Abstract
Solid lipid nanoparticles (SLNs) have shown promise as drug delivery carriers due to their unique features, which include increased stability, bioavailability, and controlled release. These sub-micron colloidal carriers, ranging in size from 50 to 1000 nm, are made up of physiological lipid and are suspended in water or in aqueous surfactant solution. Furthermore, the impact of SLN characteristics on bioavailability and pharmacokinetics is discussed, with an emphasis on the potential of sustained and targeted medication delivery. This includes characteristics of SLN stability, particularly drug incorporation models and SLN release patterns. The study covers recent developments in encapsulation techniques, combining a variety of therapeutic agents like small molecules, proteins, and nucleic acids, in addition to reviewing the present status of SLN research. It focuses especially on the use of SLNs in the treatment of particular illnesses such cancer, neurological conditions, and infectious diseases.
Keywords
Solid lipid nanoparticle, Drug incorporation, Homogenization, Colloidal drug carriers, Drug Delivery
Introduction
Lipid-based nanotechnology can be used to encapsulate and distribute active substances in a variety of models, such as solid lipid nanoparticles (SLNs), nanoliposomes, nanosuspension, nanoemulsions and nanostructured lipid carriers (NLCs) [1-5]. Introduced in 1991, solid lipid nanoparticles (SLN) offer an alternative to conventional colloidal carriers such polymeric micro- and nanoparticles, liposomes, and emulsions [6-7]. SLNs are colloidal carrier systems made up of a liquid surfactant coating over a high-melting-point lipid core. A surfactant concentration of roughly 0.5-5?n improve the dispersion stability of SLNs [8,9]. As an alternative particulate carrier system, solid lipid-based nanoparticles have gained significant interest as innovative colloidal drug carriers for intravenous applications. SLN, or sub-micron colloidal carriers, are made up of physiological lipid that has been dispersed in water or an aqueous surfactant solution [10]. Their sizes range from 50 to 1000 nm. Small size, vast surface area, high drug loading, and phase interaction at the interface are some of the distinctive qualities of SLN, which make them appealing due to their potential to enhance pharmaceutical performance. [11] The liquid lipid was substituted with a solid lipid, which was then converted into solid lipid nanoparticles, in order to solve the problems related to the liquid condition of oil droplets.
- Lipids lessen plasma profile fluctuation and improve oral absorption.
- More accurate lipoid excipient characterisation.
- A stronger capacity to handle the two main challenges of manufacturing scale-up and knowledge transfer
Solid lipid nanoparticles represent a novel approach for colloidal carrier systems, serving as a substitute for polymers in parenteral nutrition. Fig. 1 illustrates this substitution, where the liquid lipid in the emulsion is swapped out for a solid lipid. Solid lipid nanoparticles provide several benefits, including minimal toxicity, excellent biocompatibility, improved delivery of lipophilic medicines, and physical stability of the system. [12]
ADVANTAGES: -
- Enhanced Bioavailability: By enhancing the solubility and dissolution rates of poorly water-soluble drugs, SLN can improve their bioavailability.
- Stability of Encapsulated Drugs: Solid lipid provide protective matrix for encapsulated drugs, protecting them from environmental factor. This enhances the stability of the drugs during storage and transportation.
- Controlled release kinetics: SLN enable controlled and sustained release of drug and prolonged therapeutic effect.
- Excellent biocompatibility.
- No special solvent required.
- Versatility in formulation
- Reduced side effects
DISADVANTAGES: -
- Limited loading efficiency for hydrophilic compounds.
- The discharge of drugs following a polymeric transformation in storage.
- Polydispersity.
AIM OF SOLID LIPID NANOPARTICLE: -
- Feasibility of drug targeting and controlled release.
- Higher drug payload increased drug stability.
- Incorporating lipophilic and hydrophilic drugs.
- Avoiding of organic solvent.
- No carrier biotoxicity.
- Sterilization and large-scale manufacturing are not difficult tasks.
- Enhanced bioavailability of bioactive substances that are encapsulated.
METHOD OF PREPARATION OF SOLID LIPID NANOPARTICLE
1. High Pressure Homogenization
A. Hot homogenization
B. Cold homogenization
2. Ultrasonication high speed homogenization
A. Probe ultrasonication
B. Bath ultrasonication
3. Solvent evaporation method
4. Solvent emulsification-diffusion method
5. Supercritical fluid method
6. Microemulsion based method
7. Double emulsion method
8 Precipitation technique
9. Film-ultrasound dispersion
10. Solvent Injection Technique
11. Using Membrane contractor
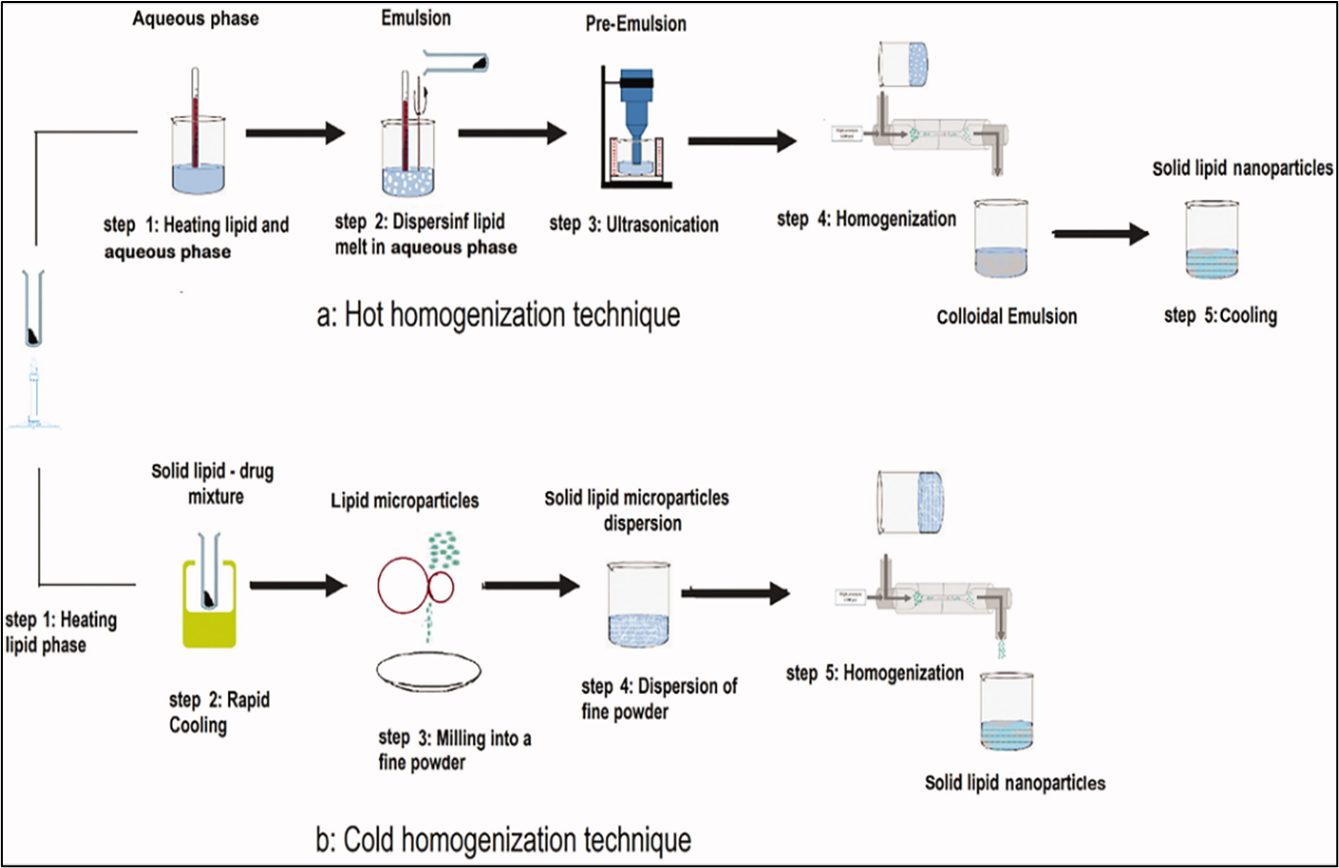
1. High Pressure Homogenization
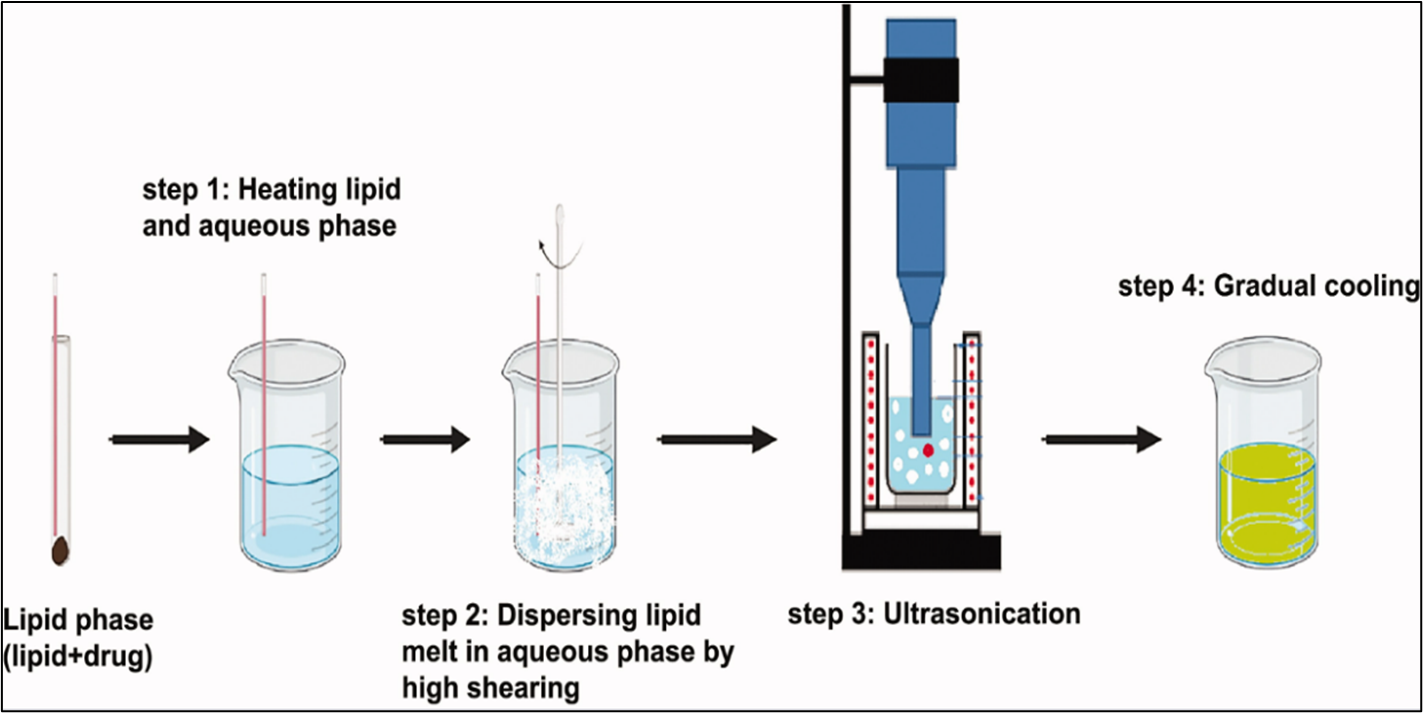
a. Hot homogenization
This process involves heating the lipid phase to 90oc and combining it with an aqueous phase that contains surfactants at the same temperature then the hot lipid is dispersed in aqueous phase containing surfactants with same temperature. After that, the pre-emulsion is homogenized three times at 5×107 Pa at 90oc using a high-pressure homogenizer. The solid lipid nanoparticles then solidify when the resultant oil-in-water emulsion is cooled to room temperature. [10,13]
b. Cold homogenization
In the cold homogenization method, the lipid phase is maintained at a lower temperature, typically room temperature or below, and then dispersed into an aqueous phase containing surfactants at the same cooler temperature. The pre-emulsion is homogenized under these cold conditions using appropriate equipment. Finally, the resulting emulsion is allowed to solidify into solid lipid nanoparticles (SLNs) at or near room temperature. [14]
2. Ultrasonication / high speed homogenization
Solid lipid nanoparticles (SLNs can be produced using ultrasonication or high-speed homogenization methods. Achieving a smaller particle size often necessitates a combination of both ultrasonication and high-speed homogenization, aiming to minimize shear stress. However, this approach comes with drawbacks, such as the risk of metal contamination and physical instability, such as particle growth during storage. To address these concerns, the use of a probe sonicate or bath sonicate is employed. [15]
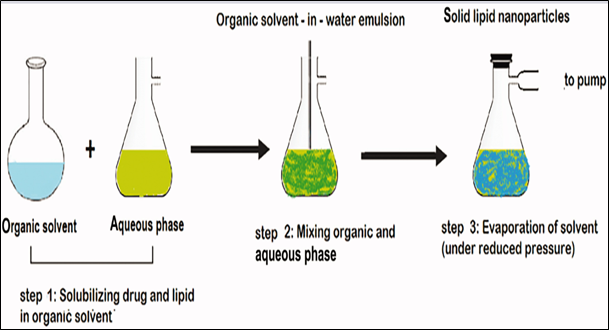
3.Solvent evaporation method
In this approach, the lipid phase is dissolved in an organic solvent, such as acetone (constituting the organic phase). Subsequently, the organic phase is introduced into the aqueous phase, which comprises a surfactant solution in water, with continuous stirring at a temperature range of 70-80 °C. The stirring process persists until the complete evaporation of the organic phase. The resulting nano emulsion is then cooled to a temperature below 5 °C, facilitating the solidification of lipid nanoparticles. [13,14]
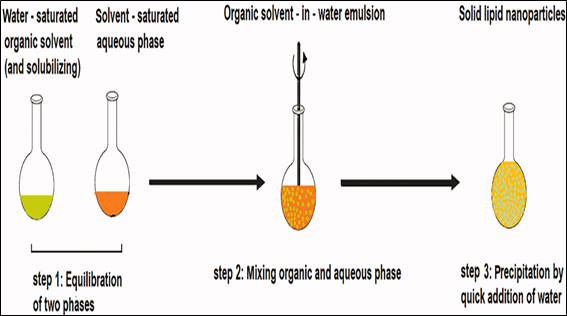
- Solvent emulsification-diffusion method
This technique enables the generation of particles with average diameters ranging from 30 to 100 nm. The key advantage lies in effective heat dissipation during the preparation process. Typically, the lipid is dissolved in the organic phase within a water bath at 50 °C. An acidic aqueous phase is employed to adjust the zeta potential, inducing coacervation of solid lipid nanoparticles (SLNs), facilitating their easy separation through centrifugation. The resulting SLN suspension is rapidly produced, and the entire dispersed system can be centrifuged and subsequently re-suspended in distilled water. [15,16]
- Supercritical fluid method
This technique for producing SLNs is relatively new and offers the advantage of solvent-free processing. There are multiple iterations of this platform technology available for the preparation of powders and nanoparticles. SLNs can be synthesized using the rapid expansion of supercritical carbon dioxide solutions (RESS) method, with carbon dioxide (99.99%) being a favourable solvent choice for this approach [17,18].
- Microemulsion based method
This method relies on the dilution of microemulsions, which are two-phase systems consisting of inner and outer phases, such as oil-in-water (o/w) microemulsions. These microemulsions are formed by blending a transparent mixture at temperatures ranging from 65 to 70°C, typically containing ingredients like a low-melting fatty acid (e.g., stearic acid), an emulsifier (e.g., polysorbate 20), co-emulsifiers (e.g., butanol), and water. The hot microemulsion is then dispersed in cold water (2-3°C) while stirring. The resulting SLN dispersion can serve as a granulation fluid for incorporation into solid products (e.g., tablets, pellets) via the granulation process. However, if the particle content is low, excessive water removal may be necessary. Rapid lipid crystallization and prevention of aggregation are facilitated by high-temperature gradients. Nevertheless, due to the dilution step, achievable lipid contents are notably lower compared to formulations based on high-pressure homogenization (HPH) [19,20].
- Double emulsion method
A novel method based on solvent emulsification-evaporation has been used for the preparation of hydrophilic loaded SLN [21]. Here, the drug is encapsulated with a stabilizer to prevent drug partitioning to the external water phase during solvent evaporation in the external water phase of w/o/w double emulsion [22,23].
- Precipitation technique
The glycerides are dissolved within an organic solvent, such as chloroform, and subsequently emulsified within an aqueous phase. Upon evaporation of the organic solvent, the lipids precipitate, forming nanoparticles [17,24].
- Film-ultrasound dispersion
A lipid film was formed after the lipid and drugs were introduced after the organic solutions had decompressed, rotated, and evaporated. The emulsifier-containing aqueous solution was then added. Finally, ultrasound with the diffuser probe was employed, resulting in the formation of Solid Lipid Nanoparticles (SLN) characterized by small and uniform particle size [25,26].
- Solvent Injection Technique
Using this method, lipids are dissolved in a water-miscible solvent and then injected with a needle into a swirling aqueous solution that might also contain a surfactant. Parameters for synthesizing nanoparticles using this method encompass the type of solvent used for injection, lipid concentration, quantity of lipid solution injected, viscosity, and the diffusion rate of the lipid solvent phase into the aqueous phase [27,28].
- Using Membrane contractor
This technique involves utilizing a membrane contactor to create solid lipid nanoparticles (SLNs). Here, a lipid is pushed through the membrane's pores at a temperature surpassing its melting point, while water flows past the pores, carrying the resulting droplets of melted lipid. Subsequently, the mixture is cooled to room temperature [29,20].
Influence of ingredient composition on product quality [30]
- Influence of the lipid:
Higher melting lipid have been observed to increase the average particle size of SLN dispersions using heat homogenization. The increased viscosity of the dispersed phase explains these observations, which are consistent with the basic HPH concept. However, for various lipids, there will be differences in other crucial factors for the creation of nanoparticles. Examples include the rate at which lipids crystallize, their hydrophilicity (which affects their ability to self-emulsify) and their shape, which determines their surface area. Notable is also the fact that the majority of the lipids utilized are blends of several chemical substances. As a result, the composition may range between vendors and even between batches from the same provider. Small alterations in the lipid content, such as contaminants, could nevertheless, have a significant effect on the SLN dispersion’s quality (e.g., by altering the zeta potential, delaying crystallization processes, etc). particle size distributions become wider and larger particles, including microparticles, are typically the results of increasing the lipid content over 5-10%. This phenomenon, which has also been noted for lipid Nano emulsions, is caused by an increase in particle agglomeration as well as a decrease in homogenization efficiency.
- Influence of the emulsifier:
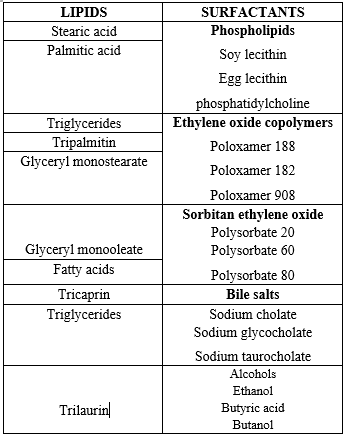
High emulsifier concentrations lower surface tension and make it easier for particles to separate during homogenization. A massive increase in surface area is associated with a decrease in particle size. During HPH, the surface area increases extremely quickly. Kinetic considerations must therefore be taken into account. The agglomeration of uncovered lipid surfaces and the process of primary covering of the new surfaces are in competition. Excess emulsifier molecules in the initial dispersion are required to quickly cover the additional surfaces. Excess emulsifier molecules can exist in a variety of forms, such as liposomes, micelles or molecular solubilized emulsifier monomers. The redistribution processes of emulsifier molecules between micelles or liposomes, water-solubilized monomers and particle surfaces occur on distinct time scales. SDS and other low molecular weight surfactants that create micelles will often quickly reach the new equilibrium. Lecithin and large molecular weight surfactants (poloxamer) will require longer redistribution times.
Sterilization and secondary production steps
Sterilization:
Sterile formulations are necessary for parenteral delivery. To attain sterility, aseptic production, filtration, ?-irradiation, and heating are typically employed. High pressure is necessary for filtration sterilizing of dispersed systems, because particles larger than 0.2 ?m cannot be filtered out. The formulation’s kinetics of drug release, chemical stability, and physical stability shouldn’t be altered by sterilization. All samples undergo free radical formation during ?-sterilization because of the high energy of the ?-rays. These radicals may recombine without changing the sample, or they may go through secondary reactions that could change the material chemically. The overall chemical reactivity determines the degree of breakdown. Moreover, the presence of oxygen and high molecular mobility (semisolid or liquid state) promote degrading events mediated by ?-sterilization. Heat sterilization is a frequently used and dependable method. It was also used in relation to liposomes. One potential issue relates to temperature-induced modification in the physical stability [31].
Lyophilization:
The maintenance of the original particle size and the avoidance of degrading events (such as hydrolysis) are two examples of the chemical and physical components of storage stability. To prevent crystal development by Ostwald ripening, it is necessary for the SLN components to have a high enough degree of chemical stability and for the particles to have a very narrow size range. The SLN formulation intended to withstand the temperature fluctuations that are inevitable during transportation. Aqueous SLN dispersion’s particle sizes have been demonstrated to remain stable over a period of 12 to 36 months. This stability, however, is not a characteristic of SLN dispersions in general, and an increase in particle size will typically be seen in a shorter amount of time. One promising strategy to improve the long-term chemical and physical stability of SLN is lyophilization. Transitioning into a solid state will avert hydrolysis reaction and Oswald ripening. Principle opportunities for incorporating SLN into pellets, pills, or capsules are also provided by lyophilization [32].
Spray drying:
spray drying could be an alternative to lyophilization for converting an aqueous SLN dispersion into a dry product. Spray drying is a less expensive option for SLN formulation than lyophilization. However, it is rarely employed. Spary drying is used to create a redispersal powder that meets the requirements for intravenous injections, including particle size and component selection. Spray drying may result in the accumulation of particles due to excessive temperatures, shear pressures, and partial melting. For spray drying, utilize lipids with melting points greater than 700c. Adding carbohydrates and minimal lipid content helps preserve colloidal particle size during spray drying. To reduce lipid melting, use ethanol-water mixes as a dispersion medium instead than pure water, which has lower inlet temperatures. The best results were obtained with 1% SLN concentrations in 30% trehalose in water or 20% trehalose in ethanol-water mixes (10/90 v/v) [33].
Model for incorporation of active compounds into SLN
There are three major models for integrating active compounds into solid lipid nanoparticles (SLN): (I) The Homogeneous Matrix Model; (II) The Drug-Enriched Shell Model; and (III) The Drug-Enriched Core Model. The formulation's composition (lipid, active ingredient, surfactant) and production conditions (hot vs. cold homogenisation) both influence the resultant structure.
- Homogenous Matrix Model:
This model is often obtained through cold homogenisation, particularly for highly lipophilic medicines. Cold homogenisation disperses the medication molecularly inside the bulk lipid. Mechanical disruption caused by high-pressure homogenisation produces nanoparticles with a homogeneous matrix structure. This structure can also result from hot homogenization if the cooling step does not cause phase separation between the lipid and the medication. For example, Prednisolone can be included into this model, resulting in a medication release profile that can last from one day to several weeks.
- Drug-Enhanced Shell Model:
This model is formed when a liquid oil droplet separates into a solid lipid nanoparticle after cooling. Initially, the lipid precipitates, resulting in a lipid core with limited drug content. As the process progresses, the concentration of the active ingredient in the residual liquid lipid rises, eventually crystallizing into a drug-rich shell. This model can be observed with substances such as coenzyme Q10, where enrichment results in fast release. This rapid release is useful for applications that require increased medication penetration through the skin by leveraging the occlusive action of SLN.
- Drug-enriched core model:
In this concept, the active component precipitates first, creating a core with a high drug concentration while the surrounding shell has less drug. This arrangement produces a membrane-controlled release profile that follows the Fick law of diffusion. The chemical properties of the active component and excipients, their interactions, and the precise manufacturing circumstances used all contribute to the structure of SLN.
Storage stability of SLN
To determine the physical parameters of SLNs during storage, measure changes in zeta potential, particle size, drug content, appearance, and viscosity over time. Long-term stability is mostly influenced by external conditions like temperature and light. To maintain physical stability, the zeta potential should be greater than -60mV.
4°C - The optimal storage temperature.
Long-term storage at 20°C did not cause aggregation or loss of medication in the SLN.
50°C - Particle size rapidly increased.
Characterization of SLNs
There are several techniques and instruments employed for the characterization of SLNs are discussed as follows
1. Visual size and distribution [34]
A. Photon corelation spectroscopy (PCS)
PCS measures the variation of scattered light by means of particle movement, depending on the diffraction angle on the particle radius, within a size range of about a few nanometres to 3 microns.
B. Dynamic light scattering
It detects fluctuations in the intensity of scattered light within microsecond time intervals. Scattering occurs due to Brownian motion and is quantified through the generation of an autocorrelation function. This coefficient enables the measurement of parameters such as size, low concentration, and viscosity.
C. Laser diffraction
Light Diffraction (LD) exhibits a notable advantage in its broad-spectrum coverage, spanning from manometers to lower millimetres. The incorporation of Polarization Intensity Differential Scattering enhances LD's sensitivity towards diminutive particles.
D. Dynamic light scattering
It records temporal differences in the intensity of scattered light within microsecond intervals. Light undergoes scattering via Brownian motion and is quantified through the generation of an autocorrelation function. This coefficient allows for the measurement of assumptions such as size, low concentration, and viscosity.
E. Static light scattering (SLS)
This process, alternatively recognized as Fraunhofer diffraction, entails the collection of scattered light patterns from a particle solution and their integration into an electromagnetic equation. It represents a rapid and approximate methodology.
2. Particle size, Electrical surface potential and pH [35]
a. Particle size
An essential factor in determining the stability of SLN is particle size. The particle size of SLNs is determined using a variety of techniques. The two key techniques are laser diffraction and photon correlation spectroscopy. Particle size is determined via PCS techniques, which measure the intensity of scattered light caused by moving particles. Particle sizes between 3 nm and 3 µm are detected. Diffraction reveals laser and particle sizes between 100 nm to 180 µm. Larger particle size microparticles are also measure by PCS.
b. Zeta potential
A zeta potential analyzer or zeta-meter can be used to analyze the measurement. The zeta potential provides information about the strength of electrostatic attraction or repulsion between the particles in the solid lipid nanoparticle (SLN) aqueous suspension.
c. PH- Sensitive probes
A pH probe is a scientific instrument utilized for quantifying the activity of H+ ions in hydrophilic solutions. By assessing pH levels, these probes offer insights into the acidic or alkaline nature of substances. The Nernst equation is employed to estimate the magnitude of electrochemical potential.
3. Shape and surface morphology [36]
a. Transmission electron microscopy
A high-energy electron beam is directed across the analyte (with a very fine granularity), facilitating interactions between these electrons and the atoms. These interactions can be utilized to discern features such as crystal structure, dislocations, and grain boundaries within the structure.
b. Scanning electron microscopy
It generates sample imagery through surface scanning using a concentrated electron beam. These electrons engage with specimens, generating diverse signals encoding surface topography and sample composition information.
c. Atomic force microscopy
This entails colloidal or resistive impedance across the specimen. The motion between the tip and surface is monitored through the topographical mapping derived from the exerted forces. This methodology facilitates the acquisition of ultrahigh resolution through sample mapping.
d. Optical microscopy
Optical microscopy, also known as light microscopy, is a modality of microscopy that employs visible light along with a system of magnifying lenses to visualize small objects. Image acquisition in this microscopy is facilitated through conventional, photosensitive cameras, yielding micrographs.
- In vitro and ex vivo method for the assessment of drug release from SLN [37]
Various methods used to Study the in vitro release of the drug are:
- Diffusion cells with biological membrane side by side.
- Diffusion technique for dialysis bags
- Reverse dialysis bag technology
- Agitation and ultracentrifugation in succession
In Vitro drug release [38]
Dialysis tubing
Dialysis tubing could be used to achieve in vitro drugs release. Closed sealed dialysis tubing that has been pre-washed is filled with the solid lipid nanoparticle dispersion. After that, the dialysis sac is dialyzed at room temperature against an appropriate dissolving medium. The samples are taken out of dissolution medium at appropriate intervals, centrifuged, and then their drug content is determined using an appropriate analytical technique.
Reverse dialysis
SLN dispersion is used in this approach to hold several tiny dialysis sacs, each holding one millilitre of dissolving medium. Next, the SLNs are moved into the medium.
Ex vivo model for determining permeability across the gut
Ahlin et al, demonstrated the passage of SLN’s across rat jejunum [11]. To put it briefly, once the study animal was sacrificed, the rat jejunum (20-30 cm distal from the pyloric sphincter) was removed from the rats. In order to conduct their permeability investigations, Qing Zhi Lu et al. removed segments of the duodenum measuring 10 cm in length, 1 cm distal to the pyloric sphincter, 15 cm to the pyloric sphincter, 20 cm proximal to the cecum, and 2 cm distal to the cecum. These segments were promptly cannulated and ligated on both sides [39].
Administration Pathways and in vivo outcomes
- Peroral administration
SLN can be administered orally through aqueous dispersions or standard dosage forms as tablets, pellets, and capsules. The stomach acidity and strong ionic strength create an ideal environment for particle aggregation. Although food is expected to have a significant impact on SLN performance, there is currently no published experimental data on the subject [40]. The impact of stomach and pancreatic lipases on SLN breakdown in vivo remains unclear. SLN can be administered orally through watery scattering or in traditional measuring shapes such as pills, pellets, containers, or powders in sachets. Fluid SLN scattering can be used to generate tablets instead of granulation fluids. Fluid scatterings are a type of SLN planning taught through an oral course. SLNs come in many dosage forms, including pills, pellets, and cases. The stomach’s microenvironment, with its high ionic quality and causticity, promotes partial aggregation. Nutrition can significantly impact SLN performance, which is not uncommon [41].
- Parenteral administration
SLN has been delivered intravenously to animals. In rats, including doxorubicin in to SLN resulted in higher blood levels compared to a commercial medication solution following intravenous injection. The SLN resulted in increased drug concentrations in the lungs, spleen, and brain, while the solution mostly affected the liver and kidneys. SLNs are typically administered intravenously to creatures. Conveyance of SLN resulted in higher drug fixations in the lung, spleen, and cerebrum as well as increased dissemination to the liver and kidney. After intravenous administration, SLN increased blood levels compared to a business sedate setup. Parenteral organization requires sterile SLN scatterings. In these instances, sterile filtering is unrealistic during to the average particle size [42].
Absorption of blood protein onto particle surfaces is thought to facilitate the brain’s uptake of SLN by mediating its adhesion [43].
- Transdermal application
SLN dispersion with low lipid content (up to 5%) exhibit the lowest particle size. The low concentration and viscosity of the dispersed lipid make it difficult to administer to the skin. To distribute SLN dispersion to the skin, it is typically essential to incorporate it into an ointment or gel formulation. The integration process reduces the lipid content even further. Increasing the solid lipid concentration in SLN dispersion creates semisolid, gel like solutions suitable for direct skin application. Unfortunately, higher lipid content typically leads larger particles sizes. Surprisingly, high concentration (30-40%) semisolid cetyl palmitate formulations maintain colloidal particle size. Increasing the lipid content significantly improves elastic characteristics [44]. The smallest particle sizes are monitored for SLN scatterings with low lipid content (up to 5%). Dermal organization has drawbacks, such as low lipid grouping and thickness. Joining the SLN scattering in a treatment or gel is crucial for creating a skin-specific treatment strategy [45].
- Pulmonary administration
This application has the potential to become an aspirational organization for SLN. SLN powders cannot be delivered to the lungs due to their small particle size, which causes them to be expelled during breathing. Aerosolization of fluid SLN scatterings is a basic process [46]. It is important to note that the SLN should not be completely consumed during aerosolization. Vaporized beads were collected by impacting them against a measuring glass. This demonstrates that SLN are suitable for lung conveyance. Medication can be released from lipid particle in the bronchial tube and alveoli under regulated conditions [47].
- Rectal Organization
Rectal drug administration is simple to employ, particularly when a quick pharmacological effect is required, it is commonly utilized for paediatric patients parenteral or rectal organization may be preferred. Rectal medicines demonstrated greater efficacy and plasma levels when compared to oral or intramuscular delivery in comparable measurements. There are limited reports available on rectal medication organization using SLN in writing. Diazepam was concentrated into SLN for rectal organization in order to achieve fast activity. The study found that rectal distribution of diazepam is ineffective when using the lipid network, which solidifies at body temperature. They made the decision to conduct their upcoming studies using lipids that breakdown at body temperature. When administered rectal, PEG coating is a dependable technique for increasing bioavailability [48].
CONCLUSION
In the early 20th century, Paul Ehrlich proposed the “magic bullet” idea, which is whereby drugs would exactly target the right spot in the body at the right timing and concentration, with no side effects en route to the therapeutic target or during clearance. Solid lipid nanoparticles (SLNs) have the ability to accomplish at least some of these lofty aims. Aside from this, SLNs effectively achieve the typical goal of regulated drug delivery. As relatively novel drug delivery technologies that have received significant attention since the early 1990s, SLNs show great promise for systematic research and development. It is expected that many patented dosage formulations containing SLNs will develop in the future
REFERENCES
- Fathi, M.; Mozafari, M. R.; Mohebbi, M. Nanoencapsulation of Food Ingredients Using Lipid Based Delivery Systems. Trends in Food Science & Technology 2012, 23 (1), 13–27.
- Hatefi, L.; Farhadian, N. A Safe and Efficient Method for Encapsulation of Ferrous Sulfate in Solid Lipid Nanoparticle for Non-Oxidation and Sustained Iron Delivery. Colloid and Interface Science Communications 2020, 34, 100227.
- López, K. L.; Ravasio, A.; González-Aramundiz, J. V.; Zacconi, F. C. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) Prepared by Microwave and Ultrasound-Assisted Synthesis: Promising Green Strategies for the Nanoworld. Pharmaceutics 2023, 15 (5), 1333.
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives. Applied Sciences 2023, 13 (3), 1726.
- Mo, K.; Kim, A.; Choe, S.; Shin, M.; Yoon, H. Overview of Solid Lipid Nanoparticles in Breast Cancer Therapy. Pharmaceutics 2023, 15 (8), 2065.
- Xuong Tuyet Nguyen; Nguyen Ngoc Vung; Tran Thu Huong; Le Hong Ky. Some Associated Factors in Hearing Loss among Children Aged 25 in Kindergartensin Hai Duong Province, Vietnam. Systematic Reviews in Pharmacy 2019, 10 (2), 22–26.
- Mishra, V.; Bansal, K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10 (4), 191.
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Advanced Pharmaceutical Bulletin 2015, 5 (3), 305–313.
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9 (10), 998.
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12 (3), 633.
- Ekambaram, P.; Abdul, A.; Sathali, H.; Priyanka, K. SOLID LIPID NANOPARTICLES: A REVIEW. Sci. Revs. Chem. Commun 2012, 2 (1), 80–102.
- Garud, A.; Singh, D.; Garud, N. Solid Lipid Nanoparticles (SLN): Method, Characterization and Applications. International Current Pharmaceutical Journal 2012, 1 (11), 384–393.
- Mohammadi-Samani, S.; Ghasemiyeh, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Research in Pharmaceutical Sciences 2018, 13 (4), 288.
- Pooja, D.; Tunki, L.; Kulhari, H.; Reddy, B. B.; Sistla, R. Optimization of Solid Lipid Nanoparticles Prepared by a Single Emulsification-Solvent Evaporation Method. Data in Brief 2016, 6, 15–19.
- Scioli Montoto, S.; Muraca, G.; Ruiz, M. E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Frontiers in Molecular Biosciences 2020, 7.
- Trotta, M.; Debernardi, F.; Caputo, O. Preparation of Solid Lipid Nanoparticles by a Solvent Emulsification–Diffusion Technique. International Journal of Pharmaceutics 2003, 257 (1-2), 153–160.
- Trucillo, P.; Campardelli, R. Production of Solid Lipid Nanoparticles with a Supercritical Fluid Assisted Process. The Journal of Supercritical Fluids 2019, 143, 16–23.
- Santo, I. E.; Pedro, A. S.; Fialho, R.; Cabral-Albuquerque, E. Characteristics of Lipid Micro- and Nanoparticles Based on Supercritical Formation for Potential Pharmaceutical Application. Nanoscale Research Letters 2013, 8 (1).
- Maria Rosa Gasco; Priano, L.; Gian Paolo Zara. Solid Lipid Nanoparticles and Microemulsions for Drug Delivery. Progress in brain research 2009, 181–192.
- Khairnar, S. V.; Pagare, P.; Thakre, A.; Nambiar, A. R.; Junnuthula, V.; Abraham, M. C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14 (9), 1886.
- Subroto, E.; Andoyo, R.; Indiarto, R.; Wulandari, E.; Wadhiah, E. F. N. Preparation of Solid Lipid Nanoparticle-Ferrous Sulfate by Double Emulsion Method Based on Fat Rich in Monolaurin and Stearic Acid. Nanomaterials 2022, 12 (17), 3054.
- Wang, J.; Wang, H.; Xu, H.; Li, J.; Zhang, X.; Zhang, X. Solid Lipid Nanoparticles as an Effective Sodium Aescinate Delivery System: Formulation and Anti-Inflammatory Activity. RSC advances 2022, 12 (11), 6583–6591.
- Shi, L.; Li, Z.; Yu, L.; Jia, H.; Zheng, L. Effects of Surfactants and Lipids on the Preparation of Solid Lipid Nanoparticles Using Double Emulsion Method. Journal of Dispersion Science and Technology 2011, 32 (2), 254–259.
- Riewe, J.; Erfle, P.; Melzig, S.; Kwade, A.; Dietzel, A.; Bunjes, H. Antisolvent Precipitation of Lipid Nanoparticles in Microfluidic Systems – a Comparative Study. International Journal of Pharmaceutics 2020, 579, 119167.
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S. P. C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7 (6). https://doi.org/10.3390/nano7060122.
- Viegas, C.; Seck, F.; Fonte, P. An Insight on Lipid Nanoparticles for Therapeutic Proteins Delivery. Journal of Drug Delivery Science and Technology 2022, 77, 103839.
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25 (20), 4781.
- Kommavarapu, P.; Maruthapillai, A.; Palanisamy, K. Preparation, Characterization and Evaluation of Elvitegravir-Loaded Solid Lipid Nanoparticles for Enhanced Solubility and Dissolution Rate. Tropical Journal of Pharmaceutical Research 2015, 14 (9), 1549–1556.
- Charcosset, C.; El-Harati, A.; Fessi, H. Preparation of Solid Lipid Nanoparticles Using a Membrane Contactor. Journal of Controlled Release 2005, 108 (1), 112–120.
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles. Advanced Drug Delivery Reviews 2012, 64, 83–101.
- N.J. Zuidam, S.S.L. Lee, D.J.A. Crommelin, Sterilization of liposomes by heat treatment, Pharm. Res. 10 (1992) 1591–1596
- H. Rupprecht, Physikalisch-chemische Grundlagen der Gefriertrocknung, in: D. Essig, R. Oschmann (Eds.), Lyophilisation. Paperback APV, Band 35, Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, 1993, pp. 13–38
- C. Freitas, R.H. Müller, Spray-drying of solid lipid nanoparticles (SLN), Eur. J. Pharm. Biopharm. 46 (1998) 145–151.
- Joshi S.A, R. K. H. Solid Lipid Nanoparticle: A Review. IOSR Journal of Pharmacy (IOSRPHR) 2012, 2 (6), 34–44.
- Sastri, K.; Radha, G.; Pidikit, S.; Vajjhala, P. Solid Lipid Nanoparticles: Preparation Techniques, Their Characterization, and an Update on Recent Studies. Journal of Applied Pharmaceutical Science 2020, 10 (6), 126–141.
- Solid Lipid Nanoparticles: Preparation Techniques, Their Characterization, and an Update on Recent Studies. Journal of Applied Pharmaceutical Science 2020, 10 (6), 126–141.
- Lingayat, V. J.; Zarekar, N. S. Review. Nanoscience and Nanotechnology Research 2017, 4 (2), 67–72.
- Alessandro Bargoni, Roberto Cavalla, Otto Caputo and M. R Gasco, Pharm. Res., 15(5), 745-750 (1998).
- Qing Zhi Lu, Aihua Yu, Yanwei Xi and Houli Li, Zhimei Song, Jing Cui and Fengliang Cao, Guangxi Zhai, Int. J. Pharm., 372, 191 – 198 (2009).
- Pinto, J. and Muèller, R, “Pellets As Carriers 0f Solid Lipid Nanoparticles for Oral Administration 0f Drugs”, Die Pharmazie, 506-509. 1999.
- Loxley, A, “Solid Lipid Nanoparticles for The Delivery of Pharmaceutical Actives”, Drug Delivery Technology, 9 (8). 2009
- Zara, G. P.; Cavalli, R.; Fundarò, A.; Bargoni, A.; Caputo, O.; Gasco, M. R. Pharmacokinetics Of Doxorubicin Incorporated In Solid Lipid Nanospheres (Sln). Pharmacological Research 1999, 40 (3), 281–286.
- R.N. Alyautdin, V.E. Petrov, K. Langer, A. Berthold, D.A. Kharkevich, J. Kreuter, Delivery of loperamide across the blood–brain barrier with Polysorbate 80-coated polybutylcyanoacrylate nanoparticles, Pharm. Res. 14 (1997) 325–328
- A. Lippacher, R.H. Müller, K. Mäder, Investigation on the viscoelastic properties of lipid based colloidal drug carriers, Int. J. Pharm. 196 (2000) 227–230.
- Bhaskar, K., Anbu, J. Ravichandiran, V. and Venkateswarlu, Y, “Lipid Nanoparticles For Transdermal Delivery of Flurbiprofen Formulation, In Vitro, Ex Vivo and In Vivo Studies”, Lipids In Health and Disease, 8(6). 2009.
- Yadav, N., Khatak, S. And Singh, S., “Solid Lipid Nanoparticles A Review”, International Journal of Applied Pharmaceutics, 5(2). 8-18. 2013.
- Ekambaram, P., Sathali, A. and Priyanka, K, “Solid Lipid Nanoparticles: A Review”, Scientific Reviews and Chemical. Communication, 2(1). 80-102. 2012.
- Sanap, G. And Mohanta, G, “A Review Solid Lipid Nanoparticle A Potential Drug Delivery Carrier”, International Journal of Chemical and Pharmaceutical Analysis, (2). 52-62.2014.


 Nilesh Wakshe *
Nilesh Wakshe *
 Ashish Jain
Ashish Jain





 10.5281/zenodo.13330039
10.5281/zenodo.13330039