Abstract
This study developed and validated a straightforward, accurate, and sensitive head space-gas chromatography method for measuring the content of isopropyl alcohol in mesalamine prolonged-release tablets. The method uses Agilent Head space-gas chromatography, which has a flame ionization detector, capillary mid-polar stationary phase [DB-624 6% cyanopropyl, 94% polydimethylsiloxane (30 m*0.53 mm, 3µm)] with nitrogen as the carrier gas, and a linear velocity flow rate of 27 ml/min with a 1:5 spilt ratio and an injector temperature of 150 °C. The experiment ran for a total of twelve minutes, and the isopropyl alcohol retention time (RT) was 4.010 min. The developed method was validated according to the current International Conference on Harmonization (ICH) Q2R1 validation guidelines and Q3C standards for residual solvents. This method shows that it is specific, linear, precise, sensitive, rugged, reproducible, and effectively applied for the quantification of isopropyl alcohol in mesalamine prolonged-release tablets and quality control practices.
Keywords
Mesalamine; Isopropyl alcohol; Quantification; Gas chromatography-Head space; Method development and validation
Introduction
Mesalamine is a synthetic derivative of salicylic acid, it is chemically known as 5-Amino-2-Hydroxy benzoic acid and mesalazine with molecular formula C7H7NO3 and molecular weight 153.15g/mol its structure is represented in Fig 1. A. This is the first-line medication to treat ulcerative colitis. Usually used to induce or maintain remission of mildly to moderately active ulcerative colitis. Absorption of mesalamine is similar in fasted and fed subjects. The absorbed mesalamine is rapidly acetylated in the gut mucosal wall in the liver and excreted by the kidney as N-Acetyl-5-amino salicylic acid [1-3] Residual Solvents are the organic volatile chemicals that are used or produced in the manufacture of drug substances or excipients in the preparation of drug products. These solvents are not completely removed by practical manufacturing techniques (usually under increased temperature or decreased temperature) in use, even after such process some solvents still remain in small quantities. These quantities of organic solvents are commonly known as "Organic Volatile Impurities (OVIs) or Residual Solvents (RS). These solvents have no therapeutic value and are toxic to the human body if intake goes beyond the limit. Quantification of residual solvents is an integral part of quality control in pharmaceuticals. Different analytical techniques are available for the estimation of residual solvents in pharmaceuticals and other products including, Loss on drying (LOD), Thermal gravimetric analysis (TGA), Different scanning colorimetry (DSC), Different thermal analysis (DTA), Thermal desorption (TD), Chemical sensors and some spectrometric and spectroscopic procedures. However, these methods have low sensitivity, but gas chromatography-based test procedures are the most popular and are chemically specific for residual solvents [4-6]. According to ICH guidelines residual solvents have been classified into four types based on their toxicity profile. Their acceptable limits are listed in Table 1[7].
Table 1: Limit of residual solvents and toxicity criteria as per ICH (ICH Q3C)

(a):Solvents to be avoided. (b):Solvents to be limited. (c):Solvents with low toxic potential.
Isopropyl alcohol (Propan-2-ol):
Isopropyl alcohol (Fig.1.B) is categorized as a Class –3, Solvents with low toxic potential. It is a colorless, flammable organic solvent with a pungent alcoholic odor. A molecular formula C3H8O, Boling point is 82.3? From the literature, several analytical techniques are accessible in mesalamine quantification like UV spectroscopic methods [8], RP-HPLC [9], Stability indicating RP-HPLC [10], HPLC-ESI-MS/MS method [11], RP-UPLC [12], RP-UHPLC [13]. Therefore, in this research, we strive to develop a simple, sensitiveand rapid method for quantification of isopropyl alcohol content in mesalamine prolonged-release tablets using a gas chromatographic method. Head Space-Gas chromatography was selected for initial separations from the knowledge of the properties of the compound.
Fig.1: Structure of Mesalamine (A) and Isopropyl alcohol (B)

A

B
Initial chromatographic conditions:
Column, oven temperature, Sample line temperature, pressurize gas pressure, equilibrating time, pressuring time, pressure equilibration time, injection time, needle flush time, injection mode, flow control mode, pressure, total flow, column flow, purge flow, split ratio, oven program, makeup gas, make up flow, hydrogen flow and airflow. Above mentioned parameters needed to be tuned based on the physiochemical properties of the sample. After careful optimization of the method for the quantification of "Isopropyl alcohol". The optimized method is to be validated as per ICH guidelines.
MATERIALS AND METHODS
Reagents and chemicals used:
Solvents used were of ? 99.8% purity N, N Dimethyl formamide and Isopropyl alcohol were purchased from Merck Life Sciences Private Limited; PADM Laboratories provided Mesalamine prolonged-release tablets.
Instruments and apparatus:
Chromatographic elution was carried out in Gas chromatography (Agilent technologies 6890N Series) with a Flame ionization detector (FID) and Headspace sampler (Agilent technologies G18888 Series), Separation was achieved using a DB-624 6% cyanopropyl, 94% polydimethylsiloxane Stationary phase (30 m*0.53 mm, 3µm). Mettler Toledo Analytical balance, Microbalance, and Vortex Genie 2 were used for sample preparation.
Chromatographic conditions:

Solutions preparation:
Preparation of Blank solution:
Transferred 5 ml of N, N Dimethylformamide into a 20ml GC Headspace Vial and immediately sealed the Vial.
Diluent:
N, N Dimethyl formamide
Preparation of Standard stock solution:
Weighed accurately about 510.0 mg of Isopropyl alcohol WS into a 50ml volumetric flask containing 25 ml of diluent and shake well then diluted up to the mark with diluent.
Preparation of Standard solution:
Taken 1 ml of the standard stock solution into a 50 ml volumetric flask containing 25 ml of diluent and shake well then diluted up to the mark with diluent, and transferred 1 ml of the above solution into 20 ml GC Headspace vial and immediately sealed using a rubber septa and metallic ring closure.
Preparation of Sample solution:
Weighed accurately and transferred 200 mg of sample into 20 ml of GC Headspace vial and added 5 ml of diluent immediately sealed the vial, vortex for 5 mins.
METHOD DEVELOPMENT:
Method development was carried out through a series of trials by changing various stationary phases and diluents based on their physiochemical properties of analyte that was optimized by final trial by using a DB-624 mid-polar stationary phase with composition of 6% cyanopropyl, 94% polydimethylsiloxane (30mt ×0.53mm, 3µm) and N, N Dimethyl formamide used as a diluent. As stated in the limits of ICH, all the parameters were optimized by injecting blank, standard, and sample solutions into the optimized chromatographic conditions. (Refer supporting info) The retention of diluent (N, N Dimethylformamide) was 8.82mins as shown in Figure 2, the retention time of standard was observed as 4.02 as shown in Figure 2 and the Retention time of sample solution was observed at 3.99 as shown in Figure 2.

Fig.2: Representative Chromatograms for blank (A), Placebo (B), Standard Solution (C) and Sample Solution (D).
Method validation:
Specificity
Specificity of the method The ability of the method to measure the analyte in the presence of compounds such as process impurities, degradation impurities, and matrix components, by comparing the chromatograms of blank, placebo, standard, sample, and spiked sample solution. Fig.2 shows the typical chromatograms of blank, placebo, standard, and spiked sample solutions. Under the optimized chromatographic conditions the typical retention time of isopropyl alcohol was 3.98. There is no significant interference was observed due to blank, placebo, and other unknown peaks at the retention time of isopropyl alcohol, the resolution between isopropyl alcohol and closet impurity peak in specificity solution is 14.26. Based on the above results, the method is specific.
System suitability
Injected the blank and standard solution (six replicates) recorded the plate count of isopropyl alcohol from the standard injection and calculated the % RSD for the peak response of isopropyl alcohol from the six replicate standard injections. The theoretical plate number for the isopropyl alcohol peak in the standard solution was found 17580 and the % RSD for the peak areas of isopropyl alcohol calculated on six replicates of the standard solution is 1.01% (Table 2).

Table 2: System suitability of six replicated standard injections by optimized method
Precision
Precision was considered at three levels, repeatability, intermediate, and reproductively precision.
System precision
To determine the method precision six replicate injections of the standard solution will be made by the optimized method and the standard deviation and relative standard deviation (% RSD) of the six replicate injections will be calculated and reported then %RSD is not more than 15%. The % RSD of method precision was 1.01. As depicted in Table 2, hence the method was deemed suitable and precise.
Method precision
Method repeatability will be performed by injecting one unspiked and six spiked sample preparations. The % RSD for isopropyl alcohol content from the six sample preparations should be now more than 15% and the results are found to be 10.43%. The values are displayed in the table 3.
Intermediate precision
Intermediate precision is the degree of reproducibility of the test results attained same sample analysis and the method has been studied by evaluating the variations from the analyst and different stationary phases. It will be performed by one unspiked and six spiked sample preparations. Calculate the relative standard deviation peak response of six replicate injections should be not more than 15% and the values are displayed in table 4.
Table 3: Results of method precision (In ppm) *ppm-Parts per million

Table 4: Results of Intermediate precision (In ppm)

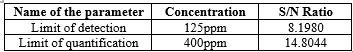
Detection Limit (DL) and Quantitation Limit (QL)
Instrumental and statistical approaches were used to determine the LOD and LOQ. The limits of detection (LOD) and quantification (LOQ) are identified by the detectors for the instrument approach. LOD and LOQ have been established by three injections of LOD levels and six injections by LOQ level. The concentration and signal-to-noise ratio was established in Table 5.
Table 5: the values for the LOD and LOQ
Linearity and Range
The linearity of an analytical procedure is its ability (within a given range) to obtain test results that are directly (i.e., after mathematical transformation) proportional to the concentration (amount) of analyte in the sample. Demonstrated the linearity parameter of the analytical method from the range 50% to 150% of the working concentration of the method by covering three different concentrations and drown a plot between concentration Vs average area response of isopropyl alcohol linearity level solutions.
From the statistical analysis, correlation coefficient, and regression equations, linearity is known as shown in Table 6 and the linearity graph was shown in figure 3. The isopropyl alcohol correlation coefficient values were noted to be higher than 0.9996 and the calibration curve was linear within the linear range.
Table 6: Results of Linearity and Range

Fig 3: Linearity graph of Isopropyl alcohol

Accuracy (Recovery)
The accuracy of an analytical method is the closeness of the sample results obtained from the method to the truevalue. Accuracy may often be expressed as the percent of recovery by the assay of known added amounts of analyte to the matrix. Accuracy is a measure of the exactness of the analytical method. Accuracy will be performed from the range of QL to 150% of the specification level of isopropyl alcohol. Recoveries of 50%, 100%, and 150% were performed by mixing known amounts of standard drug solution, and the results are presented in Table 7. From the accuracy data, the %recovery of residual solvents was found within the limits and the %RSD for the area did not exceed 10.0 for isopropyl alcohol. The outcomes show that the strategy achieves a satisfactory degree of accuracy.
Table 7: Results of accuracy for Isopropyl alcohol

Robustness
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage. Robustness of the method was evaluated by checking the system suitability parameters by deliberately varying the chromatographic conditions such as setting Oven Temperature 86? and 94? instead of 90?, the cumulative % RSD for isopropyl alcohol met to the acceptance criteria that are below 15% and the values are depicted in table 8 then Figure 4 shows the typical chromatogram of robustness of oven temperature. Optimal column flow was 4.01ml/min is changed to 3.8ml/min and 4.2ml/min respectively the consequence of the evaluation was good and the robustness of column flow chromatograms represented in figure 5 then %RSD for area of isopropyl alcohol below 15% and the values are summarized in table 9.
Table 8: System suitability for change in initial oven temperature (?)

Figure 5: Representative chromatogram for Robustness of initial temperature (?)

Table 9: System suitability for column flow (ml/min)

Figure 6: Typical Chromatogram for Robustness of column flow

CONCLUSION
The newly developed HS-GC-FID method underwent a validation successfully according to ICH Guidelines and was demonstrated to be specific, linear, precise, accurate, and robust for the estimation of isopropyl alcohol levels in mesalamine prolonged-release tablets. Therefore, this method is suitable for the conduct of testing in regulated quality control (QC) laboratories.
ACKNOWLEDGEMENTS
I sincerely wish to thank Mr. R. Sasikumar for providing all the necessary resources and facilities in PADM Laboratories Pvt ltd without which it would not have been possible to complete this project. I would also like to give a special thanks to Principal and Professor Department of pharmaceutical analysis, Sri Vijay Vidyalaya College of pharmacy for their continuous support and understanding when undertaking my research work.
REFERENCES
- Nakashima J, Preuss CV, Mesalamine (USAN); in stat pearls publishing. www/ncbi.nlm.nih.gov/books/NBK551714/
- Dash AK and Brittain H G. Mesalamine. kaluo Florey, editor. Analytical profile of drug substances and excipients. Vol-25, New Jersey: Academic Press, 1998.P.210-242.
- C. Cheng S. Liu, B J. Mueller, Z. Yan. A generic static headspace gas chromatography method for determination of residual solvents in drug substances, J Chromatogr. A, 1217 (2010)6413-6421. DOI: 10.1016/j.chroma.2010.08.016
- Kumaraswamy D., Stephenrathinaraj B. Process validation of analytical method development and validation for omeprazole capsules and Blend. Journal of Chromatographic Analysis, 2010, Vol-66, Issue5, pp234-237
- B'Hymer C. Residual solvent testing: a review of gas-chromatographic and alternative techniques. Pharm Res. 2003 Mar; 20(3):337-44.DOI: 10.1023/a: 1022693516409. PMID: 12669951.
- ICH harmonization tripartite guidelines on impurities: guidelines for residual solvents (ICH Q3A [R5]), international conference on harmonization of technical requirements for registration of pharmaceuticals for human use, 4 February 2011.
- D.Umamaheswari, Neha Gupta.T, M. Kumar, B.S.Venkateswarlu, Int. J. Pharm. Sci. Rev. Res., 69(1), July - August 2021; Article No. 01, Pages: 1-8.DOI:10.47583/ijpsrr.2021.v69i01.001
- Patel K, Patel C, Panigrahi B, Parikh A, Patel H. Development and validation of spectrophotometric methods for the estimation of mesalamine in tablet dosage forms. J Young Pharm. 2010 Jul;2(3):284-8. PMID: 21042487; PMCID: PMC2964759.doi: 10.4103/0975-1483.66789
- Ankit Awasthi, Ankit Kumar, Rajan Kumar, Sukriti, Rubiya Khursheed, Jaskiran Kaur, Leandehcorrie, Bimlash Kumar, RP-HPLC method development for simultaneous of mesalamine and curcumin in bulk form as well as nanostructured lipid carrier, South African journal of botany, Volume 151, Part B, 2022, Pages 529-537, ISSN 0254-6299.doi.org/10.1016/j.sajb.2022.05.044.
- Nalini Kanta Sahoo, Madhu Smitha Sahu, Podilapu Srinivasa Rao, Gotam Ghosh, validation of stability indicating RP-HPLC method for the estimation of mesalamine in bulk and tablets dosage form, Pharmaceutical Methods, Volume 4, Issue 2, 2013, Pages 56-61, ISSN 2229-4708.http://dx.doi.org/10.1016/j.phme.2013.12.003
- Esisabetta Pastorini, Mercello Locatelli, Patrizia Simoni, Giulia Roda, Enrico Roda, Aldo Roda, Development and validation of an HPLC-ESI-MS/MS Method for determination of 5-Aminosalicylic acid and its major metabolite N-acetyl-5-Aminosalicylic acid in human plasma, Journal of chromatography B, Volume 872, Issue 1-2, 2008, Pages 99-106, ISSN 1570-0232. https://doi.org/10.1016/j.jchromb.2008.07.026
- Trivedi Rakshit Kanubhai, Patel Mukesh, Khankar Amit R, Determination of mesalamine related impurities from drug product by Reversed-phase validated UPLC Method, E-Journal of Chemistry, 2011,8(1), 131-148, ISSN 0973-4975,http://dx.doi.org/10.1155/2011/382137
- Jayagopal Balaji and Murgesh Shivas hanker, Development and validation of RP-UHPLC procedure for estimation of 5-amino salicylic acid in 5-amino salicylic acid rectal suppositories 2017 IopConf.Ser.: Mater.Sci.Enj 263 02 2025. DOI:10.1088/1757-899X/263/2/022025
- Sushila Dagadu Chavan , and Deepa Mahendra Desai. Analytical method validation: A brief review. DOI: http/s://doi.org/10.30574/wjarr.2022.16.2.1165
- Michael E. Swartz, Ira S. Krull. Handbook of Analytical validation, 2012 by Taylor & Francis Group, LLC, International Standard Book Number: 978-0-8247-0689-0.


 Radharavi K * 1
Radharavi K * 1
 P.D. Gokulana 1
P.D. Gokulana 1
















 /10.5281/zenodo.11354677
/10.5281/zenodo.11354677