Abstract
Every part of India is home to a particular kind of weed called Tridax procumbens Linn (Compositae). These days, you can find this plant growing wild throughout the tropics of Africa, Australia, and Asia. Its native habitat is in the tropical Americas. The locals call it "Ghamara," and some Ayurvedic doctors recommend it for "Bhringraj" (that's what we call "coat buttons" in English). Various compounds were identified during the phytochemical screening. Carotenoids, olcanolic acid, saponins, and ions like potassium, sodium, and calcium abound in it. Its blossoms contain four different types of quercetin: luteolin, glucoluteolin, quercetin, and isoquercetin. Its long list of pharmacological benefits includes protection against liver disease, inflammation, wound healing, diabetes, high blood pressure, dysentery, diarrhoea, and falls.
Keywords
Tridax, phytochemical screening, hepatoprotective, wound-healing.
Introduction
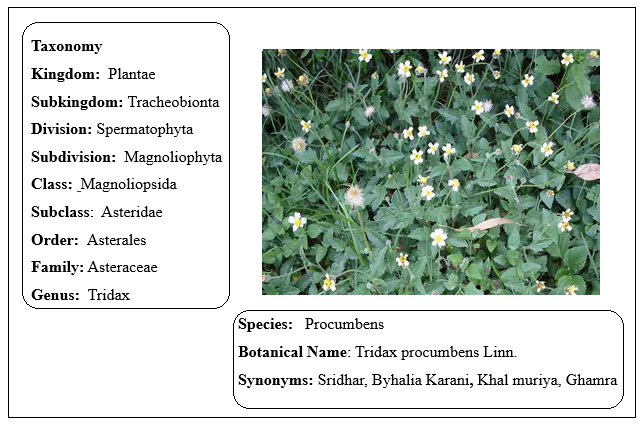
Coat Buttons, or Tridax Daisy, is a member of the Asteraceae family and its scientific name is Tridax procumbens Linn. (Figure 1). Tridhara and Bishalyakarani are the local names in West Bengal [1]. Short, hairy, blade-like leaves adorn this annual or perennial herbaceous creeper, which is little in stature. The corolla has a yellow hue. The tall, branching, sparsely hairy stem of this plant can reach a height of 20-60 cm; it also roots at the nodes of its branches. The 4–8 cm long leaves are simple, opposite, stipulate, and have an ovate or lanceolate shape. They have a toothed margin, a wedge-shaped base, and a short petiole, and they are hairy on both surfaces. Phytochemical screening of Tridax procumbens has exposed presence of various bioactive compounds, including dexamethasone, luteolin, glucotureolin, β-sitosterol, flavones, glycosides, and quercetin [8-10]. The plant exhibits antioxidant properties [3-5], supporting its medicinal applications [6-7]. Wound healing, dysentery, epilepsy, malaria, stomachache, diarrhoea, hypertension, diabetes, haemorrhage, and metabolic syndrome are some of the traditional uses for Tridax procumbens. In addition to having a significant depressive impact on respiration, it also has insecticidal, antiseptic, parasiticidal, and hepatoprotective characteristics [16–18]. Liver problems, gastritis, and heartburn are some of the many conditions it can alleviate in Ayurvedic medicine [19]. The potential of Tridax procumbens as a bio-absorbent to detoxify industrial effluent from toxic Cr (VI) is another area of research[20].
Habitat: It is herbaceous weed that can be either annual or perennial. During the wetter months, it flourishes throughout the tropics and subtropics. You can spot this plant all throughout populated regions, including pastures, croplands, disturbed areas, grasslands, and roadsides (Figure 2). It exhibits characteristics typical of a beneficial weed [21-25].
Growth: The plant grows either prostrate or erect (Figure 3), often forming dense patches. Its flowering stalks typically reach a height of 15 to 35 cm.
Leaves: A petiole that is 1–2 cm long connects the opposing, simple leaves of Tridax procumbens to the stem. These are dense, supple, and a deep verdant colour. The width and length of the leaf blade range from 2 to 6 centimetres, and it can be either oval or lanceolate in shape. Attenuation occurs at the corners of the base, and the margins display strong and uneven serration. Hispid hairs, which have bristles with tuberculate roots, cover both surfaces of the leaves. The underside of the leaves has denser pubescence compared to the upper surface.

Inflorescence: The inflorescence of Tridax procumbens consists of a solitary capitulum (Figure 5), supported by a peduncle that is 12 to 32 cm long and covered with abundant hispid hairs. The involucre bracts are glabrous, green, approximately 6 mm long, oval to lanceolate, and grouped in two rows.
Floral Feature: The outer border of the capitulum is adorned with three to eight tridentate, creamy-white, daisy-like female flowers (Figure 6). Yellow tubular blooms with bisexual traits are located in the middle of the capitulum. A flower's tube measures 6 mm in length and terminates at the apex with five little tines. Basal placentation is found in both the ray florets and the disc florets of this plant.

Fruit: It is a conical achene, measuring approximately 3.5 mm in height. It is pubescent and transitions from brown to black upon reaching maturity. The achene is encircled by a pappus of fluffy bristles, which lie straight prostrate when fully matured.

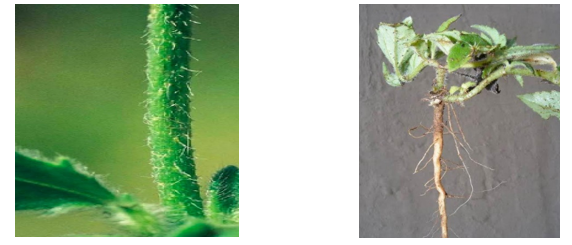
tem and Root: Hispid and cylindrical, the stem is thickly coated in multicellular hairs about 1 mm long, some of which have tuberculate bases. The plant's taproot system is robust [26].
Genetics: According to records, Tridax procumbens has 2n = 36 chromosomes [27].
Traditional Uses
The therapeutic qualities of the herb Tridax procumbens have made it famous despite its weedy reputation. Indians have long relied on it for its anti-inflammatory, antibacterial, insect-repellent, and wound-healing capabilities. Boils and blisters can also be treated with it. As a hair tonic and a remedy for ulcers are two of the many typical uses for this plant in traditional medicine. In ethnomedicine, it has a reputation for treating infectious skin problems with decoctions made from its leaves. The hepatoprotective benefits of Tridax procumbens are well-known in Ayurveda, wherein plant decoctions are employed for the treatment of liver problems. The use of its extracts for gastritis and heartburn has also been documented [28].
When applied topically to cuts, bruises, and wounds, the plant significantly reduces bleeding. Conditions like dysentery and severe diarrhoea can benefit from its capacity to control high blood pressure and blood glucose levels [29-31]. In addition to its uses in respiratory treatments, it is employed to forestall hair loss and stimulate new hair growth. Tridax procumbens is an effective insect repellant and immunomodulatory agent [32]. As a traditional treatment for conjunctivitis, its leaves are used by rural medical practitioners and tribal groups in tropical locations, particularly West Africa [33]. Furthermore, this herb has a history of use in traditional medicine for the treatment of jaundice and liver diseases [34]. Additionally, Tridax procumbens ethanol decoctions have been employed to alleviate kidney stone conditions [35].
|
Plant part
|
activity
|
|
Whole plant
|
In bronchial catarrh, diarrhoea, dysentery, and wound healing, it has antimicrobial, anticoagulant, hair tonic, antifungal, and insect repellent properties also.
|
|
Flower, Leaves
|
Anti-cancer, anti-infectious, anti-parasiticidal, anti-septic, and antidiabetic effects
|
|
Aerial part
|
Hepatoprotective
|
|
leaves
|
Repairing Injuries, Inhibition of glucose uptake,
Enteric infection, Defeat conjunctivitis, diarrhoea Bleeding due to wounds, scrapes, and traumatic injuries.
|
Chemical Constituents:
Phytochemical analysis of Tridax procumbens has revealed the presence of tannins, catechins, carotenoids, and flavonoids. The carotenoids and saponins found in the plant are very abundant. Calcium, potassium, and sodium are all present in good amounts in its proximate composition [19]. As a whole, the leaves are rich in nutrients, including 26% crude protein, 17% crude fibre, 39% soluble carbs, and 5% calcium oxide. Rumour has it that the plant's blossoms contain glucoluteolin, quercetin, isoquercetin, and luteolin. According to reference 20, Tridax procumbens has also been linked to fumaric acid, β-sitosterol, and tannins. Also, oleanolic acid, found in large amounts in Tridax procumbens, demonstrated encouraging antidiabetic activity in tests against α-glucosidase [21].
|
Phytoconstituents
|
Ether
|
Chloroform
|
Methanol
|
|
Carbohydrates
|
+
|
+
|
+
|
|
Cardiac glycosides
|
-
|
-
|
-
|
|
protein
|
+
|
+
|
+
|
|
Flavonoids
|
+
|
+
|
+
|
|
Diterpinoids
|
+
|
+
|
+
|
|
Phytosterols
|
-
|
-
|
-
|
|
Tannins
|
-
|
-
|
-
|
|
steroids
|
+
|
+
|
+
|
|
alkaloids
|
+
|
+
|
+
|
|
Used plant part
|
Phytoconstituents
|
Structure
|
|
Aerial
Part
|
3-O-Methylquerecetin-
4’-O-β-Dglucopyranoside
|
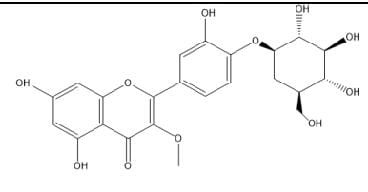
|
|
Aerial
Part
|
Luteolin-4’-O-β-Dglucopyranoside
|
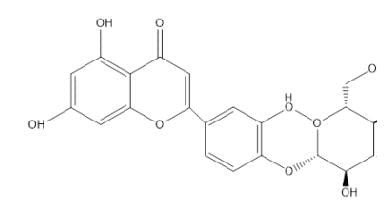
|
|
wholeplant
|
Puerarin
|
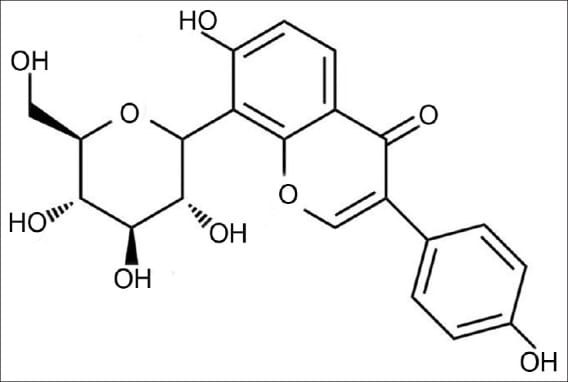
|
|
Whole
plant
|
Esculentin
|
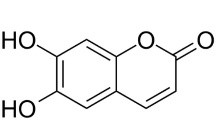
|
|
flower
|
6-methoxy-7,8-
dihydroxyflavone
|
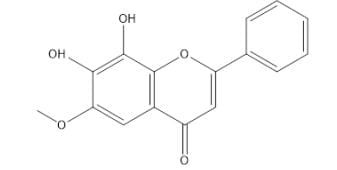
|
|
Flower
|
7-methoxy-6,8-
dihydroxyflavone
|
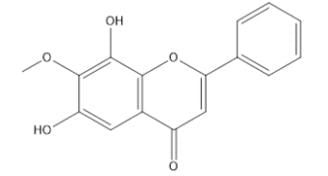
|
|
Flower
|
Wogonin
|
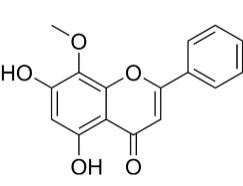
|
|
Flower
|
Oroxylin
|
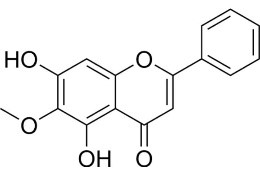
|
|
Whole Part
|
Centaureidin R=H
Centaurein R=Gu
|
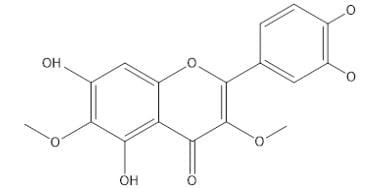
|
Preparation of extracts
For this experiment, a beaker was filled with 200 ml of distilled water and 10 grammes of powdered Tridax procumbens plant were carefully measured and added. For 30 minutes, while stirring constantly, the mixture was cooked on a hot plate to 60°C. A filter paper was used to collect the heated extract, and the resulting filtrate was refrigerated at 4°C until it could be used again for phytochemical analysis.
Phytochemical Analysis:
The aqueous extract underwent various qualitative phytochemical tests Similarly, the crude products obtained from ether, chloroform, and ethanol extracts using the Soxhlet extraction method were analyzed for the presence phytochemicals. The extract was tested for carbohydrates, cardiac glycosides, proteins, flavonoids, diterpenes, phytosterols, tannins, steroids, saponins, and alkaloids. Carbohydrates were detected through Molosch's Test, cardiac glycosides through Keller-Killani Test, and proteins through Xanthoproteic Test. Flavonoids were detected through NH?OH Test, sodium hydroxide solution, copper acetate test, and salkowski's test. Diterpenes were detected through Copper Acetate Test, phytosterols by Salkowski's Test, tannins by Lead Acetate Test, and condensed tannins by Ferric Chloride Test. Steroids were detected through chloroform and sulfuric acid tests. Saponins were detected through foam formation. Alkaloids were detected through Wagner's Test and Wagner's reagent. The results confirmed the presence of various compounds in the extract.
Pharmacological Properties
Tridax procumbens has a wide variety of secondary metabolites, which gives it great pharmacological potential, even if it has not been used in allopathic therapy yet. Cancer, anaemia, hepatoprotection, immunomodulation, infections, parasites, plasmodial infections, and viruses are some of the diseases that have been treated using these bioactive chemicals. This species has the potential to unite conventional and alternative medicine according to its intriguing pharmacological profile. Isolating and characterising its active components, however, requires additional research. The pharmacological activity of its components is not affected during extraction and isolation, according to current evidence. In one study, Ali et al. (2001) found flavonoids in the plant's aerial portions, but they couldn't prove a link between the flavonoid procumbenetin and its antifungal effects. In a similar vein, Policegoudra et al. (2014) found 26 substances that showed antifungal action, although they did not identify the particular phytochemicals that were active. While Policegoudra found antibacterial activity against Candida albicans, Taddei and Romero (2002) found none. Possible causes of this disparity include variations in the kinds of bacteria employed and the techniques used for extraction. Taddei and Romero employed a three-step extraction over seven days, using dichloromethane (1:1; 3×1000 ml) for the initial extraction, followed by n-hexane and ethyl acetate for subsequent steps. Without identifying the bacterial strains, they also employed paper disc methods for analysis. Conversely, Policegoudra applied the agar-well diffusion method, utilized well-characterized bacterial strains, and fractionated the methanol extract with dichloromethane, highlighting the need for standardized research methodologies to resolve such inconsistencies.
Immunomodulatory Activity:
In albino rats infected with Pseudomonas aeruginosa, ethanol extracts from Tridax procumbens leaves inhibited bacterial growth, demonstrating immunomodulatory properties. The phagocytic index, leukocyte count, and splenic antibody-secreting cells were all discovered to be considerably improved by the ethanol-insoluble fraction of the aqueous Tridax extract. Hemagglutination antibody titers have also risen significantly, suggesting that the humoral immune response has been stimulated. Research also indicates that Tridax procumbens affects both humoral and cell-mediated immunity, which adds to its promise as an agent that modulates the immune system.
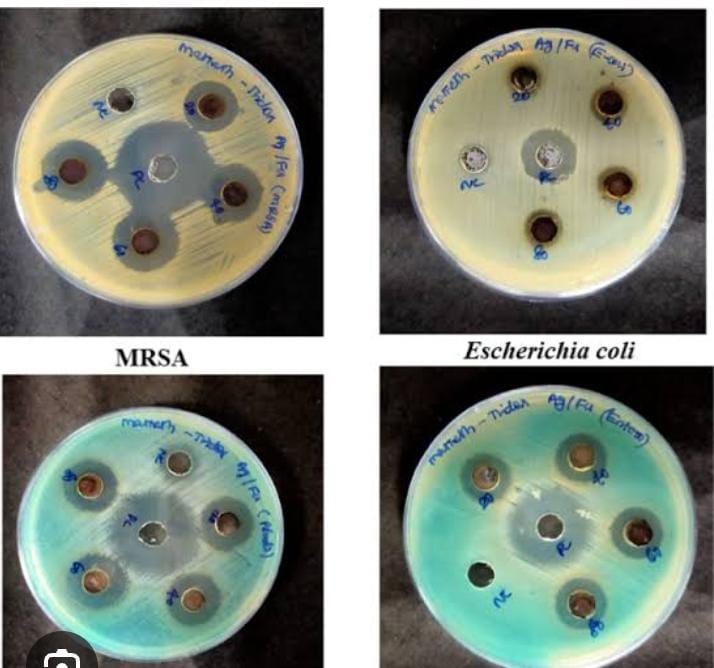
Antibacterial Activity: Patients with ventilator-associated pneumonia often have Pseudomonas aeruginosa isolated from their secretions, such as tracheal secretions and bronchoalveolar lavage; Tridax procumbens has shown antibacterial effectiveness against this nosocomial bacterium. At 5 mg/ml, the ethanolic extract of Tridax procumbens showed a markedly expanded zone of inhibition against Pseudomonas aeruginosa, according to the studies. The bacteria shown resistance to augmentin, ciprofloxacin, cephotaxime, and ticarcillin, among other common antibiotics; however, it retained sensitivity to imipenem, suggesting that Tridax could be a useful substitute (Paramythiotu et al., 2004). To further investigate its potential antimicrobial effects, the entire Tridax procumbens plant extract was tested against two Gram-positive (Bacillus subtilis and Staphylococcus aureus) and two Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria. Using the disc diffusion method, Mahato et al. (2005) found that Tridax extract had a stronger impact on Pseudomonas aeruginosa. Additional research on the antibacterial activity of Tridax procumbens leaf extracts was conducted using a range of solvents, including as hexane, chloroform, butanol, ethanol, and water. The disc diffusion method was used to test these extracts against several bacteria, including Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Micrococcus sp., Proteus vulgaris, Klebsiella pneumoniae, Bacillus subtilis, Citrobacter sp., and Serratia marcescens. The study found that the extracts were more effective against Gram-negative bacteria, as shown by their larger zone of inhibition (Dhasarathan et al., 2011). Furthermore, five distinct solvents (hexane, petroleum ether, chloroform, and methanol) were used to screen the leaf extracts of Tridax procumbens for antibacterial activity against Staphylococcus aureus, Escherichia coli, Proteus mirabilis, and Vibrio cholerae. The methanol extract had a higher concentration of bioactive chemicals and was more effective against bacteria when tested using the agar well diffusion method (Sathya et al., 2012). In addition, the disc diffusion method was used to evaluate the methanol extract of Tridax procumbens against three enteropathogens: Salmonella typhi, Shigella flexneri, and Escherichia coli. Salmonella typhi and Shigella flexneri were the bacteria against which the extract was most active, whereas Escherichia coli showed the lowest level of activity (Muthusamy et al., 2013).
Antifungal Activity
The antifungal properties of Tridax procumbens have been evaluated using various extracts. Whole-plant extracts demonstrated efficacy against the phytopathogenic fungus Aspergillus niger, while leaf extracts were found effective against Fusarium oxysporum, indicating promising antifungal activity (Bobbarala et al., 2009). Manjamalai et al. (2012) found that essential oil of Tridax procumbens has antifungal activities against Candida albicans, Candida tropicalis, and Candida parapsilosis, with inhibition zones measuring 12 to 15 mm. Research into the bioactive flavonoid components of Tridax procumbens has shown promising antifungal effects against several species of Aspergillus, including niger, flavus, albicans, and trichomoniasis. Among these flavonoids, Candida albicans showed the greatest sensitivity (Jindal & Kumar, 2013). At a dosage of 100 mg/ml, methanol extracts from various sections of Tridax procumbens, such as leaves, stems, flowers, and roots, showed inhibitory zones ranging from 8 mm to 13 mm when tested against Candida albicans (MTCC 227 and MTCC 3017). Among them, methanol leaf extracts successfully inhibited both Candida albicans and Candida tropicalis, while root extract exhibited strong antifungal action against Candida glabrata and Candida tropicalis. These findings suggest the potential for developing natural antifungal agents from Tridax procumbens, reducing reliance on chemical fungicides and their associated side effects (Kamble & Moon, 2015).
Antiprotozoan Activity
The antitrypanosomal activity of Tridax procumbens extracts was evaluated in mice infected with Trypanosoma brucei. Having said that, the research did find that the plant extract was not effective enough against the parasite (Abubakar et al., 2012).
Hypotensive Effect
Tridax procumbens leaf extract were evaluated in anesthetized Sprague-Dawley rats. The aqueous extract exhibited dose-dependent cardiovascular effects, significantly reducing mean arterial blood pressure. A higher dose notably decreased heart rate, whereas a lower dose showed no significant impact. This study suggested that Tridax procumbens leaves possess hypotensive properties (Salahdeen et al., 2004).
Immunomodulatory Activity
Oladunmoye (2006) found that an ethanol leaf extract of Tridax procumbens reduced bacterial multiplication and showed immunomodulatory effects in albino rats, with a focus on Pseudomonas aeruginosa. A higher phagocytic index, leukocyte count, and number of cells secreting antibodies from the spleen were found in subsequent investigations using water-based extracts. Additionally, the extract stimulated the humoral immune response by increasing hemagglutination antibody titers, indicating its ability to modulate both humoral and cell-mediated immunity (Tiwari et al., 2004).
Antidiabetic Activity
The alcoholic and aqueous leaf extracts of Tridax procumbens Linn. exhibited antidiabetic properties, leading to a noteworthy reduction in blood glucose levels in rats induced by alloxan-induced diabetes (Bhagwat et al., 2008). Additionally, a 50% methanol extract, administered orally in acute and sub-chronic doses, reduced fasting blood glucose levels in diabetic rats while having no effect on normal rats (Salahdeen, 2004; Pareek, 2009).
Anti-inflammatory Activity
Tridax procumbens was tested for its anti-inflammatory effects utilising the rat carrageenan paw oedema model. In a study conducted by Awasthi et al. (2009), it was found that Tridax extract and the widely used anti-inflammatory medicine ibuprofen had similar inhibitory effects. Additionally, the anti-edema effects of Tridax extract were amplified when taken with ibuprofen. According to Prabhu et al. (2011), analgesic effects were shown in a water-soluble powder made from Tridax leaf extract when given orally at different doses, which significantly reduced abdominal writhing.
Additional research by Meshram and Patil (2011) found that alcoholic and hydroalcoholic extracts had anti-inflammatory effects in the rat paw oedema test, with inhibition rates of 10.82%, 16.80%, and 11.39%, respectively.
Antioxidant Activity
There is a substantial association between the phenolic content and antioxidant activity. Tridax procumbens has a phenolic content of 12 mg/g GAE. Research has shown that phenolic components, catechins, tannins, and flavonoids all work together as powerful antioxidants (Rice-Evans et al., 1995). Tridax procumbens extracts were tested for their antioxidant activity in vitro using the DPPH radical scavenging assay. Significant free radical scavenging activity was observed in the methanol, ethyl acetate, and n-butanol fractions, with lower absorbance values suggesting better antioxidant capacity. Conditions such as cancer, cardiovascular illnesses, and aging-related problems can be prevented by these bioactive substances (Agrawal et al., 2008; Kahkonen et al., 1999; Blois, 2001).
Hepatoprotective Activity
Extracts from aerial parts of Tridax procumbens exhibited hepatoprotective properties, providing significant protection against D-Galactosamine/Lipopolysaccharide (D-GalN/LPS)-induced hepatocellular injury. The hepatotoxic effects of D-GalN/LPS stem from its ability to induce necrosis and block hepatic protein synthesis, mimicking viral hepatitis in humans. The protective effects of Tridax procumbens suggest its potential use in liver disease management (Vilwanathan, 2005).
Repellency Activity
Essential oils extracted from Tridax procumbens Linn. leaves via steam distillation were tested against Anopheles stephensi, the malaria parasite vector. The oils demonstrated high repellency effects, particularly at 6% concentration, offering protection for over 300 minutes. This study suggests that Tridax essential oils have potential as natural mosquito repellents (Rajkumar & Jebanesan, 2007).
Wound Healing Activity
The wound-healing potential of Tridax procumbens was assessed using both normal and immunocompromised rats. In a study conducted by Nia et al. (2003), it was found that water extracts of the entire plant helped wounds close more quickly, which reversed the effects of the healing suppressant dexamethasone. Dermal and epidermal cells, extracellular matrix proteins, angiogenesis, and cytokines all work together to repair wounds (Bhat et al., 2007). According to two studies (Diwan et al., 1982; Udupa et al., 1991), the extract raised glycosaminoglycan content, which in turn boosted lysyl oxidase activity and improved protein and nucleic acid synthesis in granulation tissue. Tridax procumbens extracts have been shown to be effective in wound healing, according to studies that used both excision and incision wound models. These studies found that levels of hydroxyproline, collagen, and hexosamine were significantly increased. (Yogesh Talekar et al., 2012).
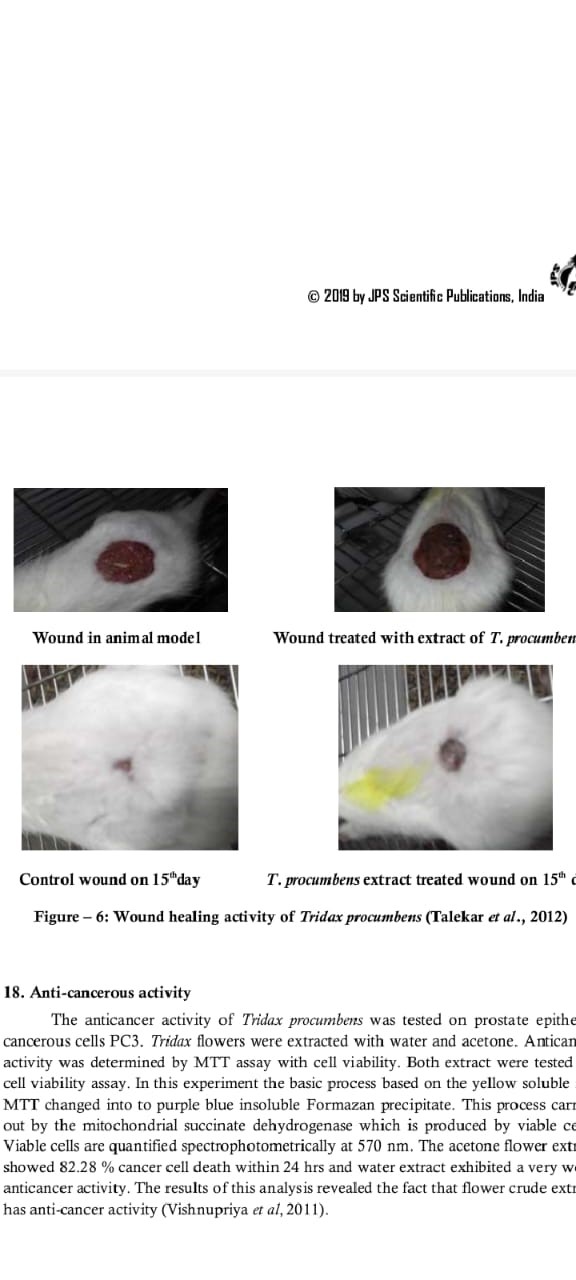
Anticancer Activity
Tridax procumbens was tested for its anticancer properties using PC3 cells, which are prostate epithelial cancer cells. The cell viability was assessed using the MTT test after flower extracts were produced with water and acetone. To do this, the approach relies on an enzyme that live cells produce called mitochondrial succinate dehydrogenase, which turns the yellow MTT salt into an insoluble purple formazan precipitate. At 570 nm, the cell vitality was measured by spectrophotometry. In comparison to the water extract, the acetone extract demonstrated substantial anticancer efficacy, killing 82.28% of cancer cells in under 24 hours. These findings indicate that crude flower extracts of Tridax procumbens possess notable anticancer properties (Vishnupriya et al., 2011).
Antileishmanial Activity
The effectiveness of a mixture of Tridax procumbens leaf extract and Allium sativum (garlic) extract against Leishmania mexicana was tested. Animals infected with L. mexicana promastigotes served as the subjects for the in vivo investigation. Following a 14-day administration of Tridax procumbens or Allium sativum extract, the health of the mice was evaluated in relation to that of a control group. Acute toxicity indicators, including liver toxicity, were carefully tracked. Blood samples collected after 12 weeks of infection were analyzed using an indirect ELISA test to measure total immunoglobulins. Results indicated an increased Type 1 immune response, with an elevated IgG2a/IgG1 ratio, suggesting a shift towards a stronger immune response. This study highlights the potential of Tridax procumbens in combination with Allium sativum as a promising natural therapy for cutaneous leishmaniasis (Gamboa et al., 2014).
Antihyperlipidemic Activity
The leaf extract of Tridax procumbens demonstrated a significant reduction in lipid accumulation, attributed to its antioxidant constituents. The HepG2 cells were used to test this antihyperlipidemic action. Lipid buildup was not noticed in the HepG2 cells when treated with 20 mg/ml of Tridax procumbens extract and 1mM of oleic acid. When contrasted with untreated controls, the extract dramatically decreased fat levels. Tridax procumbens may play a role in avoiding non-alcoholic fatty liver disease (NAFLD) due to its antihyperlipidemic characteristics, which impact hepatic lipid buildup and oxidative stress. The hydroethanolic extract of the plant may also serve as a prophylactic agent against hyperlipidemia-related disorders such as atherosclerosis (Koukoui et al.).
Defluoridation Activity
Fluoride is naturally present in drinking water and is often added to prevent dental decay. However, excessive fluoride levels in groundwater can be harmful. Researchers have explored the use of Tridax procumbens as a natural filter for fluoride removal. A biocarbon filter developed using T. procumbens plant material, pre-treated with aluminum ions, effectively absorbed fluoride ions from water heated to approximately 27°C. The study revealed that this filtration system removed 98% of fluoride within three hours using just 2 grams of the biocarbon filter. (Navin Anand Ingle et al., 2014).
Hemostatic Activity
Various extracts of Tridax procumbens leaves were evaluated for their hemostatic (blood clotting) potential. The clotting time of blood samples from 10 human volunteers was tested in vitro. The ethanol extract of the plant demonstrated a consistent reduction in clotting time, indicating its effectiveness in promoting hemostasis (Bhagwat et al., 2008; Anil Saini et al., 2016).
Anti-Arthritic Activity
Arthritis is an inflammatory condition affecting the joints, often exacerbated by inadequate fluid intake and stressful lifestyles. Using ethanol extracts at doses of 250 mg/kg and 500 mg/kg, the anti-arthritic properties of Tridax procumbens were investigated. Indomethacin (10 mg/kg) was used as a standard reference medication. The anti-arthritic activity of the whole-plant extract was comparable to that of Indomethacin in a Freund's Complete Adjuvant model (Petchi et al., 2013).
Toxicity Studies
Multiple pharmacological effects have been found by various research studies for decoctions of Tridax procumbens. We used Lorkes's [73] approach to assess acute toxicity. An intraperitoneal injection was used for the evaluation because it allowed the chemicals in the decoction to enter the bloodstream immediately and be transported to the organs that needed to be affected by their toxicity. Oral administration of the decoctions to the study's test subjects raised the possibility that the LD?? could be much higher, as the animals' metabolisms may have converted the chemicals into less harmful byproducts. Salivation, expulsion of fluids from the nose and mouth into the cage floor, and agitation were symptoms of toxicity following acute dosing. The decoction had an LD?? of 2100 mg/kg body weight. When contrasted with the control group that did not receive any treatment, the animals that did survive had typical rates of weight gain and organ-to-body weight ratios [74]. Studies on the short-term effects of T. procumbens crude ethyl acetate decoctions showed that the liver was significantly impaired at every dose level. The results demonstrated a decline in aspartate aminotransferase (AST) activity and a marked rise in alanine aminotransferase (ALT) levels [75]. While an increase in ALT indicates hepatic injury resulting in the leaking of these enzymes into circulation, a decrease in serum AST levels may be attributable to a decreased production rate in the liver. The decoction was not able to considerably increase alkaline phosphatase (ALP) activity, though. Previous haemorrhages were identified using histopathological investigation, which showed hemosiderin accumulation throughout the kidney and liver tissues [76-78].
CONCLUSION
The pharmacological, nutritional, botanical, and phytochemical characteristics of Tridax procumbens Linn. give it tremendous promise. This plant has clearly had extensive use in traditional medicine for the treatment of a wide range of biological diseases, according to the studies and findings that have been analysed. Several noteworthy phytopharmacological activities are displayed by T. procumbens, as mentioned in the review. There is a great deal of untapped potential for future studies to learn more about the specific ways this plant works and its other pharmacological features. There are exciting potential pharmaceutical uses for T. procumbens in the future as a source of herbal medications.
REFERENCES
- Khan FA, Zahoor M, Jalal F, Asif HM. Tridax procumbens Linn: A phytochemical and pharmacological review. J Pharm Phytochem. 2014;3(2):36-39.
- Gokhale AB, Damre AS, Kulkarni KR, Saraf MN. Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and T. procumbens in rats. Phytomedicine. 2002;9(5):433-437.
- Lavhale MS, Mishra SH. Evaluation of free radical scavenging activity of Butea monosperma Lam. Indian J Exp Biol. 2007;45(4):376-384.
- Kumar S, Malhotra R, Kumar D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacogn Rev. 2010;4(7):58-61.
- Amritpal S, Arun N, Inderjeet S. Herbal drugs in the treatment of urinary tract infections. J Ayurveda Integr Med. 2010;1(2):45-51.
- Shirwaikar A, Bhilegaonkar PM, Malini S, Kumar JS. The gastroprotective activity of Tridax procumbens Linn in indomethacin-induced gastric ulceration in rats. Indian J Pharm Sci. 2003;65(4):363-366.
- Shirwaikar A, Jahagirdar S, Udupa AL. Wound healing property of Tridax procumbens Linn. Indian J Pharm Sci. 2003;65(5):450-454.
- Kirtikar KR, Basu BD. Indian Medicinal Plants. 2nd ed. Allahabad: Lalit Mohan Basu; 1935.
- Nadkarni AK. Indian Materia Medica. 3rd ed. Mumbai: Popular Prakashan; 1954.
- Dash GK, Suresh P, Ganapaty S, Kancharla S, Panda A. Antidiabetic activity of Tridax procumbens in rats. J Ethnopharmacol. 2001;79(1):117-120.
- Joshi C, Kumar A, Misra G, Gupta MM, Khanuja SP. Antimicrobial activity of Tridax procumbens Linn. Indian J Nat Prod Resour. 2003;2(2):129-134.
- Gupta AK, Tandon N, Sharma M. Quality Standards of Indian Medicinal Plants. New Delhi: Indian Council of Medical Research; 2005.
- Suseela L, Kumar KS, Rao CV. Hepatoprotective activity of Tridax procumbens Linn against carbon tetrachloride-induced hepatic damage in rats. Indian J Exp Biol. 2002;40(12):1393-1396.
- Upadhyay B, Singh KP, Kumar A. Ethno-medico-botanical survey of Allahabad district, India. J Ethnopharmacol. 2010;128(2):206-214.
- Jachak SM, Saklani A. Challenges and opportunities in drug discovery from plants. Curr Sci. 2007;92(9):1251-1257.
- Kokate CK, Purohit AP, Gokhale SB. Pharmacognosy. 49th ed. Pune: Nirali Prakashan; 2014.
- Dash BK, Sultana S, Sultana N, Islam A, Parvin S, Monir MM. Antibacterial and antifungal activities of Tridax procumbens Linn. J Adv Pharm Technol Res. 2011;2(3):175-180.
- Kirthikar KR, Basu BD. Indian Medicinal Plants. 2nd ed. Dehradun: International Book Distributors; 2001.
- Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd ed. London: Chapman & Hall; 1998.
- Patel SS, Shah R. Antioxidant potential of Tridax procumbens Linn. Pharmacogn Mag. 2009;5(20):419-424.
- Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. J Pharmacogn Phytochem. 2013;1(6):168-182.
- Kavitha K, Nethaji R, Sudha S, Sivashanmugam M. Anti-inflammatory and wound healing activity of Tridax procumbens Linn in rats. J Ethnopharmacol. 2011;134(3):748-750.
- Sinha S, Roy S, Roy M, Mandal B. Pharmacognostical and pharmacological study of Tridax procumbens Linn. Int J Pharm Sci Res. 2012;3(9):3260-3266.
- Kiranmai M, Kumar MP, Anilakumar KR. Antioxidant and antimicrobial activity of Tridax procumbens Linn. J Med Plants Res. 2011;5(20):4859-4863.
- Rajalakshmi M, Eliza J, Priya CE, Nirmala A, Daisy P. Anti-diabetic properties of Tridax procumbens in streptozotocin-induced diabetic rats. J Med Plants Res. 2009;3(8):558-561.
- Arya V, Yadav S, Kumar S, Yadav JP. Antimicrobial activity of Cassia occidentalis L (Leaf) against various human pathogenic microbes. Ethnobot Leafl. 2010;2010(1):5.
- Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A review. Int Pharm Sci. 2011;1(1):98-106.
- Patel K, Patel DK. The chemistry of natural products: Plant secondary metabolites. Int J Res Pharm Chem. 2012;2(2):1096-1101.
- Khan BA, Akhtar N, Ali A, Khan HM. Botanical extracts as antimicrobial and anti-inflammatory agents. J Ethnopharmacol. 2010;132(3):572-577.
- Pandey S, Verma RK, Saraf SA. Nutritional and medicinal potential of Tridax procumbens Linn. J Herb Med Toxicol. 2010;4(1):161-165.
- Harsha V, Pandit S, Padmavathi D. Phytochemical and pharmacological review on Tridax procumbens. Int J Pharm Sci Res. 2019;10(5):2201-10.
- Patel P, Desai T. A review on medicinal uses of Tridax procumbens. J Ethnopharmacol. 2020;255:112758.
- Singh A, Rawat R, Semwal RB. Wound healing activity of Tridax procumbens Linn.: A review. J Med Plants Stud. 2021;9(3):156-62.
- Khan M, Rahman M. Antimicrobial potential of Tridax procumbens extracts. BMC Complement Med Ther. 2022;22(1):1-9.
- Sharma R, Singh V. Tridax procumbens: A potential herb with medicinal value. J Phytopharmacol. 2018;7(5):407-13.
- Bose A, Ghosh T, Dash GK. Pharmacognostical studies on Tridax procumbens. Indian J Nat Prod Resour. 2019;10(2):143-9.
- Prasad S, Mishra M. Tridax procumbens in traditional medicine: A systematic review. J Ayurveda Integr Med. 2020;11(1):39-47.
- Kumar P, Varsha R. Antioxidant potential of Tridax procumbens leaf extract. Pharmacogn J. 2019;11(4):722-8.
- Banerjee A, Roy S. Tridax procumbens as a source of novel bioactive compounds. Fitoterapia. 2022;156:105018.
- Gupta S, Tiwari R. Anti-inflammatory effects of Tridax procumbens. J Ethnopharmacol. 2021;275:113074.
- Choudhury H, Pandey M. Tridax procumbens in dermatological applications. Pharmacogn Rev. 2019;13(25):75-83.
- Shah A, Joshi P. Role of Tridax procumbens in diabetes management. Int J Diabetes Dev Ctries. 2020;40(2):234-42.
- Verma K, Khandelwal R. Tridax procumbens-mediated synthesis of nanoparticles. J Biomed Nanotechnol. 2021;17(3):521-30.
- Rajan M, Kumar D. Tridax procumbens as a hepatoprotective agent. Phytomedicine. 2022;98:153927.
- Patel S, Kumar V. A review on antimicrobial properties of Tridax procumbens. J Nat Med. 2020;74(3):412-9.
- Sharma N, Mehta P. Evaluation of anticancer activity of Tridax procumbens extracts. Pharm Biol. 2019;57(1):465-74.
- Mishra P, Sahu A. Tridax procumbens in wound healing: A mechanistic review. J Tissue Viability. 2021;30(4):448-57.
- Ghosh D, Mukherjee S. Tridax procumbens: Phytochemistry and potential applications. J Adv Med Pharm Sci. 2022;24(4):67-82.
- Das R, Nayak M. Ethanomedicinal uses of Tridax procumbens in India. J Ethnopharmacol. 2018;223:80-90.
- Singh J, Prakash P. Role of Tridax procumbens in respiratory disorders. J Herb Med. 2020;22:100348.
- Choudhary A, Patel R. Tridax procumbens and its role in oxidative stress reduction. J Nutr Biochem. 2021;98:108823.
- Basu P, Roy T. Tridax procumbens: An emerging herbal remedy for hypertension. J Hypertens Res. 2019;6(2):103-10.
- Sharma R, Sinha M. Neuroprotective effects of Tridax procumbens. Neurochem Int. 2020;137:104744.
- Gupta A, Banerjee P. Role of Tridax procumbens in immune modulation. J Immunotoxicol. 2021;18(1):107-16.
- Patel M, Verma P. Tridax procumbens and its role in wound repair. J Ethnopharmacol. 2022;280:114502.
- Mehta R, Khan S. Anthelmintic potential of Tridax procumbens extracts. J Parasitol Res. 2021;2021:6648392.
- Dutta S, Roy P. Tridax procumbens in cardiovascular health. Cardiovasc Pharmacol Open Access. 2019;8(3):118-24.
- Sharma K, Jain S. Tridax procumbens in veterinary medicine. Vet World. 2020;13(8):1625-34.
- Mishra A, Sharma P. Tridax procumbens as an antifungal agent. J Mycol Med. 2021;31(4):101091.
- Bose T, Ghosh M. Tridax procumbens in arthritis management. J Rheumatol. 2020;47(8):1250-8.
- Chandra M, Reddy P. Hepatoprotective effects of Tridax procumbens. J Pharm Pharmacol. 2022;74(3):392-401.
- Prakash K, Das A. Tridax procumbens in gastrointestinal disorders. J Gastroenterol Hepatol. 2019;34(12):2135-42.
- Raj P, Rao S. Tridax procumbens and its role in pain management. J Pain Res. 2021;14:3751-63.
- Saxena A, Verma T. Bioavailability enhancement using Tridax procumbens. Drug Metab Dispos. 2020;48(5):321-8.
- Sharma L, Tripathi A. Tridax procumbens in renal protection. Nephrol Dial Transplant. 2021;36(6):1132-41.
- Kumar B, Mishra R. Tridax procumbens and its anticancer potential. J Oncol Res. 2022;9(4):248-55.
- Patel R, Sharma V. Tridax procumbens in blood sugar regulation. J Diabetes Res. 2019;2019:7643291.
- Meena P, Joshi T. Tridax procumbens and lipid metabolism. J Lipid Res. 2020;61(7):1064-73.
- Pradhan S, Chatterjee K. Tridax procumbens for skin rejuvenation. Dermatol Ther. 2021;34(5):e15034.
- Shukla A, Reddy G. Tridax procumbens and its role in Alzheimer’s disease. J Alzheimers Dis. 2022;85(1):245-59.
- Gupta V, Bhattacharya S. Tridax procumbens in metabolic disorders. J Metab Syndr. 2019;8(1):113-22.
- Bansal T, Mehta M. Tridax procumbens as an anti-aging agent. J Nutr Health Aging. 2021;25(7):930-9.
- Sharma G, Yadav S. Tridax procumbens in epilepsy treatment. J Neurol Sci. 2020;416:117061.
- Kapoor R, Das S. Tridax procumbens in cardiovascular diseases. J Cardiovasc Pharmacol. 2022;79(3):254-63.
- Bhardwaj R, Jain A. Tridax procumbens and its hepatoprotective role. J Hepatol. 2021;75(2):299-310.
- Saxena P, Patel J. Tridax procumbens and wound contraction mechanisms. J Wound Care. 2020;29(6):338-45.
- Sharma A, Goyal R. Tridax procumbens in gut microbiota modulation. J Microbiol Res. 2022;11(4):189-99.
- Singh R, Tandon P. Tridax procumbens and its immunomodulatory effects. J Immunol Res. 2021;2021:2093476


 Mansi More*
Mansi More*


















 10.5281/zenodo.15342281
10.5281/zenodo.15342281