Abstract
The mechanical, electrical, optical, thermal, and chemical characteristics of carbon nanotubes (CNTs) are remarkable. The structure, synthesis, and manufacturing kinds of carbon nanotubes (CNTs) are examined in this paper, along with their uses in drug delivery systems, namely in the treatment of cancer. CNTs are efficient nano-carriers for targeted medication delivery because of their capacity to penetrate cell membranes and gather in malignancies. Furthermore, trans-dermal drug delivery systems (TDDS), which circumvent the first-pass effect and are patient-compliant, heavily rely on CNTs. CNTs are advantageous materials in nanotechnology because of their strong structure and distinctive qualities.
Keywords
Mechanical, optical, electrical, treatment of cancer.
Introduction
Carbon nanotubes (CNTs), discovered by Japanese scientist Iijima in 1991[1], are currently regarded as superior subjects for academic studies and a variety of industrial fields. Carbon nanotubes (CNTs) are one of the newest and most promising methods in pharmaceutical research and development when it comes to innovative drug delivery systems. One of the components of the fullerenes group is CNTs. CNTs are long, thin, cylindrical, tubular, and pure carbon molecules that range in size from two to three manometers.[2] Because of their unique architectures and qualities, CNTs can be applied to a wide range of pharmaceutical applications, including drug delivery, cancer treatment, biosensors, and biomedical imaging, as well as tissue engineering applications requiring structured materials. Moreover, CNTs are utilized in the intracellular administration of proteins, plasmids, deoxyribonucleic acid, short interfering ribonucleic acid, and small pharmacological entities. [3] CNTs are allotropes, which is having a tubular figure and prepared from graphite. They are classified into three categories,
- Single-walled (SWCNTs),
- Double-walled (DWCNTs)
3. Multi-walled (MWCNTs).[2]
Compared to traditional dosage forms, nanoparticulate drug delivery systems may have a number of benefits, such as increased bio-distribution, decreased toxicity, and better patient compliance. Subnanosize pharmaceutical nanoparticles based structure, comprised of tens or hundreds of atoms or molecules with a range of sizes (from 5nm-300nm) and morphologies (amorphous, crystalline, spherical, needles, tubes, etc.), and contain pharmacological or bioactive chemicals within them.{[1],[2],[3]This paper aims to understand the applications of carbon nanotubes in the pharmaceutical industries and what they offer in terms of improving the quality of pharmaceutical products or adding something to pharmaceutical sciences. It will cover all topics related to carbon nanotubes in pharmaceutical and pharmaceutical studies and medical uses. We'll also go over certain pharmaceutical worries regarding the utilization of carbon nanotubes in medicine production.
Carbon Nanotubes
ADVANTAGE OF CNTs [2,3,4]
- High electrical along with warm conductivity
- Very high elasticity
- Highly adaptable and flexible (~18% lengthening before disappointment)
- High perspective proportions
- Good field emanation
DISADVANTAGE OF CNT [2,3,4,5]
- More current innovation so not as much testing has been completed
- Lower lifetime (1750 hours contrasted with 6000 hours for silicon tips) Higher possibilities required for field outflow as the cylinders are not all that very much restricted so the extractor cathode must be further away.
Classification Of CNTs
• Single-walled CNTs
• Double-walled CNTs
• Multi-walled CNTs
Structure Of CNTs
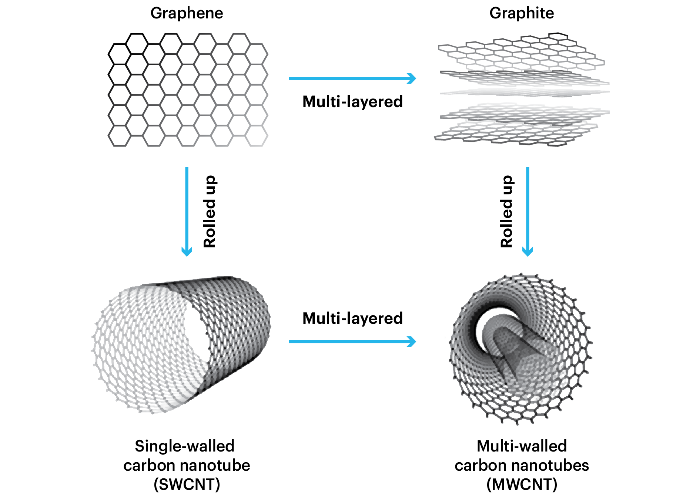
The structure of pure SWCNT, which is formed entirely of carbon, is represented as a rolled-up tubular shell of graphene sheet composed of hexagonal rings of the benzene type.
Atoms of carbon (Figure (a)). Graphene sheets, which symbolize a single atomic layer of crystalline graphite, are seamless cylinders made from a honeycomb lattice. A stack of graphene sheets wrapped into concentric cylinders is known as a MWCNT. A single molecule made up of millions of atoms, a nanotube can have a diameter as small as 0.7 nm and a length of tens of micrometers [6]. SWCNTs typically have a circum circumference of just 10 atoms and a tube thickness of only one atom.
They have an aspect ratio (length-to-diameter ratio) of roughly 1000, which makes them almost one-dimensional structures [7].
(a)
MWCNTs are made up of numerous single-walled tubes stacked one on top of the other and are larger.
The term MWCNT is limited to nanostructures having an outer diameter of less than 15 nm; nanostructures larger than this are referred to as carbon nanotubes. CNTs are not to be confused with carbon fibers, which are strands of stacked graphite sheets rather than single molecules [8, 9].
Three distinct forms of carbon nanotubes are possible in addition to the two basic configurations (figure (b)). CNTs come in three different varieties: armchair carbon nanotubes, carbon nanotubes with chirality and zigzag patterns. How graphite is "rolled up" throughout the production process determines how different kinds of carbon nanotubes are produced. Different forms of SWCNTs are possible due to the rolling axis's adjustment in relation to the closing cylinder's radius and the hexagonal network of the grapheme sheet .
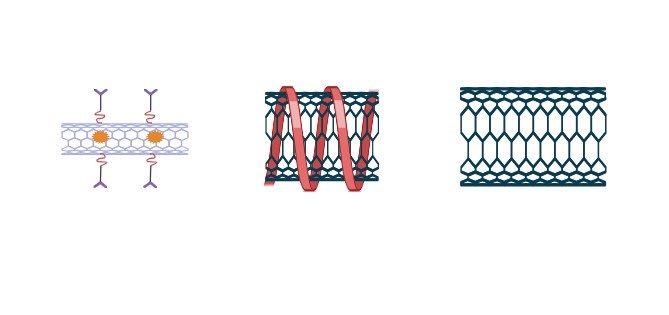
(b)
Pharmaceutical Applications Of CNTs
The main applications of CNTs in pharmacy and medicine include drug, bio-molecule, gene delivery to cells or organs, tissue regeneration, and biosensor diagnostics and analysis.The general CNT-based medication delivery procedure can be briefly repeated here. Drug is adhered to the interior or exterior of functionalized carbon nanotubes. The resultant conjugate is subsequently administered orally, intravenously, or directly to the target site by use of a magnetic conjugation; for instance, the target organ, such lymphatic nodes, could be steered to the spot by an external magnet.
Drug delivery occurs when the drug-containing CNT capsule is ingested by the cell and eventually leaks its contents into the cell [10,11]. Medication Administration. To treat malignancies, CNTs can be employed as drug carriers [11,12,13]. The systemic toxicity of anticancer medicines is not the sole factor limiting their effectiveness when taken alone. Its restricted therapeutic window, in addition to cellular penetration limitations and medication resistance. The anticancer medication carried by this vehicle will be released in situ with intact concentration since CNTs can readily pass through the cytoplasmic and nuclear membranes. As a result, the anticancer drug's activity in the tumor cell will be greater than that of standard therapy when given alone. Therefore, it is necessary to create effective delivery systems that can improve the cellular uptake of already effective medications.The CNTs' high aspect ratio provides significant benefits over the current delivery vectors because of its large surface area, which gives medications several places to attach [14]. Numerous anticancer medications, including epirubicin, doxorubicin, cisplatin, methotrexate, quercetin, and paclitaxel, have been conjugated with functionalized carbon nanotubes and successfully tested in vitro and in vivo [14, 15, 16–17]. Our group [15] has linked epirubicin with a magnetic CNTs complex obtained by fixing a layer of magnetite (Fe3O4) nanoparticles on the surface of the nanotubes with necklace-like type and on the tips of shortened MWCNTs in order to prevent the harmful effect of anticancer drug on healthy organs and cells.[15]
Toxicity Of CNTs
Generally speaking, a number of elements combine to cause the negative consequences of nanoparticles, two of which are two factors in particular: (a) the large surface area and (b) the surface's inherent toxicity[18]. Nanoparticles less than 100 nm have the ability to change the structure of proteins, escape from the body's natural defenses against phagocytic defenses, and possibly be more toxic to the lung (the portal of entry) than conventional particles. Consequently, nanoparticles may impact normal tissue function and trigger immunological and inflammatory reactions[19]. Because of their nanoscale, CNTs can be categorized as "nanoparticles" in the context of toxicity. dimensions, so coming into touch with biological systems may result in unanticipated toxicological consequences. Because of the CNT's nanoscale size, milligrams of material include a high total surface area together with a significant number of cylindrical, fiber-like particles. The degree to which the nanotubes in solution are bundled and aggregated will likewise affect this total surface area[20]. The level of surface functionalization and the distinct toxicity determine the intrinsic toxicity of CNT.
several useful groupings. Following synthesis, batches of pure CNT (non-purified and/or non-functionalized) frequently contain impurities including amorphous carbon and metallic nanoparticles (catalysts: Co, Fe, Ni, and Mo), which can potentially have extremely harmful consequences.[20]. According to Donaldson et al.[21], the structural properties of nanomaterials, such as the length, shape, and aggregation state of the CNT, can also affect the immunological reaction that occurs after exposure to CNT and the local deposition of the material in the lungs.
The bio-availability of CNT in the body is an additional significant element. The metabolism of carbon nanotubes, To gain a better understanding of the limits of such nanomaterials as medicinal ingredients, more research and attention must be paid to degradation or dissolution, clearance, and bio-accumulation. The great majority of studies on the administration of carbon nanotubes (CNT) that have been published to date have mostly focused on the toxicology of CNT, discussing the potential adverse effects of this nanomaterials on human health and the environment, especially with regard to public health and the safety of workers in CNT production plants. As the synthesis of carbon nanotubes (CNT) moves toward large-scale manufacturing, concerns about exposure risk arise from worker handling and CNT exposure (both pulmonary and cutaneous).[20]
Transdermal Drug Delivery
The trans-dermal drug delivery system (TDDS) is a viable and compelling substitute for injection or oral medication administration. Because of their high adsorption capability and small nano-needle shaped structure, CNTs have recently been studied as TDDS. In order to regulate drug release, they created an electro-sensitive TDDS of CNTs utilizing polyethylene oxide and pentaerythritol triacrylate polymers using electrospinning. CNTs enabled electro-sensitive transdermal drug delivery in this TDDS [22]. High loading efficiency and improved transdermal drug penetration with the application of a tiny electrical bias to provide a programmable drug delivery system are two benefits of using CNTs in TDDS.
Future research on f-CNTs in TDDS offers a workable remedy to the drawbacks of the existing therapies for opioid withdrawal symptoms and smoking cessation [23,24].
Ophthalmic Drug Delivery
A number of strategies have been used to create a pleasant, safe, and bio-compatible nanovehicle that can transport drugs to the eyes in a regulated manner while improving patient compliance and minimizing toxicity. Just 1% to 5% of administered treatment may get through the cornea and into the intraocular tissues, and ophthalmic medications have a brief residence period (less than five minutes). Few eye drugs' bio-availability has been observed to be improved by CNTs-based ocular drug administration, which circumvents the drawbacks of conventional ocular drug delivery techniques [25]. Additionally, CNTs might be employed to target various therapeutic medicines to the various eye locations. Regretfully, there aren't many reports accessible in this field [26].
Applying "Kajal," often referred to as kohl or surma, is a custom in Indian households.
Diagnosis
With their low toxicity and immunogenicity, carbon nanotubes (CNTs) are showing great promise as innovative drug delivery and diagnostic nano-vector tools. They may be used alone or in conjunction with other nano-carriers such as dendrimers, nanoparticles, and quantum dots. When Delogu and colleagues evaluated the echogenic properties of nanotubes in vitro, they discovered that f-MWCNTs had a stronger ultrasonic signal than f-SWCNTs, graphene oxide, and pure MWCNTs. Using pigs as an experimental animal model, the authors investigated the liver and heart of the animals and discovered that the MWCNTs are highly echogenic. As a result, nanotubes may prove to have great promise as theragnostics, which combine therapeutic and diagnostic modalities, and as ultrasound contrast agents [27].
CNTs As Carrier for Drug Delivery
Among the cells that Amphotericin B targets, CNTs are investigated. Cisplatin discharge in an aqueous environment has been seen to be slowed down by a medication similar to cisplatin encapsulating oxidized SWCNHs. Cisplatin discharge has been effective in stopping the growth of human lung cancer cells, where SWCNHs have been employed as a carrier system, however their anticancer efficacy has not been demonstrated. Because of the CNTs' regulated lipophobicity, cancer treatments like Polyphosphazene platinum, which are known to penetrate, distribute, and maintain their medication in brain tumors, have better results.
CNT-specified antibiotic, such as doxorubicin, is seen to improve intracellular component and permeability. Hydro-gel, a blend of gelatin and carbon nanotubes, is a crucial biomedical carrier system.Because erythropoietin is denaturated by GIT conditions and enzymes, the carrier system based on CNTs may be utilized as an effective oral route of administration of erythropoietin (EPO). However, this is not practical.[28]Researchers Zhuang Liu et al have worked on using carbon nanotubes for drug delivery in the treatment of cancer in vivo. Chemical-functional single-walled carbon nanotubes (SWNT) have demonstrated promise in tumor-targeted accumulation in mice and exhibit minimal toxicity, bio-compatibility, and excretion. Where they had previously A cleavable ester bond is employed to bind branch polyethylene glycol chains on star nanotubes (SWNts) with paclitaxel (PTX), a commonly used cancer chemotherapy medication, to produce a water-soluble SWNT-PTX conjugation. In a murine 4T1 model of breast cancer, WNT-PTX is more effective than clinical Taxol at suppressing tumor development. This is because WNT-PTX causes extended blood circulation and 10-fold greater tumor absorption of PTX by SWNT delivery, most likely due to enhanced permeability and retention. Medicinal compounds transported into the reticuloendothelial system are freed from SWNTs and eliminated via the biliary pathway without posing a hazard to organs that are healthy.[29]
CNTs: Functionalization for Biomedical Applications
Pristine CNTs' very hydrophobic surfaces prevent them from dissolving in aqueous liquids. For CNTs to be soluble and to have minimal toxicity and bio-compatibility for use in medicine, surface functionalization is necessary [31]. The process of CNT functionalization may be separated into based on the kind of bio-molecule attached to the carbon nanotube, into two primary strategies: non-covalent attachment (physio-adsorption) and covalent attachment (chemical bond formation) [30,32]. CNTs are typically covalently functionalized by oxidation with strong acids (HNO3). Carboxyl (-COOH) groups are created at the open throughout the process. SWCNT or MWCNT's sides (tips) and sidewall flaws, followed by further covalent conjugation with amino acid. Nitrene cycloaddition, arylation with diazonium salts, or 1,3-dipolar cycloadditions are often used to produce -COOH on the sidewalls of carbon nanotubes [30,32]. Coating CNTs with amphiphilic surfactant molecules or polymers (polyethylene-glycol) allows for the non-covalent functionalization of CNTs. The big, fragrant Carbon nanotubes are perfect for non-covalent interactions with appropriate complimentary molecules and macrobiomolecules (DNA) due to their hydrophobic surface (electron).
Both the interior and exterior of CNTs may experience these interactions. Nevertheless, macro-molecules cannot be joined internally [30, 32, 33]. CNTs become hydrophilic after functionalization and are prepared to be delivered into the target cells or organs by being coupled with medications or bio-molecules (genes, DNA, proteins, enzymes, biosensors, etc.).
Pharmacokinetics and Metabolism of CNTs
Research on the Pharmacokinetics and metabolism of various CNT forms has been conducted, and some review articles have recently been published in the literature [34,37].
Nanoparticles' physicochemical properties, including surface functionalization, solubility, form, aggregation, and chemical composition, determine their bio-distribution and Pharmacokinetics. Two investigations on the bio-distribution of water-soluble carbon nanotubes (CNTs) in mice (SWCNT or/and MWCNT) have been published in the literature [35,36,37]. There are no fatalities or harmful adverse effects mentioned in any of these investigations. In order to observe their bio-distribution in mice,111Indium or 125Iodine were employed as radio-tracers in both investigations [35,36]. 125I-hydroxylated-SWCNT (125I-SWCNT-OH) was administered orally, intraperitoneally, intravenous, or SC in the first one.According to this study, 94% of the unmodified nanotubes were eliminated in the urine and 6% in the feces, indicating that the delivery method had no discernible effect on the CNT bio-distribution and that the 125I-SWCNT-OH spread swiftly throughout the body [35, 37]. The stomach, kidneys, and bones were the organs that were most favored for accumulation. There were no reports of tissue injury or discomfort. Two types of 111Indium-functionalized SWCNT or MWCNT were administered intravenously (IV) only to mice in the second trial. Thirty minutes after treatment, the bio-distribution patterns for the two types of functionalized CNTs were found to be quite comparable, demonstrating a predilection for the kidneys, muscle, skin, bone, and blood [34, 36].
Nonetheless, it was shown that all forms of CNTs had a maximum blood circulation half-life of 3.5 hours and were quickly removed from all tissues [37]. Transmission electron microscopy showed that both SWCNT and MWCNT were intact in the expelled urine and that they were eliminated by the renal pathway [35]. However, some researchers have recently demonstrated that myeloperoxidase (MPO), an enzyme present in mouse neutrophils, may degrade CNTs [38]. Their findings challenge the long-held notion that CNTs are not degraded by the body. Since it is evident that endogenous MPO may degrade CNTs, the process by which MPO transforms CNTs into water and carbon dioxide has potential medical applications and therefore marks a milestone in nanotechnology and nanotoxicology [39].
The future of pharmaceutical studies involving carbon nanotubes (CNTs)
looks incredibly promising due to their unique properties and versatile applications. Here are some key areas where CNTs are expected to make significant impacts:
- Drug Delivery: CNTs can be used as carriers for drug delivery due to their high surface area, ability to penetrate cells, and capacity to be functionalized to target specific tissues. This can enhance the efficacy and reduce the side effects of drugs.[40]
- Cancer Treatment: CNTs have shown potential in targeting cancer cells specifically, allowing for more precise delivery of chemotherapy drugs and reducing damage to healthy cells.[41]
- Tissue Engineering: CNTs can be used to create scaffolds that support the growth and regeneration of tissues. Their mechanical strength and biocompatibility make them ideal for applications in regenerative medicine.[40]
- Biosensors and Bioimaging: CNTs can be used to develop highly sensitive biosensors for detecting diseases at an early stage. They also have applications in bioimaging, helping to visualize biological processes in real-time.[40]
- Reducing Toxicity: Ongoing research is focused on reducing the cytotoxicity of CNTs to make them safer for use in medical applications. Functionalization techniques are being developed to improve their biocompatibility.[41]
The integration of CNTs in pharmaceuticals is still in the research phase, but the advancements so far indicate a transformative potential in medicine and healthcare.
CONCLUSIONS
The pharmaceutical business greatly benefits from the utilization of carbon nanotubes. Carbon nanotubes may be one of the most dependable ways to enhance medicine quality, lessen adverse effects, and enhance drug delivery systems while competing with drug production technologies, according to recent advancements. Carbon nanotubes are predicted to be able to successfully compete with nanotechnology in the near future. But there are still a lot of important questions. One of the biggest obstacles that medicine production and the application of nanoparticle technology may encounter in the near future. The use of carbon nanotubes in the pharmaceutical industry has many benefits, but there are also significant challenges facing this field. Here are some of the main challenges:
1.Toxicity: Carbon nanotubes may be toxic to living cells, raising concerns about their safety when used in medical applications.
2.Biodegradability: Carbon nanotubes are not easily biodegradable within the body, which can lead to their accumulation in tissues and organs, thus causing long-term health problems.
3.Dispersibility in water: Carbon nanotubes suffer from poor Dispersibility in water, making them difficult to use in biological systems that depend on water.
4.High cost: Manufacturing carbon nanotubes requires advanced and expensive technologies, which increases the cost of production and limits their widespread use.
5.Interaction with bio-molecules: There are concerns that carbon nanotubes may interact with proteins and DNA within the body, which could lead to undesirable effects on health.
REFERENCES
- Iijima S., Helical microtubules of graphitic carbon, Nature. (1991) 354, no. 6348, 56–58, 2-s2.0-0342819025.
- Rajwant, K. Pooja, V. Mandeep, K. (2018). Carbon Nanotubes: A Review Article. Indian Journal of Research in Applied Sciences Engineering,6(5), pp. 5075-5079.
- Beatriz, R.C.M. Karla, F. R. et.al. (2019). Recent advances on the use of carbon nanotubes as smart biomaterials. Journal of Materials Chemistry B. 1(2), pp. 1-20.
- Valentin, N. P. (2004). Carbon nanotubes: properties and application. Materials Science and Engineering R.43 (2), pp. 61–102.
- Iijima, S. Ichihashi, T. (1993). Single-shell carbon nanotubes of 1-nm diameter. Nature. 1(4), pp. 363- 603. http://dx.doi.org/10.1038/363603a0.
- M. Meyyappan, L. Delzeit, A. Cassell, and D. Hash, “Carbon nanotube growth by PECVD: a review,” Plasma Sources Science and Technology, vol. 12, no. 2, pp. 205–216, 2003.
- M. S. Dresselhaus, G. Dresselhaus, and A. Jorio, “Unusual properties and structure of carbon nanotubes,” Annual Review of Materials Research, vol. 34, pp. 247–278, 2004.
- T. Lin, V. Bajpai, T. Ji, and L. Dai, “Chemistry of carbon nanotubes,” Australian Journal of Chemistry, vol. 56, no. 7, pp. 635–651, 2003.
- R. Saito, G. Dresselhaus, and M. S. Dresselhaus, Physical Properties of Carbon Nanotubes, London and Imperial College Press, 1998.
- B. G. P. Singh, C. Baburao, V. Pispati et al., “Carbon nanotubes. A novel drug delivery system,” International Journal of Research in Pharmacy and Chemistry, vol. 2, no. 2, pp. 523–532, 2012.
- W. Zhang, Z. Zhang, and Y. Zhang, “The application of carbon nanotubes in target drug delivery systems for cancer therapies,” Nanoscale Research Letters, vol. 6, pp. 555–577, 2011.
- H. Liao, B. Paratala, B. Sitharaman, and Y. Wang, “Applications of carbon nanotubes in biomedical studies,” Methods in Molecular Biology, vol. 726, pp. 223–241, 2011.
- A. M. A. Elhissi, W. Ahmed, I. U. Hassan, V. R. Dhanak, and A. D’Emanuele, “Carbon nanotubes in cancer therapy and drug delivery,” Journal of Drug Delivery, vol. 2012, Article ID 837327, 10 pages, 2012.
- Z. Chen, D. Pierre, H. He et al., “Adsorption behavior of epirubicin hydrochloride on carboxylated carbon nanotubes,” International Journal of Pharmaceutics, vol. 405, no. 1-2, pp. 153– 161, 2011.
- D. Xiao, P. Dramou, H. He et al., “Magnetic carbon nanotubes: synthesis by a simple solvothermal process and application in magnetic targeted drug delivery system,” Journal of Nanoparticle Research, vol. 14, pp. 984–995, 2012.
- S. Y. Madani, N. Naderi, O. Dissanayake, A. Tan, and A. M. Seifalian, “A new era of cancer treatment: carbon nanotubes as drug delivery tools,” International Journal of Nanomedicine, vol. 6, pp. 2963–2979, 2011.
- R. Li, R. Wu, L. Zhao, M. Wu, L. Yang, and H. Zou, “Pglycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells,” ACS Nano, vol. 4, no. 3, pp. 1399–1408, 2010
- Kostarelos K. Rational design and engineering of delivery systems for therapeutics:biomedical exercises in colloid and surface science. Adv. Colloid Interface Sci. 2003; 106:147–168.
- Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ. Nanotoxicology. Occup Environ M 2004; 61:727-728.
- Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv. Drug. Deli. Rev. 2006; 58:1460-1470
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G. Carbon nanotubes: a review of their properties in relation to their pulmonary toxicology and workplace safety. Toxicol Sci 2006; 92: 5-22.
- Im J.S. The effect of carbon nanotubes on drug delivery in an electro-sensitive transdermal drug delivery systems. Biomaterials. 2010;31:1414.
- Degim I.T. Carbon nanotubes for transdermal drug delivery. J.Microencapsulation. 2010;27:669–681.
- Strasinger C.L. Carbon nanotubes membranes for use in the transdermal treatment of nicotine addiction and opioid withdrawal symptoms. Res. Treatment. 2009;3:31–39.
- Chauhan, A. and Gulsen, D. (2009) EP1534202.
- Beg S. Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J. Pharm. Pharmacol. 2011;63:141–163.
- Delogu L.G. Functionalized multiwalled carbon nanotubes as ultrasound contrast agents. Proc. Natl. Acad. Sci. 2012;109:4116612.
- Sinnott, S.B. Andrews, R. (2001). Carbon Nanotubes: Synthesis, properties and applications. Critical Reviews in Solid State Mat. Sci. 26(4), pp.145–249.
- Pantarotto, D. Partidos, C. Hoebeke, J. Brown, F. Kramer, E. Briand, J. (2003) Immunization with peptide- functionalized carbon Nanotubes enhances virus-specific neutralizing antibody responses. Chem Biol. 10(5), pp. 961-966.
- Y. Zhang, Y. Bai, and B. Yan, “Functionalized carbon nanotubes for potential medicinal applications,” Drug Discovery Today, vol. 15, no. 11-12, pp. 428–435, 2010.
- Z. Liu, S. Tabakman, K. Welsher, and H. Dai, “Carbon nan otubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery,” Nano Research, vol. 2, no. 2, pp. 85– 120, 2009.
- W. Yang, P. Thordarson, J. J. Gooding, S. P. Ringer, and F. Braet, “Carbon nanotubes for biological and biomedical applications,” Nanotechnology, vol. 18, Article ID 412001, 12 pages, 2007.
- M. S. Digge, R. S. Moon, and S. G. Gattani, “Applications of carbon nanotubes in drug delivery: a review,” International Journal of PharmTech Research, vol. 4, no. 2, pp. 839–847, 2012.
- ] S. Yang, J. Luo, Q. Zhou, and H. Wang, “Pharmacokinetics, metabolism and toxicity of carbon nanotubes for bio-medical purposes,” Theranostics, vol. 2, no. 3, pp. 271–282, 2012.
- H. Wang, J. Wang, X. Deng et al., “Biodistribution of carbon single-wall carbon nanotubes in mice,” Journal of Nanoscience and Nanotechnology, vol. 4, no. 8, pp. 1019–1024, 2004.
- R. Singh, D. Pantarotto, L. Lacerda et al., “Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers,” Proceedings of the National Academy of Sciences of the United States of America, vol. 103, no. 9, pp. 3357– 3362, 2006.
- R. Hirlekar, M. Yamagar, H. Garse, M. Vij, and V. Kadam, “Carbon nanotubes and its applications: a review,” Asian Journal of Pharmaceutical and Clinical Research, vol. 2, no. 4, pp. 17–27, 2009.
- V. E. Kagan, N. V. Konduru, A. A. Shvedova et al., “Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation,” Nature Nanotechnology, vol. 5, no. 5, pp. 354–359, 2010.
- ] B. G. P. Singh, C. Baburao, V. Pispati et al., “Carbon nanotubes. A novel drug delivery system,” International Journal of Research in Pharmacy and Chemistry, vol. 2, no. 2, pp. 523–532, 2012.
- https://link.springer.com/referenceworkentry/10.1007/978-3-319-70614-6_39-1.
- https://link.springer.com/article/10.1007/s42823-022-00364-4


 Hezam Saleh Mohammed Dhaifallah *
Hezam Saleh Mohammed Dhaifallah *


 10.5281/zenodo.14450464
10.5281/zenodo.14450464