Abstract
An easy-to-use, sensitive and accurate RP-HPLC technology has been developed and validated to detect Vonoprazan and Domperidone simultaneously in pharmaceutical dosage forms. The acetonitrile-based mobile phase buffer is supplied at a flow rate of 1.0 ml/min at a ratio of 65:35, with a detection wavelength of 230 am. The retention durations for vonoprazan and domperidone were 7.185 and 11.365 minutes, respectively. The method was developed and approved in accordance with ICH guidelines.
Keywords
Vonoprazan, Domperidone, RP-HPLC, Validation
Introduction
From the fields of development and validation, quality control, and quality assurance, the ongoing and interdependent tasks include the creation of analytical methods and validation. Analytical methods are essential for risk management and equivalency assessments. It supports the stability of outcomes and the development of acceptability criteria. The analytical procedure's suitability for the intended use should be demonstrated through validation. An essential tool for characterizing and validating a method is experiment design. To optimize the analytical method, it should be easy for analytical professionals to employ. Significant gains in accuracy and a decrease in errors can be obtained by well-developed analytical methods and their validation. Avoiding time-consuming and expensive exercises can be effective.[1] Vonoprazan fumarate 1-[5-(2-fluorophenyl)-1- ( pyridine -3 -yl sulfonyl ) – 1 H-pyrrol- 3 – yl ]- N - methyl methanamine fumarate , a chemical derivative of pyrroles, The potassium-competitive acid blocker Vonoprazan prevents acid secretion by competitively preventing potassium from being available to hydrogen-potassium ATPase. When fasting or fed, Vonoprazan is rapidly absorbed and acid stable, taking 1.5–2.0 hours to reach its Cmax. It slowly separates from its target, with a half-life of roughly 7.7 hours[3] Due to its high pKa (>9), it is more likely to accumulate in the canalicular region of parietal cells, where it inhibits resting and active proton pumps in a competitive manner[2] There is interindividual variation in the effects of CYP2C19, age, sex, and dose[2] It is not advised to change the dosage in cases of hepatic or renal illne
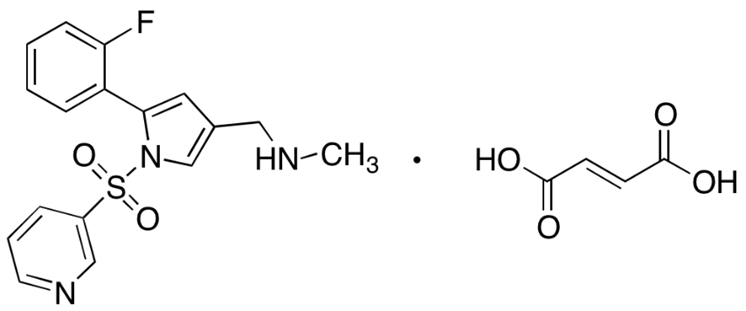
Structure of Vonoprazan
Peripheral dopamine2-receptor antagonist domperidone has been demonstrated to have some effects on the motor function of the oesophagus in addition to regulating the motility of gastric and small intestine smooth muscle. Because it blocks dopamine receptors in the chemoreceptor trigger zone, it also possesses antiemetic properties. In patients with symptoms of diabetic gastropathy, domperidone relieved symptoms (anorexia, nausea, vomiting, abdominal pain, early satiety, bloating, and distension) more effectively than placebo in controlled clinical trials; Additionally, dopamine-containing medications avoided the gastrointestinal and emetic side effects of antiparkinsonian therapies, prevented nausea and vomiting linked to emetogenic chemotherapy, and temporarily relieved symptoms in patients suffering from dyspepsia or gastroesophageal reflux. Domperidone barely penetrates the blood-brain barrier, therefore reports of negative effects on the central nervous system, like dystonic responses, are rare.
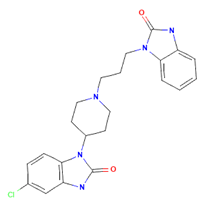
Structure of Domperidone
MATERIALS AND METHODS
RP-HPLC method
Materials and reagents
Vonoprazan and Domperidone from Sai Mirra Pharma company, Chennai, Tamilnadu, India were received as a gift sample. Acetonitrile of HPLC grade from Qualigens fine chemicals, Mumbai, India. All the reagents and chemicals used were of analytical and HPLC grade. Water (HPLC grade) was obtained from Milli Q RO system.
Instruments and Chromatographic Conditions
Shimadzu HPLC system was used for method development and validation. Data acquisition was performed on Data ace HPLC software. The separation were achieved on C8 (250 × 4.6 mm, 5?m) column at ambient temperature and the eluent was monitored at 230 nm with flow rate of 1.0 mL/min using UV detector. The mobile phase mixture of 35 parts Acetonitrile & 65 parts Water was filtered through nylon 0.22 ?m membrane filters and was degassed before use (30 min). A volume of 20 ?L of each sample was injected into column.
Preparation of Buffer:
4.14 g of Sodium dihydrogen orthophosphate in 1000 mL of water adjust pH 6.5 with Sodium hydroxide or Ortho phosphoric acid.
Diluent:
Water: Acetonitrile (65:35)
Preparation of standard solution
Weigh accurately 67 mg of Vonoprazan fumarate WRS and 25 mg Domperidone WRS transfer to a 50 ml volumetric flask. Dilute 5 mL into a 50 ml with diluent. Dissolve and make up the volume with mobile phase
Sample preparation :
Transfer 210 mg of sample to a 100 ml volumetric flask, add 70 ml of diluents and sonicate for 25 minutes with intermittent shaking. Allow to cool and dilute to volume. Filter through 0.45 µm membrane filter. Dilute 5 ml into a 10 ml with diluent.
Procedure:
Separately inject 20 µL blank, 6 replicates of the standard solution and check the system suitability. If the system suitability passes the acceptance criteria, proceed with the injection of sample solutions.
System suitability:
Relative standard deviation: NMT 2.0% for the 6 areas of Vonoprazan and Domperidone peaks from 6 replicate standard injections.
Column efficiency:
Not less than 2500 theoretical plates for both Vonoprazan and Domperidone
Resolution: Not less than 5.0 between Vonoprazan and Domperidone peaks. Calculate the content of Vonoprazan per tablet using the formula :
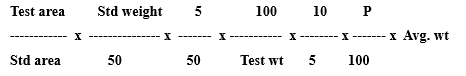
Where, P = Purity of Vonoprazan fumarate WRS
Calculate the content of Domperidone in % of label claim using the
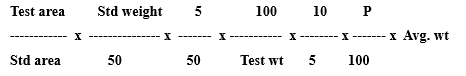
P= Purity of Domperidone WRS
METHOD VALIDATION
Procedure:
Separately inject the standard solution in 6 replicates and sample solution and record the chromatograms. The order of elution will be as follows:
VONOPRAZAN
DOMPERIDONE
Linearity:
The calibration curve was plotted between concentration and peak area. Vonoprazan and Domperidone were linear in the concentration range of 50-150mcg/ ml and 25-75 mcg/ ml respectively. All the solution were filtered through 0.22 µm membrane filter Aliquots (20 ?l) of each solution were injected under the operating chromatographic condition described above [Number of replicates (n = 6)]. A calibration graph was plotted between the mean peak area Vs respective concentration and regression equation was derived.
System suitability
The developed procedure was verified in compliance with ICH guidelines [7] Measurements were made of the system suitability characteristics in order to assess the system performance. The precision of the system was assessed using six identical injections of the same setup. Asymmetry and the number of theoretical plates were measured [8,9]. By calculating the peak retention time, peak area, theoretical plates, tailing or asymmetry factor for Vonoprazan and Domperidone. the system's appropriateness was ensured.
Relative standard deviation:
NMT 2.0% for the 6 areas of Vonoprazan and Domperidone peaks from 6 replicate standard injections.
Column efficiency:
Not less than 2500 theoretical plates for both Vonoprazan and Domperidone were used for determining the system suitability.
Resolution:
Not less than 5.0 between Vonoprazan and Domperidone peaks
Precision
The reproducibility of an analytical procedure under typical operating conditions is known as precision. Repeatability (intra-day) and intermediate precision (inter-day) were used to determine the assay's precision; the results were expressed as a percentage R.S.D. for a statistically significant number of replicate readings. By comparing the assays on three separate days, the intermediate precision was investigated, and the results were recorded as standard deviation and % R.S.D. The % RSD for the six Assay determinations is NMT 2.0% were used for determining the precision
Accuracy
Recovery studies verified the method's accuracy. A known amount of standard Vonoprazan and Domperidone were added to the previously examined formulation, and the recovery % was computed. The recovery at various levels is between 98.0% and 102.0% ii. The RSD for Recovery of triplicate samples at various levels is not more than 2.0% were used for determining the Accuracy
Specificity
The ability to accurately and specifically measure the target analyte in the presence of other substances that would be expected to be present in the sample matrix is known as specificity. It measures the level of interference from other active ingredients and excipients, among other things, to make sure that a peak reaction is exclusively caused by one ingredient and that there is no co-elution. Specificity parameters were assessed using standard, blank, and placebo solutions to see whether Vonoprazan and Domperidone retention times were impacted in any way. There should be no interference of the placebo which determines the specificity.
RESULTS AND DISCUSSION
Linearity:
For linearity, Construction of the calibration curves for the drugs Vonoprazan and Domperidone are assessed by the combined standard solutions in range of 50-150mcg/ml and 25-75mcg/ml respectively. The responses were linear throughout the range for all two analytes {r?2;=0.999,r?2;=0.997,respectively}
Correlation Coefficient square – NLT
0.995 y-intercept : Not more than ± 2.0%
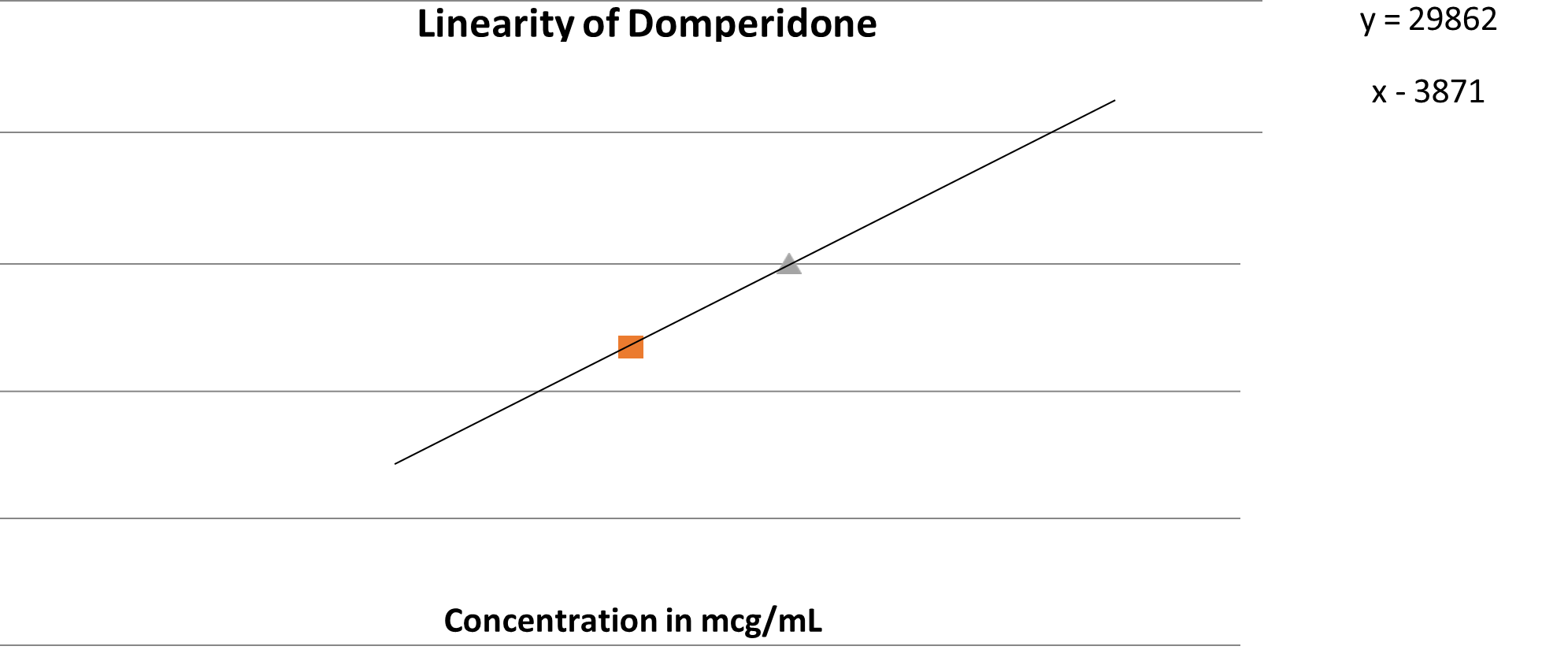
Calibration curve of Domperidone
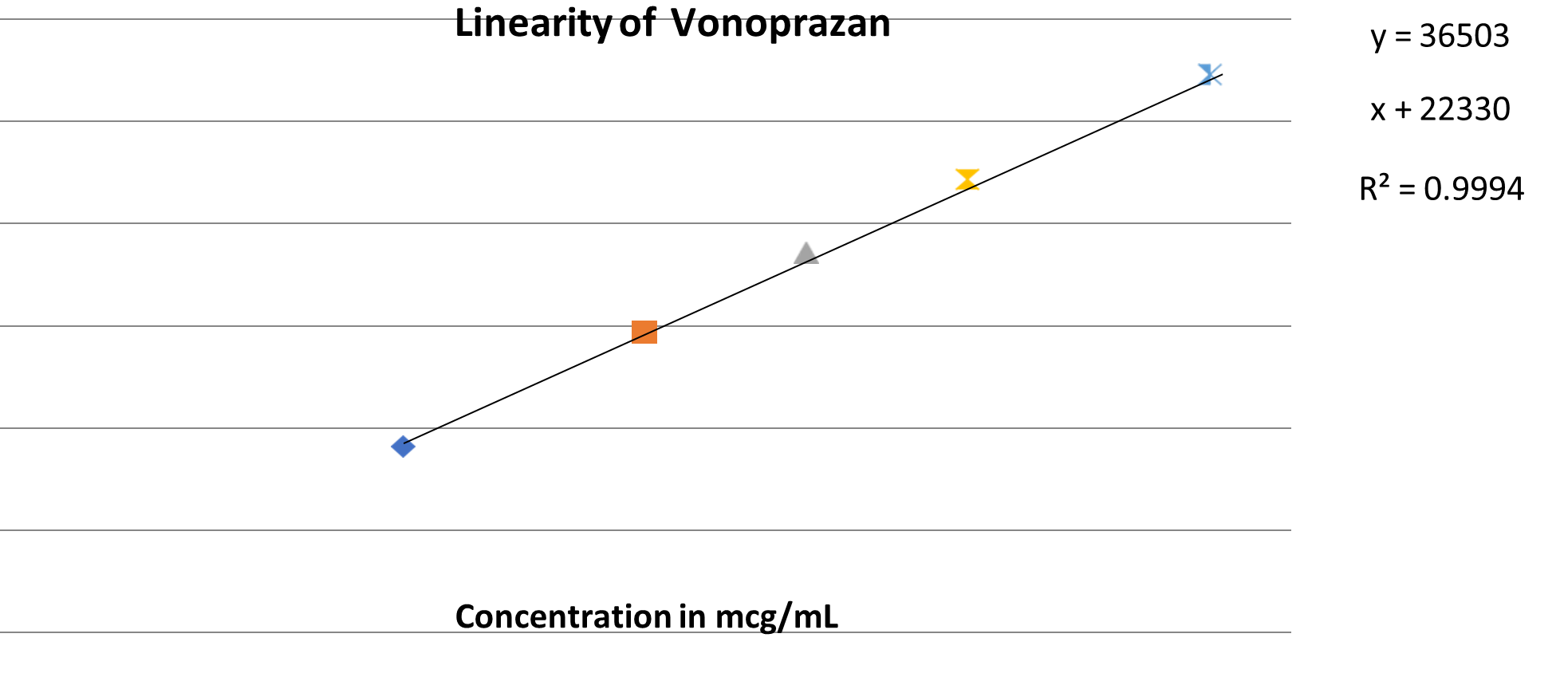
System suitability:
In the present HPLC method, Acetonitrile and distilled water (65:35 v/v) were used as mobile phase. In a preliminary separate analysis study of Vonoprazan and Domperidone C8 column was successfully used; therefore, C8 was used simultaneous determination. The described chromatographic conditions resulted in Vonoprazan and Domperidone retention at about 7.167 and 11.256 minutes,

Precision:
The reproducibility of an analytical procedure under typical operating conditions is known as precision. Repeatability (intra-day) and intermediate precision (inter-day) were used to determine the assay's precision; the results were expressed as a percentage R.S.D. for a statistically significant number of replicate readings. By comparing the assays on three separate days, the intermediate precision was investigated, and the results were recorded as standard deviation and percent R.S.D.
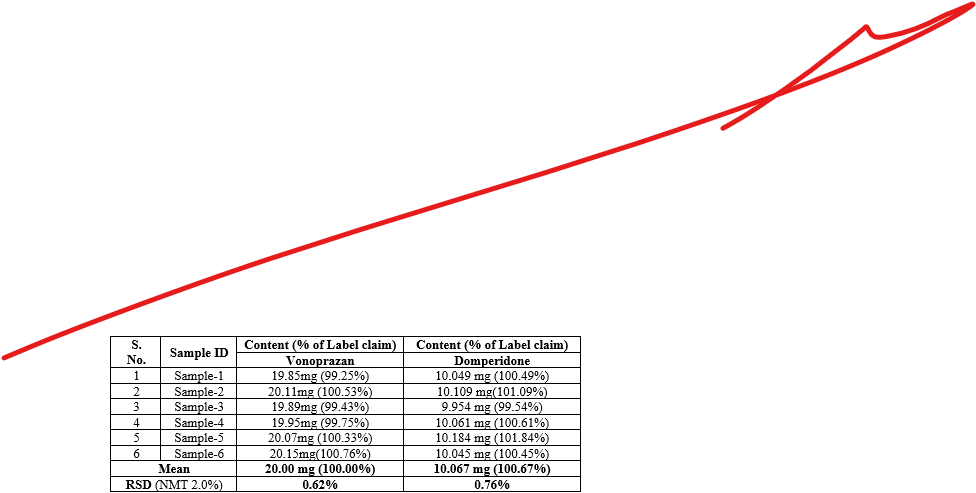
Accuracy:
According to FDA, percentage recovery method was used to determine Accuracy. The recovery at various levels is between 98.0% and 102.0% ii. The RSD for Recovery of triplicate samples at various levels is not more than 2.0% were used for determining the Accuracy
For Vonoprazan:
20 mg drug solutions was taken in three different flask label A1,A2,A3.spiked 50%,100%,150% of standard solution in it and diluted .the area of each solution at 230 nm. the amount of vonoprazan was calculated at each level and percentage recoveries were calculated.
For Domperidone:
10 mg drug solution was taken in three different flask label A1,A2,A3. Spiked 50%,100%,150% of standard solution in it and diluted. The area of each solution at 230 nm. The amount of domperidone was calculated at each level and percentage recoveries were calculated.
Vonoprazan
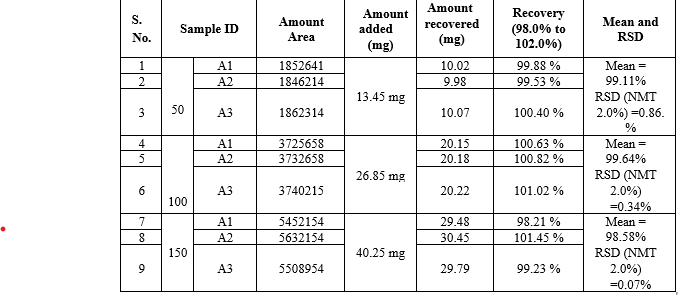
Data of Accuracy of Vonoprazan
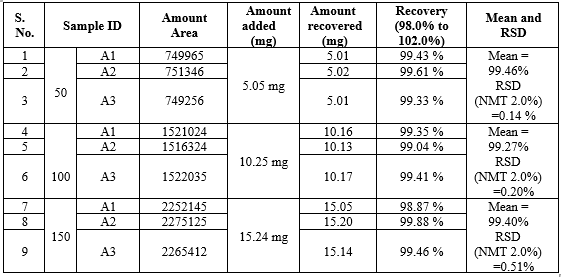
Data of Accuracy of Domperidone
Remarks: The recovery and RSD for recovery at each level meets the acceptance criteria
Specificity:
The specificity of the drug Vonoprazan and Domperidone were assessed using standard, blank, placebo solutions which impacts the retention time.
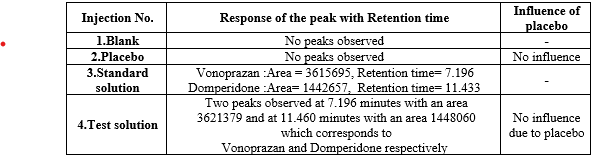
Remarks:
There is no interference of the placebo. Hence the method is specific
CONCLUSION
In accordance with ICH guidelines, the current RP-HPLC developed method for the simultaneous evaluation of Vonoprazan and Domperidone was verified. It was discovered that the developed RP-HPLC method was easy to use, fast, accurate, selective, and precise for the simultaneous quantification of all two drugs. Because the validated approach yielded no incorrect findings, it can be utilized for routine examination of two drugs.
REFERENCES
- Bharti Mittu A., & Chauhan P. Analytical Method Development and Validation: A Concise Review. Journal of Analytical & Bioanalytical Techniques, 2015; 06(01). doi: 10.4172/2155- 9872.1000233.
- Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: Pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet 2016;55:409–418.
- Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-Nmethylmethanamin e monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther 2010; 335:231–238.
- Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/ non-Japanese subjects. Clin Transl Gastroenterol 2015;6:e94.
- National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 45375887, Vonoprazan Fumarate. Retrieved August 27, 2024
- Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999 Apr;33(4):429-40
- ICH Proceedings of the International Conference on Harmonisation of Technical Requirement of Registration of Pharmaceuticals for Human Use (ICH Harmonised Tripartite Guidelines). Validation of Analytical Procedures: Methodology, Q2B.
- Bishnoi RS, Kumar M, Shukla AK, Jain CP, Development and validation of novel HPLC method for the estimation of Rutin in crude hydromethanolic leaf extract of Prosopis cineraria, Journal of Drug Delivery and Therapeutics. 2018; 8(6):68-7
- Kumar M, Shukla AK, Bishnoi RS, Jain CP, Development of UV Spectrophotometric Method for The Determination of Benidipine Hydrochloride By Using Quality By Design (QbD) Approach. International Journal of Applied Pharmaceutics, 2018; 10(4):92-97.
- Kim D., Yousaf A., Li D., Kim J., Yong C., Cho K. and Choi H. Development of RP-HPLC method for simultaneous determination of docetaxel and curcumin in rat plasma: Validation and stability. Asian Journal of Pharmaceutical Sciences, 2017; 12(1):105-113.
- Kavathia A. and Misra M. Development and validation of RPHPLC and UV-spectrophotometric methods for rapid simultaneous estimation of amlodipine and benazepril in pure and fixed dose combination. Arabian Journal of Chemistry, 2017; 10:S3021-S3028.
- Shabir GA. Validation of high-performance liquid chromatography methods for pharmaceutical analysis: understanding the differences and imilarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. Journal of Chromatography A 2003; 987(1):57–66.
- Beludari M., Prakash K. and Mohan G. RP-HPLC method for simultaneous estimation of Rosuvastatin and Ezetimibe from their combination tablet dosage form. International Journal of Chemical and Analytical Science, 2013; 4(4):205-209
- Gupta V, Jain AD, Gill NS, Gupta K. Development and validation of HPLC method - a review. International Research Journal of Pharmaceutical and Applied Sciences. 2012; 2(4):17-25.
- Sonia K, Nappinnai M. Development and validation of HPLC and UV-visible spectrophotometric method for the pharmaceutical dosage form and biological fluid –review. European Journal of Biomedical and Pharmaceutical sciences. 2016; 3(3): 382-391.
- Sabir AM, Molony M,Parminder SB. HPLC Method Development and validation: A Review. International research Journal of pharmacy. 2013; 4(4):39-46.


 P. Kanaga*
P. Kanaga*


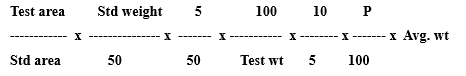







 10.5281/zenodo.13712320
10.5281/zenodo.13712320