Abstract
Antibiotic resistance is an important concern for the public health authorities at global level. Review on Hospital data showed higher and varied spectrum of resistance in different levels of health care. There is an urgent need to develop and strengthen antimicrobial policy, standard treatment guidelines, plan for containment of antimicrobial resistance and research related to public health aspects of antimicrobial resistance at hospital level. Antibiotic stewardship is a practice that promote rational use of antibiotic which means prescribing antibiotics only when necessary and, when antibiotics are considered necessary, promoting use of the appropriate agent(s), dose, duration, and route of therapy to optimize clinical outcomes while minimizing the unintended consequences of antibiotic use. This study discusses different principles and guideline for implementation of antibiotic stewardship in the hospital.
Keywords
Antibiotics, Antibiotic resistance, Rational use, Antibiotic stewardship.
Introduction
Antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antimicrobial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections [1][2] They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity.[3][4] Antibiotics are not effective against viruses such as the common cold or influenza [5]. Antibiotics are used to treat or prevent bacterial infections [6], and sometimes protozoan infections. When an infection is suspected of being responsible for an illness but the responsible pathogen has not been identified, an empiric therapy is adopted [7]. This involves the administration of a broad-spectrum antibiotic based on the signs and symptoms presented and is initiated pending laboratory results that can take several days.[6][7] Antibiotics may be given as a preventive measure and this is usually limited to at-risk populations such as those with a weakened immune system (particularly in HIV cases to prevent pneumonia), those taking immunosuppressive drugs, cancer patients, and those having surgery [6]. Their use in surgical procedures is to help prevent infection of incisions. They have an important role in dental antibiotic prophylaxis where their use may prevent bacteremia and consequent infective endocarditis. Antibiotics are also used to prevent infection in cases of neutropenia particularly cancer-related [9][10].
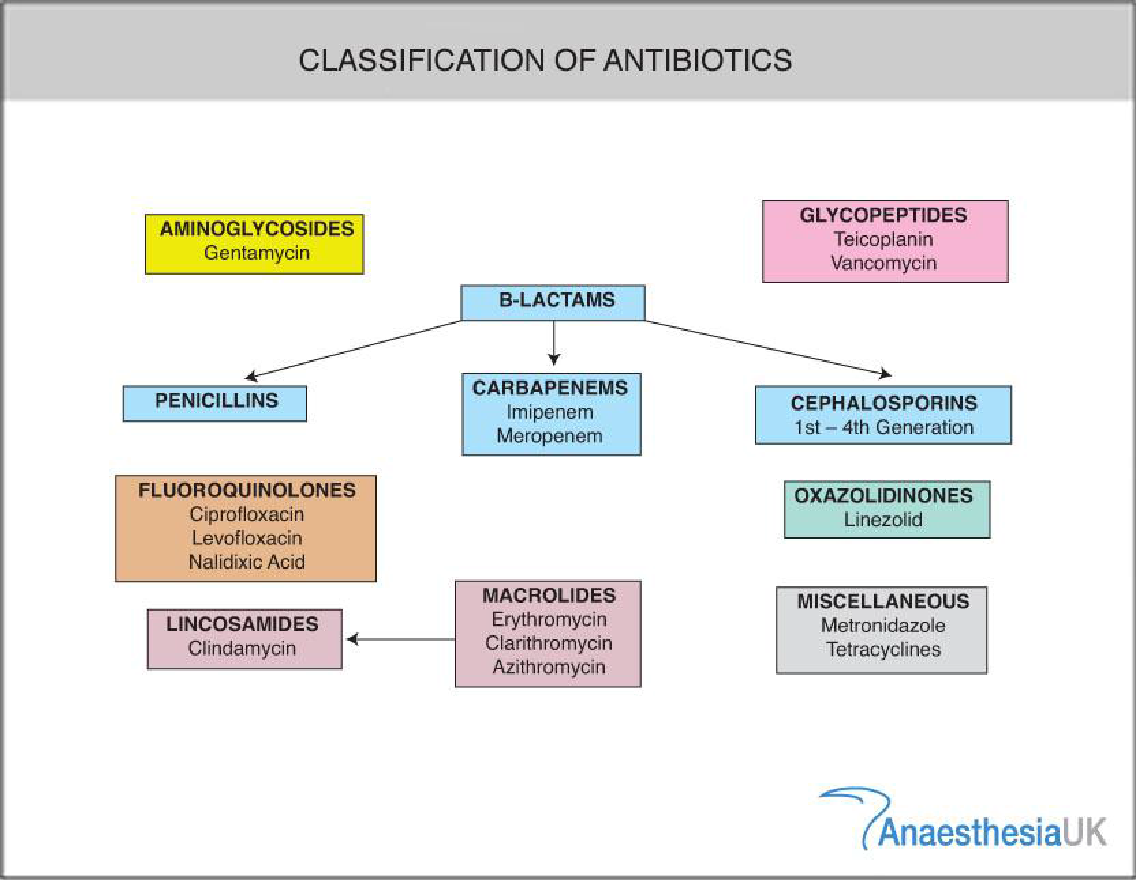
Antibiotic Resistance
Antibiotic resistance is the ability of a bacterium or other microorganism to survive and reproduce in the presence of antibiotic doses that were previously thought effective against them. The successful use of any therapeutic agent is compromised by the potential development of tolerance or resistance to that compound from the time it is first employed. Wide range of biochemical and physiological mechanisms may be responsible for resistance. Antibiotic resistance applies specifically to bacteria that become resistant to antibiotics[11]. Resistance in bacteria can arise naturally by genetic mutation, or by one species acquiring resistance from another [12]. Inappropriate antibiotic treatment and overuse of antibiotics have contributed to the emergence of antibiotic-resistant bacteria. Self-prescribing of antibiotics is an example of misuse [14]. Many antibiotics are frequently prescribed to treat symptoms or diseases that do not respond to antibiotics or that are likely to resolve without treatment. The overuse of antibiotics, like penicillin and erythromycin, has been associated with emerging antibiotic resistance since the 1950s[17][18] .Widespread usage of antibiotics in hospitals has also been associated with increases in bacterial strains and species that no longer respond to treatment with the most common antibiotics[18]. Antibiotic resistance leads to higher medical costs, prolonged hospital stays, and increased mortality. Antibiotic resistance is rising to dangerously high levels in all parts of the world. New resistance mechanisms are emerging and spreading globally, threatening our ability to treat common infectious diseases.
Antibiotic Stewardship
Antibiotic stewardship is the effort to measure and improve how antibiotics are prescribed by clinicians and used by patients. Improving antibiotic prescribing and use is critical to effectively treat infections, protect patients from harms caused by unnecessary antibiotic use, and combat antibiotic resistance. Hospital antibiotic stewardship programs can increase infection cure rates while reducing:
- Treatment failures
- C. difficile infection
- Adverse effects
- Antibiotic resistance
- Hospital costs and lengths of stay
Data shows high levels of unneeded antibiotic prescribing across health care settings. For example, 1 in 3 antibiotic prescriptions written in doctors’ offices, emergency rooms, and hospital-based clinics—about 47 million prescriptions annually—is completely unnecessary. Effective antibiotic stewardship can reduce this type of misuse and help slow the emergence of resistance. Stewardship programs also help ensure that when antibiotics are needed, they’re used appropriately. Antibiotics can also kill off “good” bacteria in the gut, which enables previously latent “bad” bacteria to multiply and increases a patient’s chances of developing secondary illnesses such as a Clostridium difficile infection that can result in life-threatening diarrhea and colitis. Antibiotic stewardship helps address these dangers by ensuring that antibiotics are used only when needed. Stewardship also ensures that patients receive the most effective treatment option and that it is administered correctly, which is key to protecting individual’s health and helping them recover as quickly as possible. This is especially crucial for sepsis patients, who can rapidly suffer tissue damage, organ failure and even death if they do not receive antibiotics quickly enough or if their antibiotic treatment does not work.[19]
Aims Of Antibiotic Stewardship
Antibiotic Stewardship has two primary goals:[19][20]
- To ensure effective treatment for patients with bacterial infection.
- To reduce unnecessary antibiotic use and minimize collateral damage
General Principles Of Antibiotic Stewardship
- Accurately identify patients who need antibiotic therapy
- Use local epidemiology to guide the selection of empiric therapy
- Avoid agents with overlapping activity
- Adjust antibiotics when culture results become available
- Monitor for toxicity
- Optimize dose, route and duration of therapy
- Ongoing evaluation of need for antibiotics
Core Elements Of Antibiotic Stewardship
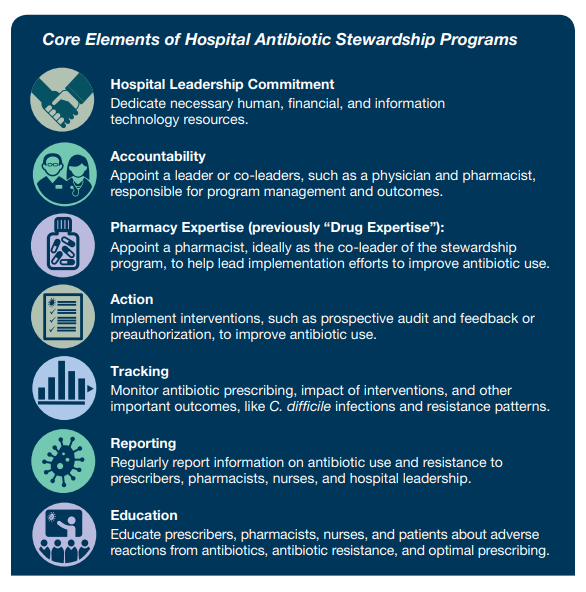
Optimizing the use of antibiotics is critical to effectively treat infections, protect patients from harms caused by unnecessary antibiotic use, and combat antibiotic resistance. Antibiotic stewardship programs can help clinicians improve clinical outcomes and minimize harms by improving antibiotic prescribing. In 2019, CDC updated the hospital Core Elements to reflect both lessons learned from five years of experience as well as new evidence from the field of antibiotic stewardship. Major updates to the hospital Core Elements include:
- Hospital Leadership Commitment: Dedicate necessary human, financial and information technology resources.
- Accountability: Appoint a leader or co-leaders, such as a physician and pharmacist, responsible for program management and outcomes.
- Pharmacy Expertise: Appoint a pharmacist, ideally as the co-leader of the stewardship program, to lead implementation efforts to improve antibiotic use.
- Action: Implement interventions, such as prospective audit and feedback or preauthorization, to improve antibiotic use.
- Tracking: Monitor antibiotic prescribing, impact of interventions, and other important outcomes like C. difficile infection and resistance patterns.
- Reporting: Regularly report information on antibiotic use and resistance to prescribers, pharmacists, nurses, and hospital leadership.
- Education: Educate prescribers, pharmacists, and nurses about adverse reactions from antibiotics, antibiotic resistance and optimal prescribing.
Hospital Leadership Commitment
Support from the senior leadership of the hospital, especially the chief medical officer, chief nursing officer, and director of pharmacy, is critical to the success of antibiotic stewardship programs. A lack of necessary resources is commonly cited as the top barrier to success by stewardship programs. Hospital leadership can play a critical role in helping the stewardship program get the resources needed to accomplish its goals.
- Giving stewardship program leader(s) time to manage the program and conduct daily stewardship interventions.
- Providing resources, including staffing, to operate the program effectively. Staffing suggestions for hospital antibiotic stewardship programs are available from the Veterans Administration [21] and a survey published in 2018 .[22]
- Having regular meetings with leaders of the stewardship program to assess the resources needed to accomplish the hospital’s goals for improving antibiotic use.
- Reporting stewardship activities and outcomes (including key success stories) to senior leadership and the hospital board on a regular basis (e.g. including stewardship measures in hospital quality dashboard reports).
- Integrating antibiotic stewardship activities into other quality improvement and patient safety efforts, such as sepsis management and diagnostic stewardship.
Stewardship programs are greatly enhanced by strong support from the following groups:
Clinicians: It is vital that all clinicians are fully engaged in and supportive of efforts to improve antibiotic use. Department or program heads: Support from clinical department heads, as well as the director of pharmacy, is especially important in embedding stewardship activities in daily workflow. Pharmacy and therapeutics committee can play a key role in helping to develop and implement policies that will improve antibiotic use (e.g., incorporating stewardship into order sets and clinical pathways). Some hospitals have created a multidisciplinary stewardship subcommittee of the Pharmacy and Therapeutics Committee.
Infection preventionists and hospital epidemiologists can assist with educating staff and with analyzing and reporting data on antibiotic resistance and C. difficile infection trends [24].
Nurses: There is growing recognition of the importance of engaging nurses in hospital stewardship efforts. Nurses can play an especially important role in:
- Optimizing testing, or diagnostic stewardship. For example, nurses can inform decisions about whether or not a patient has symptoms that might justify a urine culture.
- Assuring that cultures are performed correctly before starting antibiotics
- Prompting discussions of antibiotic treatment, indication, and duration.
- Improving the evaluation of penicillin allergies.
Accountability
The antibiotic stewardship program must have a designated leader or co-leaders who are accountable for program management and outcomes. Effective leadership, management and communication skills are essential for the leaders of a hospital antibiotic stewardship program [25].It is important for physician leaders who do not work full time at the hospital. Antibiotic prescribing is ultimately under the direction of the medical staff. If a non-physician is the leader of the program, it is important that the hospital designate a physician who can serve as a point of contact and support for the non-physician program leader. Training in infectious diseases and/or antibiotic stewardship benefits stewardship program leaders. Larger facilities have achieved success by hiring full-time staff to develop and manage stewardship programs while smaller facilities report other arrangements, including use of part-time or even off-site expertise, sometimes referred to as tele stewardship.
Pharmacy expertise
It was previously named ‘Drug Expertise’ and was renamed ‘Pharmacy Expertise’ to reflect the importance of pharmacy engagement for leading implementation efforts to improve antibiotic use. Highly effective hospital antibiotic stewardship programs have strong engagement of pharmacists, either as a leader or co-leader of the program. It is important to identify a pharmacist who is empowered to lead implementation efforts to improve antibiotic use. Infectious diseases trained pharmacists are highly effective in improving antibiotic use and often help lead programs in larger hospitals and healthcare systems [26]. In hospitals without infectious disease trained pharmacists, general clinical pharmacists are often co-leaders or pharmacy leaders. General clinical pharmacists are more effective when they have specific training and/or experience in antibiotic stewardship. There are a variety of resources to support the antibiotic stewardship efforts of clinical pharmacists, ranging from posters highlighting key stewardship interventions for pharmacists to formal training and certificate programs in stewardship for pharmacists.
Action
Antibiotic stewardship interventions improve patient outcomes. An initial assessment of antibiotic prescribing can help identify potential targets for intervention. Stewardship programs should choose interventions that will best address gaps in antibiotic prescribing and consider prioritizing prospective audit and feedback, preauthorization and facility-specific treatment guidelines.
Prospective audit and feedback are an external review of antibiotic therapy by an expert in antibiotic use, accompanied by suggestions to optimize use, at some point after the agent has been prescribed.
Facility-specific treatment guidelines
Guidelines can optimize antibiotic selection and duration, particularly for common indications for antibiotic use like community-acquired pneumonia, urinary tract infection, intra-abdominal infection, skin and soft tissue infection and surgical prophylaxis. Recommendations may be based on national guidelines but should reflect hospital treatment preferences based on local susceptibilities, formulary options, and patient mix. Ideally, the recommendations should also address diagnostic approaches, such as when to send diagnostic samples and what tests to perform, including indications for rapid diagnostics and non-microbiological tests (e.g., imaging, procalcitonin). The development of treatment guidelines is a good way for the stewardship program to engage prescriber stakeholders to develop consensus on antibiotic use.
Tracking
Measurement is critical to identify opportunities for improvement and to assess the impact of interventions. Measurement of antibiotic stewardship interventions may involve evaluation of both processes and outcomes. For example, a program will need to evaluate if policies and guidelines are being followed as expected (processes) and if interventions have improved patient outcomes and antibiotic use (outcomes).
Outcome measures
C. difficile infections are an important target for stewardship programs, given the evidence that improved antibiotic use can prevent these infections. C.difficile infection prevention is multifaceted and creates an opportunity for stewardship programs to collaborate with other groups, such as the laboratory and infection prevention.
Antibiotic Resistance: Improving antibiotic use is important to reduce antibiotic resistance, which presents another option for measurement. The development and spread of antibiotic resistance is multi-factorial and studies assessing the impact of improved antibiotic use on resistance rates have shown mixed results. The impact of stewardship interventions on resistance is best assessed when measurement is focused on pathogens that are recovered from patients after admission when they are under the influence of hospital stewardship interventions.
Process measures for quality improvement
Process measures can focus on the specific interventions being implemented at the hospital. Priority process measures include:
- Tracking the types and acceptance of recommendations from prospective audit and feedback interventions, which can identify areas where more education or additional focused interventions might be useful.
- Monitoring of preauthorization interventions by tracking agents that are being requested for certain conditions and ensuring that preauthorization is not creating delays in therapy.
- Monitoring adherence to facility-specific treatment guidelines. If feasible, consider tracking adherence by each prescriber.
Reporting
Antibiotic stewardship programs should provide regular updates to prescribers, pharmacists, nurses, and leadership on process and outcome measures that address both national and local issues, including antibiotic resistance. Antibiotic resistance information should be prepared in collaboration with the hospital’s microbiology lab and infection control and healthcare epidemiology department. The local or state health department’s healthcare infection control and antibiotic resistance program is also an important resource for local information on antibiotic resistant threats. Summary information on antibiotic use and resistance along with antibiotic stewardship program work should be shared regularly with hospital leadership and the hospital board.
Education
Education is a key component of comprehensive efforts to improve hospital antibiotic use; however, education alone is not an effective stewardship intervention. There are many options for providing education on antibiotic use such as didactic presentations, which can be done in formal and informal settings, messaging through posters, flyers and newsletters, or electronic communication to staff groups. Education is most effective when paired with interventions and measurement of outcomes. Case-based education can be especially powerful, so prospective audit with feedback and preauthorization are both good methods to provide education on antibiotic use. This can be especially effective when the feedback is provided in person, for example through handshake stewardship. Some hospitals review de-identified cases with providers to help identify changes in antibiotic therapy that could have been made. Education is most effective when tailored to the action(s) most relevant to the provider group, such as education on community acquired pneumonia guidelines for hospitalists or education on culture techniques for nurses.
CONCLUSION
Antibiotic stewardship evolved continuously, and is now a global drive, with organizations aiming to implement interventions to rationalize antibiotic use in secondary care. The importance of antibiotic stewardship and surveillance of antimicrobial resistance, including the use of molecular biology methods, for maintaining the effectiveness of antibiotics and limiting the spread of multidrug resistant bacterial pathogens. Data on the prevalence of bacterial resistance and the results of molecular genetic analysis of multidrug-resistant strains must form the basis for practical antibiotic stewardship. Antibiotic stewardship has come a long way, but there is a long way to go. With recognition that antibiotic stewardship programs are a core function for medical facilities, we can start to reimagine our place in health care. Success of antibiotic stewardship program dependents on defined leadership and a coordinated multidisciplinary approach to implement improvement strategies, monitor antibiotic prescribing, and educate.
REFERENCES
- Anderson A. Analysing incompliant attitudes towards antibiotic prescription completion in the UK. Journal of Antimicrobial Chemotherapy. 2020 Mar 1;75(3):756-63.
- Majeed J, Sharma K, Acharya PC, Sharma PC. Antiamoebic drugs. In Medicinal Chemistry of Chemotherapeutic Agents 2023 Jan 1 (pp. 397-429). Academic Press.
- Gallagher JC, MacDougall C. Antibiotics simplified. Jones & Bartlett Learning; 2022 Jul 11.
- McMillan M, McDonough J, Angliss M, Buttery J, Saunders L, Mathew SM, Shaw D, Gordon D, Warner MS, Nelson R, Hannah R. Exploring the Health-Related Quality of Life and the Lived Experience of Adolescents following Invasive Meningococcal Disease. In Healthcare 2024 May 24 (Vol. 12, No. 11, p. 1075). MDPI.
- MacDougall C. Antibiotics Simplified. Jones & Bartlett Publishers; 2018.
- Surbhi L, Christine L.T, et al .antimicrobial therapy, National Library of Medicine. Available at: https://www.ncbi.nlm.nih.gov/books/NBK535443/ Published : February 2011 -Accessed: November 25, 2022.
- Rollins KE, Varadhan KK, Neal KR, Lobo DN. Antibiotics versus appendicectomy for the treatment of uncomplicated acute appendicitis: an updated meta?analysis of randomized controlled trials. World journal of surgery. 2016 Oct;40(10):2305-18.
- Flowers CR, Seidenfeld J, Bow EJ, Karten C, Gleason C, Hawley DK, Kuderer NM, Langston AA, Marr KA, Rolston KV, Ramsey SD. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology. 2013 Feb 20;31(6):794-810.
- Alali M, Mayampurath A, Dai Y, Bartlett AH. A prediction model for bacteremia and transfer to intensive care in pediatric and adolescent cancer patients with febrile neutropenia. Scientific Reports. 2022 May 6;12(1):7429.
- Freitas AR, Werner G. Nosocomial Pathogens and Antimicrobial Resistance: Modern Challenges and Future Opportunities. Microorganisms 2023, 11, 1685 [Internet]. 2023.
- General Background: About Antibiotic Resistance (2013) Tufts Now. Available at: https://now.tufts.edu/2013/03/26/new-study-identifies-unique-mechanisms-antibiotic-resistance (Accessed: November 26, 2022).
- Arulkumaran N, Routledge M, Schlebusch S, Lipman J, Conway Morris A. Antimicrobial-associated harm in critical care: a narrative review. Intensive care medicine. 2020 Feb;46:225-35.
- Slama TG, Amin A, Brunton SA, File Jr TM, Milkovich G, Rodvold KA, Sahm DF, Varon J, Weiland Jr D, for Appropriate C. A clinician’s guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. The American journal of medicine. 2005 Jul 1;118(7):1-6.
- Antibiotic Resistance Fast-Growing Problem Worldwide Availablefrom:https://www.voanews.com/a/a-13-2007-02-28-voa33/405785.html Published on November 01,2009: Accessed on November 27 2022.
- Hawkey PM. The growing burden of antimicrobial resistance. Journal of Antimicrobial Chemotherapy. 2008 Sep 1;62(Supplement 1).
- Hand K. Antibiotic Stewardship. Clinical Medicine 2013. 2013 Oct;13(5):499–503
- Clancy C, Shine C, Hennessy M. Spending Review 2022 Hospital Performance: An Analysis of HSE Key Performance Indicators.
- Liao JX, Appaneal HJ, Menon A, Lopes V, LaPlante KL, Caffrey AR. Decreasing antibiotic resistance trends nationally in gram-negative bacteria across United States Veterans Affairs Medical Centers, 2011–2020. Infectious Diseases and Therapy. 2023 Jul;12(7):1835-48.
- Doernberg SB, Abbo LM, Burdette SD, Fishman NO, Goodman EL, Kravitz GR, et al. Essential Resources and Strategies for Antibiotic Stewardship Programs in the Acute Care Setting. Clin Infect Dis. 2018 Sep 28;67(8):1168-74
- Rohde JM, Jacobsen D, Rosenberg DJ. Role of the Hospitalist in Antimicrobial Stewardship: A Review of Work Completed and Description of a Multisite Collaborative. Clinical therapeutics. 2013 Jun 5
- Moody J, Cosgrove SE, Olmsted R, Septimus E, Aureden K, Oriola S, et al. antimicrobial stewardship: a collaborative partnership between infection preventionists and health care epidemiologists. American journal of infection control. 2012 Mar;40(2):94
- Cosgrove SE, Hermsen ED, Rybak MJ, File TM, Jr., Parker SK, Barlam TF. Guidance for the knowledge and skills required for antimicrobial stewardship leaders. Infection control and hospital epidemiology: the official journal of the Society of Hospital Epidemiologists of America. 2014 Dec;35(12):1444-51
- Bessesen MT, Ma A, Clegg D, Fugit RV, Pepe A, Goetz MB, et al. Antimicrobial Stewardship Programs: Comparison of a Program with Infectious Diseases Pharmacist Support to a Program with a Geographic Pharmacist Staffing Model. Hospital pharmacy. 2015 Jun;50(6):477-83.
- MacBrayne CE, Williams MC, Levek C, Child J, Pearce K, Birkholz M, Todd JK, Hurst AL, Parker SK. Sustainability of handshake stewardship: extending a hand is effective years later. Clinical Infectious Diseases. 2020 May 23;70(11):2325-32.
- Pickens CI, Wunderink RG. Principles and practice of antibiotic stewardship in the ICU. Chest. 2019 Jul 1;156(1):163-71


 Nithish Shyamlal V. K.*
Nithish Shyamlal V. K.*


 10.5281/zenodo.14329665
10.5281/zenodo.14329665