieces: one from the right, one
from the left and one from the middle. The length of each strip is measured. The following equation is used to determine flatness:
Construction (%) = (I1 – I2) × 100 (4)
where I1 = strip initial length, I2=strip final lenght
11.Tensile Strength:
A modified pulley system is utilized to study the tensile strength of the film. The system measures the force needed to break the film, providing valuable information about its tensile strength.
12.Shear Adhesion Test:
This test is conducted to assess the cohesive strength of the adhesive polymer. In this method, the adhesive-coated patch is applied over a smooth surface, and a specific weight is hung from thpatch parallel to the surface. The duration taken to pull off the patch from the surface measures its shear adhesion property, indicating the strength of the adhesive bond.
13.Quick stick (Peel Tack) Test:
The peel force required to break the bond between the adhesive and the substrate is measured by pulling the tape away from the substrate through 90? at a speed 12 inches per minute. The contact test is performed using a probe that is pushed forward and touched with the adhesive surface and then retracted at a predefined speed. The force required to break the bond after a short period of contact is measured. The test may be performed with the help of Texture Analyser.
14.Ball Rolling Test
In this test, a 7/16 inch steel ball is rolled down a horizontal plane with a horizontal patch facing up and the contact surface. horizontal distance on the patch. . The distance traveled by the ball is an indication of the stickiness or stickiness of the glue Adhesion is a measure of the adhesive's ability to quickly adhere
15.Stability studies
Tests are performed according to ICH (International Council for Harmonization) guidelines. The manufactured transdermal patches are stored at a temperature of 40 ± 0.5 degrees Celsius and a humidity of 75 ± 5% for a period of six months. Samples are taken at the designated time points, i.e., 0, 30, 60, 90 and 180 days, and analyzed to determine drug content as needed. This test ensures the stability and quality of the transfer fabrics for a long time under the specified storage conditions.
CONCLUSION:
The route of drug delivery through the skin is considered safe and effective compared to other cardiovascular drugs, have been formulated into transdermal drug delivery systems (TDDS) to reduce gastrointestinal side effects and first pass. . Due to the advantages and increasing popularity of transdermal drug delivery systems, researchers are focusing on introducing new drugs into this delivery format. Despite its benefits, it is important to understand that the skin acts as a protective barrier for the internal organs. These changes are minimized when designing a course. A comprehensive understanding of the physiology and anatomy of the skin is essential for progress in this area. To achieve optimal skin delivery, however, it is necessary to understand and understand the interactions between the various polymers and the skin components. This knowledge is important for designing and optimizing a transdermal drug delivery system. This knowledge is important for designing and optimizing a transdermal drug delivery system.
REFERENCES
- Phatale, V., Vaiphei, K. K., Jha, S., Patil, D., Agrawal, M., & Alexander, A. (2022). Overcoming skin barriers through advanced transdermal drug delivery approaches. Journal of controlled release, 351, 361-380.
- Jeong, W. Y., Kwon, M., Choi, H. E., & Kim, K. S. (2021). Recent advances in transdermal drug delivery systems: A review. Biomaterials research, 25(1), 24.
- Pastore, M. N., Kalia, Y. N., Horstmann, M., & Roberts, M. S. (2015). Transdermal patches: history, development and pharmacology. British journal of pharmacology, 172(9), 2179- 2209.
- Panda A, Vanathi M, Kumar A, Dash Y, Priya S. Corneal graft rejection. Survey of ophthalmology. 2007 Jul 1;52(4):375-96.
- Al Hanbali OA, Khan HM, Sarfraz M, Arafat M, Ijaz S, Hameed A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharmaceutica. 2019 Jun 30;69(2):197-215.
- Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—A review. International journal of pharmaceutics. 2011 Aug 30;415(1-2):34-52.
- Guerra A, Etienne-Mesmin L, Livrelli V, Denis S, Blanquet-Diot S, Alric M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends in biotechnology. 2012 Nov 1;30(11):591-600.
- Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annual review of pharmacology and toxicology. 2012 Feb 10;52(1):275-301.
- Naziya Shaikh, Richa Srivastava A review on transdermal drug delivery through patches Ip Indian journal of Clinical and Experimental Dermatology 2024;10(2):113-121.
- Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annual review of pharmacology and toxicology. 2012 Feb 10;52(1):275-301.
- Saroj AK, Khan R, Sharma B. Transdermal drug delivery system (Patch). World Journal of Pharmaceutical Research. 2019 Jun 25;8:325-43 Chambers ES, Vukmanovic?Stejic M. Skin barrier immunity and ageing. Immunology. 2020 Jun;160(2):116-25.
- Chambers ES, Vukmanovic?Stejic M. Skin barrier immunity and ageing. Immunology. 2020 Jun;160(2):116-25.
- Kumar MA. The skin. Techniques in Small Animal Wound Management. 2024 Feb 29;1.
- Boulais N, Misery L. Merkel cells. Journal of the American Academy of Dermatology. 2007 Jul 1;57(1):147-65.
- Mercuri M, Fernandez Rivas D. Challenges and opportunities for small volumes delivery into the skin. Biomicrofluidics. 2021 Jan 1;15(1).
- Supe S, Takudage P. Methods for evaluating penetration of drug into the skin: A review. Skin research and technology. 2021 May;27(3):299-308.
- Souto EB, de Souza AL, Dos Santos FK, Sanchez-Lopez E, Cano A, Zieli?ska A, Staszewski R, Karczewski J, Gremião MP, Chorilli M. Lipid nanocarriers for hyperproliferative skin diseases. Cancers. 2021 Nov 10;13(22):5619.
- Aleksandrowicz H, Owczarczyk-Saczonek A, Placek W. Venous leg ulcers: advanced therapies and new technologies. Biomedicines. 2021 Oct 29;9(11):1569. Oliveira R, Almeida IF. Patient-centric design of topical dermatological medicines. Pharmaceuticals. 2023 Apr 19;16(4):617.
- Geboers B, Scheffer HJ, Graybill PM, Ruarus AH, Nieuwenhuizen S, Puijk RS, van den Tol PM, Davalos RV, Rubinsky B, de Gruijl TD, Miklav?i? D. High-voltage electrical pulses in oncology: irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology. 2020 May;295(2):254-72.
- Li MK, Liu C, Hsu JT. The use of lasers and light devices in acne management: an update. American Journal of Clinical Dermatology. 2021 Nov;22:785-800.
- Zablotskii V, Polyakova T, Dejneka A. Effects of high magnetic fields on the diffusion of biologically active molecules. Cells. 2021 Dec 28;11(1):81.
- Ghadge RS. Transdermal Drug Delivery System. Journal of Research in Agriculture and Animal Science. 2022;9(6):31-48.
- Saroha, K., Yadav, B., & Sharma, B. (2011). Transdermal patch: A discrete dosage form. Int J Curr Pharm Res, 3(3), 98-108.
- Kandavilli S, Nair V, Panchagnula R. Polymers in transdermal drug delivery systems. Pharmaceutical technology. 2002 May;26(5):62-81.
- Bala P, Jathar S, Kale S, Pal K. Transdermal drug delivery system (TDDS)-a multifaceted approach for drug delivery. J Pharm Res. 2014 Dec;8(12):1805-35.
- Yu YQ, Yang X, Wu XF, Fan YB. Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: novel strategies for effective transdermal applications. Frontiers in bioengineering and biotechnology. 2021 Mar 29;9:646554.
- Taub MB. A mechanics approach to the study of pressure sensitive adhesives and human skin for transdermal drug delivery applications. Stanford University; 2003.
- Shi, G. M., Feng, Y., Li, B., Tham, H. M., Lai, J. Y., & Chung, T. S. (2021). Recent progress of organic solvent nanofiltration membranes. Progress in Polymer Science, 123, 101470.
- Wang Q, Chen W, Zhu W, McClements DJ, Liu X, Liu F. A review of multilayer and composite films and coatings for active biodegradable packaging. npj Science of Food. 2022 Mar 11;6(1):18.
- Ashfaq A, Riaz T, Waqar MA, Zaman M, Majeed I. A comprehensive review on transdermal patches as an efficient approach for the delivery of drug. Polymer-Plastics Technology and Materials. 2024 May 23;63(8):1045-69.
- Alam, M. I., Alam, N., Singh, V., Alam, M. S., Ali, M. S., Anwer, T., & Safhi, M. M. (2013). Type, preparation and evaluation of transdermal patch: a review. World journal of pharmacy and pharmaceutical sciences, 2(4), 2199-2233.
- Kalia, Y. N., & Guy, R. H. (2001). Modeling transdermal drug release. Advanced drug delivery reviews, 48(2-3), 159-172.
- Al Hanbali, O. A., Khan, H. M. S., Sarfraz, M., Arafat, M., Ijaz, S., & Hameed, A. (2019). Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharmaceutica, 69(2), 197-215.
- Ghadge RS. Transdermal Drug Delivery System. Journal of Research in Agriculture and Animal Science. 2022;9(6):31-48.
- Mishra B, Bonde GV. Transdermal drug delivery. InControlled drug delivery systems 2020 Feb 28 (pp. 239-275). CRC Press.
- Snežana IS, Nikoli? L, Vesna N, Ili? D, Risti? IS, Ta?i? A. Polymeric matrix systems for drug delivery. InDrug Delivery Approaches and Nanosystems, Volume 1 2017 Nov 15 (pp. 95-131). Apple Academic Press.
- Patel RP, Patel G, Patel H, Baria A. Formulation and evaluation of transdermal patch of aceclofenac. Research Journal of Pharmaceutical Dosage Forms and Technology. 2009;1(2):108-15.
- Alam, M. I., Alam, N., Singh, V., Alam, M. S., Ali, M. S., Anwer, T., & Safhi, M. M. (2013). Type, preparation and evaluation of transdermal patch: a review. World journal of pharmacy and pharmaceutical sciences, 2(4), 2199-2233.
- Thirunavukkarasu, A.; Nithya, R.; Jeyanthi, J. Transdermal drug delivery systems for the effective management of type 2 diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2022, 194, 10999 .
- Barry B. Transdermal drug delivery. In: Aulton E, editor. The science of dosage forms design, 2nd edn. . Churchill Livingstone, New York:Harcourt publishers; 2002. p. 499– 533.
- Das Kurmi, B., Tekchandani, P., Paliwal, R., & Rai Paliwal, S. (2017). Transdermal drug delivery: opportunities and challenges for controlled delivery of therapeutic agents using nanocarriers. Current drug metabolism, 18(5), 481-495


 Bharat Jadhav *
Bharat Jadhav *
 Gabhad kanchan
Gabhad kanchan
 Dhas Swati
Dhas Swati
 Dhapate prashanth
Dhapate prashanth






 10.5281/zenodo.14678689
10.5281/zenodo.14678689