Abstract
Herbal remedies are more effective and have fewer negative effects, they are frequently utilised to treat a wide range of illnesses. One new medicine delivery method that is being used a lot these days is the novel drug delivery system. In order to prepare niosomes, the current study used a variety of surfactants, including as Span and Tween, to create and assess a curcumin-loaded niosomal gel. Enhancing curcumin delivery by the ether injection method is the main goal. Turmeric's major ingredient, curcumin, is abundant in polyphenolic chemicals and volatile oils, including zingiberone, atlantone, and tumerone. These ingredients are good for treating acne and reducing lesions because they have antibacterial, anti-inflammatory, and antioxidant qualities. The TLC plate method was used to standardise curcumin, and ether injection was used to create niosomes with varying drug-to-cholesterol-to-surfactant ratios (1:1:2 and 1:1:3). The evaluation parameters of niosomal gel were assessed.
Keywords
Niosomes, Niosomal gel,curcumin, non ionic surfactant.
Introduction
Niosomes are minuscule particles. Recent research has demonstrated that niosomes significantly improve transdermal drug delivery and are also utilised in targeted drug administration. It is mostly created by adding cholesterol surfactants as an excipient. In addition, several excipients are employed. Multilamellar or unilamellar vesicles make up niosomes. They resemble liposomes quite a little. Depending on how they are prepared, niosomes can range in size from 300 nm to 5000 nm.2,3 Curcumin is found in natural product. It is a member of the zingiberaceaceae family and is commercially derived from the rhizomes of the curcuma longa plant. The spice has long been used to treat a wide range of human illnesses. Resins, proteins, and carbohydrates are other components. Curcumin, the active ingredient that has been studied the most, makes up 0.z3-5.4 percent of raw turmeric. The body's complicated biochemical reaction to viruses, damaged cells, or irritants is inflammation. Although anti-inflammatory medications are frequently used for treatment, prolonged usage frequently causes serious side effects, underscoring the need for safer and more efficient substitutes The research entails creating niosomes, refining their properties, and incorporating them into a hydrogel for topical application. In order to determine whether the finished product has the potential to be a unique treatment alternative, its physicochemical characteristics and anti-inflammatory activity will be evaluated.A new medicine delivery method called niosomes involves loading the drug inside a vesicle.
2.1 Structure of Niosomes:3,13A vesicle-forming amphiphile, or non-ionic surfactant, such as span 20, 40, 60, 80, or tween 20, 40, 60, 80, would typically make up a typical niosome vesicleMicroscopic lamellar structures called niosomes are created when cholesterol and non-ionic surfactants of the alkyl or dialkylpolyglycerol ether family are mixed together.
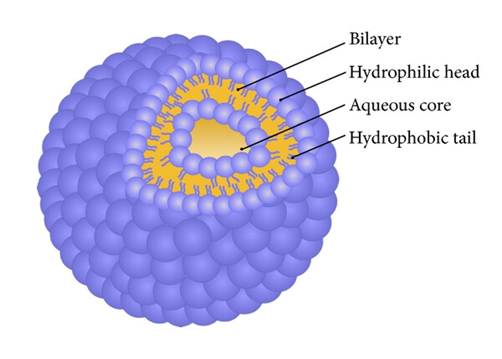
Fig no:1
2.2Compositions of Niosomes4,12
A] Cholesterol
B] Nonionic surfactants
C] Charged molecule
A] Cholesterol: It is a steroid derivative, which is used to give rigidity and proper shape, conformation to the niosomes preparations Cholesterol is a waxy, fat-like substance that's found in all the cells in your body.
B] Nonionic surfactants: The following are some non-ionic surfactants which are frequently used for the preparation of niosomes.
E.g.: 1]Spans (span 60, 40, 20, 85, 80)
2]Tweens (tween 20, 40, 60, 80)
3]Labrafil
C] Charged Molecule: Some of the charged molecules are further added to niosomes to increase stability of niosomes by electrostaticrepulsion which block the formation of coalescence
2.3 Types of Niosomes2,5
i) Multi lamellar vesicles (size=>0.05µm)
ii) Large unilamellar vesicles (size=>0.10 µm)
iii) Small unilamellar vesicles (size=0.025-0.05µm)
2.4advantages Of Niosomes4,6,17
? It is easy for storage
? They are less toxic.
? Inexpensive
?has more stability than liposomes.
2.4 Method Of Preparation Of Niosomes2,4,5
1.Sonication
2.Micro Fluidization
3 Hand Shaking Method
4.Ether Injection Method
5.Reverse Phase Evaporation Technique
6.Bubble Method
7.Emulsion Method
8.Lipid Injection Method
9.Trans Membrane Ph Gradient
3.AIM And Objectives
A] AIM
To formulate and evaluate a curcumin-loaded niosomal gel for its potential anti-inflammatory effect, enhancing the therapeutic efficacy and overcoming the bioavailability challenges of curcumin.
B] Objectives
1.Developing and assessing a topical gel formulation incorporating curcumin within niosomes for its potential anti-inflammatory activity.
2.Enhancement of bioavailability of curcumin loaded niosomal gel.
3.Analysis of efficacy of curcumin loaded niosomal gel.
4.MATERIALS AND METHODS10,15
4.1MATERIALS:
1. Active Ingredient
Curcumin:
Chemical constituents:
1.Curcumin [curcumin I]
2.Demethoxycurcumin [curcumin II]
3.Bisdemethoxycurcumin[curcumin III]
4.Cyclo Curcumin.
Role: The therapeutic agent with anti-inflammatory properties
Curcumin, a bioactive compound found in Curcuma longa (turmeric), has garnered significant attention due to its diverse pharmacological properties. Below is a detailed description of its pharmacological parameters.

Fig no: 4
4.2 Excipients
When formulating curcumin-based products, choosing compatible excipients is crucial to enhance its solubility, stability, and bioavailability. Below is a list of commonly used excipients that are compatible with curcumin for different formulations.
1. Solubilizers and Surfactants
Used to enhance curcumin’s poor water solubility:
- Polysorbates (e.g., Tween 20, Tween 80): Widely used in emulsions.
- Polyethylene Glycol (PEG): Used in various dosage forms like tablets, capsules, and injectable formulations.
- Sodium Lauryl Sulfate (SLS): Often used in oral and topical formulations.
- Pluronic F68/F127 (Poloxamers): For nanoparticle or gel formulations.
2. Lipid-Based Excipients
Enhance curcumin’s lipophilicity and absorption:
- Phospholipids (e.g., Phosphatidylcholine): Used for liposomes or lipid-based carriers.
- Medium-Chain Triglycerides (MCTs): Used in lipid-based drug delivery systems.
- Solid Lipid Excipients (e.g., Stearic Acid, Glyceryl Monostearate, Precirol ATO 5): Ideal for solid lipid nanoparticles.
- Lecithin: Enhances solubility and stability.
3. Polymers
Provide sustained release and structural integrity:
- Chitosan: A biocompatible polymer for nanoparticles.
- Eudragit: Useful in enteric coating and controlled release.
- Hydroxypropyl Methylcellulose (HPMC): Commonly used in gels and solid dispersions.
- Polyvinylpyrrolidone (PVP): Improves solubility and stability.
4. Antioxidants
Prevent degradation of curcumin due to oxidation:
- Vitamin E (Tocopherol): Often used in lipid-based systems.
- Ascorbic Acid: Acts as a stabilizer.
- Butylated Hydroxytoluene (BHT): For lipid-based formulations.
5. Stabilizers:
- Cyclodextrins (e.g., ?-Cyclodextrin, HP-?-CD): Form inclusion complexes to enhance stability and solubility.
- Silicon Dioxide: Prevents agglomeration in powdered forms.
6.Penetration Enhancers (Topical and Transdermal Formulations):
- Propylene Glycol: A commonly used humectant.
- Dimethyl Sulfoxide (DMSO): Improves skin penetration.
- Oleic Acid: Enhances transdermal absorption.
7. pH Modifiers
Ensure curcumin’s stability in formulations:
- Sodium Bicarbonate: Maintains pH for stability in aqueous solutions.
4.3 Materials for Niosome Preparation10,15
A. Non-Ionic Surfactants:
Span 60 (Sorbitan monostearate):
Role: Forms the bilayer of niosomes, ensuring vesicle stability and drug encapsulation.
B. Cholesterol
C. Hydration Medium:Phosphate-buffered saline (PBS):pH: 7.4.
Role: Used for hydrating the thin lipid film and maintaining physiological pH.
D. Organic Solvents:
Chloroform: Used for dissolving lipid components.
Methanol: A co-solvent for preparing the lipid phase.
6.2 Method Of Preparation For Niosome10,15
Ether Injection Method:
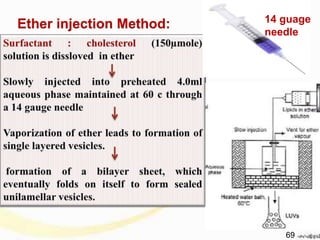
Fig no:5
4.4 Materials For Preparation For Niosomal Gel10
1. Gelling Agent:Carbopol 934
2. Neutralizing Agent:
Triethanolamine (TEA):
Role: Adjusts the pH of the gel to skin-friendly levels (5.5–7.0).
3. Humectant:
Glycerin:
4.Preservatives:Methylparaben and Propylparaben:
5.Deionized Water:: Solvent for preparing the gel.
4.5 Method For Preparation For Niosomal Gel11
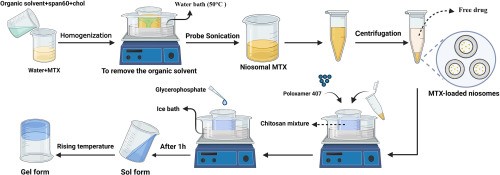
5. Evaluation Studies.
5.1 Evaluation Studies For Niosomes[17,13,15]
a. Particle Size and Size Distribution
Method: Dynamic light scattering (DLS) or Zetasizer analysis.
Criteria: Particle size should ideally be in the nanometer range (<300>
b. Zeta Potential
Method: Zetasizer or electrophoretic mobility analysis.
c. Entrapment Efficiency (EE%)
1. Centrifuge the niosomal suspension to separate free drug from encapsulated drug.
2. Analyze the supernatant for unencapsulated curcumin using UV-Vis spectroscopy.
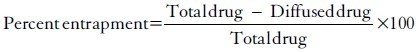
D. IN vitro Drug release study: To evaluate the release profile of curcumin from noisome.
Suspend it in phosphate buffer saline[ph 7.4]at 370. Collect sample at predetermined intervals and analyse curcumin content using uv spectroscopy.
Collect sample at predetermined intervals and analyse curcumin content using uv spectroscopy.
5.2 Evaluation of Niosomal Gel
a. Physical Appearance
Objective: To assess the visual characteristics of the gel.
Parameters: Color, transparency, and homogeneity.
Method: Visual inspection.
b. pH Measurement
Objective: To determine the pH of the gel formulation for skin compatibility.
Method: Use a calibrated digital pH meter.
Criteria: pH should be in the range of 5.5–7.0 (suitable for skin).
C. Viscosity and Rheology
Objective: To measure the gel's flow properties and spreadability.
Method: Use a Brookfield viscometer to measure viscosity.
Perform shear stress vs. shear rate analysis to assess rheological behavior.
Criteria: Gel should exhibit pseudo-plastic (shear-thinning) behavior.
d.Spreadability
Method:Place 0.5 g of gel between two glass slides and apply a specific weight.
Measure the diameter of the spread area.
Criteria: Spreadability should be optimal for topical application.
e. Drug Content Uniformity
Objective: To ensure uniform distribution of curcumin in the gel.
Method:Dissolve a known quantity of gel in a solvent.
Analyze curcumin content using UV-Vis spectroscopy or HPLC.
Criteria:Drug content should be consistent across batches.
f. Stability Studies
Objective: To evaluate the stability of the niosomal gel under various conditions.
Method:
Store the gel at different temperatures (e.g., 4°C, 25°C, and 40°C) and monitor for changes in physical appearance, pH, viscosity, and drug content over 1–3 months.
Conduct accelerated stability testing at 40°C/75% RH in a stability chamber.
6.SUMMARY & CONCLUSION
A possible method for boosting curcumin's therapeutic effectiveness in anti-inflammatory applications is curcumin-loaded niosomal gel. Important drawbacks like poor solubility, low bioavailability, and quick degradation can be successfully overcome by encapsulating curcumin in niosomes. The niosomal gel technology maximizes the anti-inflammatory effectiveness of the medicine by providing targeted delivery to inflammatory regions, enhanced skin penetration, and sustained drug release. Furthermore, niosome integration into a gel base guarantees localized activity with few systemic adverse effects, patient compliance, and ease of application. In addition to utilizing curcumin's strong natural anti-inflammatory qualities, this creative formulation demonstrates how nanotechnology can be used to improve herbal medication delivery systems. Its wider use in the treatment of inflammatory skin illnesses and associated conditions may be made possible by future research concentrating on clinical efficacy, safety, and scalability.
7. Future Prospect
1.Formulation, optimization and evaluation of curcumin loaded niosomal gel.
2.Stability study of niosomal gel
REFERENCES
- Kshitij B. Makeshwar, Suraj R. Wasankar. Niosome: a Novel Drug Delivery System. Asian J. Pharm. Res. 2013; (3)1:16-20 .
- GadhiyaP, Shukla S, Modi D, Bharadia P. A Review- Niosomesin Targeted Drug Delivery. International Journal forPharmaceuticalResearch Scholars 2012; 2: 61
- Vadlamudi HC. Niosomal drug delivery system—a review. Indo Am J Pharm Res. 2012;2(9):1-10.
- Khoee S, Yaghoobian M. Niosomes: A novel approach in modern drug delivery systems. In: Nanostructures for drug delivery. 2017 Jan 1. Elsevier. p. 207-37. DOI: 10.1016/B978-0-323-46143-6.00006-3.
- Madaan, K., Kumar, S., Poonia, N., Lather, V., & Pandita, D. (2014). Nanotechnology-based targeted drug delivery systems for breast cancer. Nanomedicine: Nanotechnology, Biology, and Medicine, 10(7), 1247–1262.
- Kumar, P., Sankar, C., Mishra, P., & Panda, S. S. (2016). Formulation, optimization, and evaluation of curcumin-loaded niosomes for enhancing its oral bioavailability. Journal of Drug Delivery Science and Technology, 33, 159-166.
- Prasad, S., et al. (2014). "Curcumin: The Future of Therapeutic Medicine." Med. Chem. [PMID: 25292019].
- Gharat, M., et al. (2016). "Niosomes as a vesicular carrier for topical drug delivery." Asian J Pharm Sci. [DOI: 10.1016/j.ajps.2016.01.002]
- Remington, J. P. (2021). Remington: The Science and Practice of Pharmacy (23rd ed.). Pharmaceutical Press.
- Aulton, M. E., & Taylor, K. (2013). Aulton's Pharmaceutics: The Design and Manufacture of Medicines (4th ed.). Elsevier.
- Devender Sharma, AashiyaAara E. Ali, Jayshree R. Aate. Niosomes as Novel Drug Delivery System: Review Article. PharmaTutor; 2018; 6(3); 58-65.
- Devender Sharma, AashiyaAara E. Ali, Jayshree R. Aate. Niosomes as Novel Drug Delivery System: Review Article. PharmaTutor; 2018; 6(3); 58-65.
- Rampal Rajera, KalpanaNagpal, Shailendra Kumar Singh, Dina Nath Mishra. Niosomes: A Controlled and Novel Drug Delivery System.
- Malhotra, M., & Jain, N. K. (1994). Niosomes as drug carriers. Indian Drugs, 31(3), 81–86.
- Lachman, L., Lieberman, H. A., & Kanig, J. L. (2009). The Theory and Practice of Industrial Pharmacy (4th ed.). CBS Publishers & Distributors.
- DidemAgSeleci, MuharremSeleci, JohannaGabrielaWalter, FrankStahl, and Thomas Schepe Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications Hindawi Publishing Corporation Journal of Nanomaterials Volume 2016
- Hussain, Z., Katas, H., & Amin, M. C. I. M. (2013). Suppression of inflammatory responses by dexamethasone-loaded nanocarriers: Gelatin nanoparticles, niosomes, and solid lipid nanoparticles.
- Moghassemi, S., & Hadjizadeh, A. (2014). "Nano-niosomes as nanoscale drug delivery systems." J Control Release.
- Nayak, A., et al. (2010). "Niosomes: Recent advancements in targeted drug delivery." J Drug Delivery Sci Technol.
- Kaur, G., et al. (2022). "Formulation of topical niosomal gel: Characterization and evaluation." Int J Pharm Sci Res.
- Shilakari Asthana, G., et al. (2016). Design and development of ethosomal transdermal gel of valsartan for improved skin permeation and anti-hypertensive activity. Journal: Journal of Liposome Research.DOI: 10.3109/08982104.2016.1151556.
- Costa, P., & Sousa Lobo, J. M. (2001). Modeling and comparison of dissolution profiles.Journal: European Journal of Pharmaceutical Sciences.DOI: 10.1016/S0928-0987(01)00095-1.
- Sharma, P. (2018). Formulation and evaluation of niosomes for topical delivery of curcumin. Master’s Thesis, XYZ University


 Awale Sainath *
Awale Sainath *
 Dr. Wajid Chaus
Dr. Wajid Chaus
 Ghalge Mahesh
Ghalge Mahesh
 Kodgire Nikita
Kodgire Nikita
 Patel Sujan
Patel Sujan




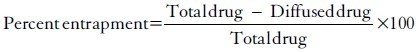
 10.5281/zenodo.14447482
10.5281/zenodo.14447482