Abstract
This study investigates the sustained release behavior of venlafaxine hydrochloride, an antidepressant agent, using Hydroxypropyl Methylcellulose (HPMC) and Sodium Carboxymethyl Cellulose (SCMC) as matrix-forming polymers. The research aimed to develop a once-daily sustained-release tablet formulation of venlafaxine hydrochloride. All formulations were prepared by direct compression and evaluated for various physical parameters, including weight uniformity, active ingredient content, friability, hardness, thickness, and in vitro dissolution profile. Dissolution studies were conducted using a USP Type II apparatus in 900 ml of distilled water as the dissolution medium. The release kinetics were analyzed using zero-order, Higuchi’s square root, and Peppas' exponential equations. All formulations complied with pharmacopoeial standards. Among them, formulation F7 demonstrated the most effective sustained drug release, with 99.73% release over 12 hours. The regression coefficient (R²) for zero-order kinetics was 0.993, and the slope of the Peppas model was 0.981 for F7, indicating that the HPMC and SCMC matrix system successfully prolonged the release of venlafaxine hydrochloride.
Keywords
Sustained Release, Matrix Tablets, Venlafaxine Hydrochloride, Hydroxypropyl Methylcellulose (HPMC), Sodium Carboxymethyl Cellulose (SCMC) ,Drug Release Kinetics, Zero-Order Release, Diffusion-Controlled Release, Dissolution Mechanism, In Vitro Dissolution Study Polymer Drug Delivery System Direct Compression Method Peppas Model Controlled Drug Release Pharmaceutical Formulation,Gel Matrix Formation,Regulation of Drug Release,Pharmacopoeial Standards,Swelling Behavior of Polymers,Kinetic Modeling of Drug Release
Introduction
The pharmaceutical industry has long recognized the benefits of administering a single dose of a drug that is released gradually over an extended period, rather than requiring multiple doses throughout the day. This approach not only helps in maintaining a near-constant or uniform drug concentration in the bloodstream, but also improves patient compliance and enhances the clinical effectiveness of the drug. Sustained-release (SR) formulations, such as matrix tablets, have revolutionized the field of novel drug delivery systems (NDDS) by offering a simpler and more efficient alternative to traditional dosage forms. Matrix tablets, in particular, have gained significant attention because they eliminate the need for complex manufacturing processes like coating and pelletization. The rate at which the drug is released from these tablets is primarily governed by the type and proportion of polymer used in their formulation. Hydrophilic polymer matrices are especially common for developing sustained-release dosage forms due to their ability to control drug release effectively.With the increasing complexity and high costs associated with bringing new drugs to market, there is a growing emphasis on the development of sustained-release or controlled-release drug delivery systems for existing drugs. Matrix systems are widely used for this purpose, as they allow for the prolonged and controlled release of a drug that is either dissolved or dispersed within the matrix. A matrix is essentially a well-mixed composite of one or more drugs with a gelling agent, typically hydrophilic polymers, which controls the release rate. Sustained-release formulations offer the advantage of maintaining therapeutic drug concentrations in the bloodstream over an extended period, which can significantly improve patient adherence to treatment regimens. Various SR oral dosage forms have been developed, including membrane-controlled systems, matrices made with water-soluble or insoluble polymers, waxes, and osmotic systems. In recent years, substantial research efforts have focused on designing SR systems for drugs with poor water solubility, further expanding the possibilities of controlled drug delivery.

Figure. 1: Chemical structure of Venlafaxine Hydrochloride
Venlafaxine hydrochloride is an antidepressant intended for oral administration, known for its high absorption and extensive metabolism in the liver. The objective of this study is to formulate and evaluate sustained-release matrix tablets of venlafaxine hydrochloride, using varying proportions of polymers to achieve controlled drug release over an extended period.
MATERIAL AND METHODS:
Venlafaxine Hydrochloride was generously provided as a gift sample by Lupin Pharmaceuticals, Aurangabad, for use in this study. All other chemicals and reagents employed in the formulation and analysis were of analytical grade, ensuring high purity and quality for the experimental procedures. These chemicals were sourced from various suppliers, as detailed in Table 1, and were used without further modification or purification. This ensured consistency and reliability in the experimental outcomes, particularly in the formulation of sustained-release matrix tablets.
Table.1.Procurement of drug and Excipients.

METHODS:
sustained-release matrix tablets of Venlafaxine Hydrochloride were prepared using the direct compression method, a widely adopted and efficient technique in tablet manufacturing. In this process, different ratios of drug to polymer were utilized to achieve the desired sustained-release effect. The polymers selected for controlling the drug release were Hydroxypropyl Methylcellulose (HPMC K-100M) and Sodium Carboxymethyl Cellulose (SCMC), both of which are well-known for their effectiveness in forming hydrophilic matrices that regulate the release of active ingredients over an extended period. The drug and polymers, along with other excipients, were carefully weighed and mixed uniformly to ensure proper distribution of the active ingredient. The mixture was then subjected to direct compression, where the powder blend was compressed into tablets using a tablet press without the need for additional granulation steps, making the process more straightforward and cost-effective. Table 2 outlines the detailed formulation of the sustained-release matrix tablets, including the specific proportions of Venlafaxine Hydrochloride, HPMC K-100M, SCMC, and other excipients used in each formulation. These variations in drug-to-polymer ratios allowed for the evaluation of the impact of polymer concentration on the release kinetics of Venlafaxine Hydrochloride, enabling the optimization of the formulation for sustained drug release.
Table 2: Formulation table
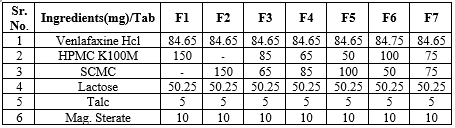
EVALUATION OF TABLETS:
Organoleptic Evaluation:
Organoleptic evaluation involves the assessment of sensory attributes such as color, odor, shape, taste, and tactile properties (touch and texture) of the tablets. These characteristics provide valuable insights into the identity, purity, and overall quality of the material. Key parameters, including solubility, swelling properties, pH, and viscosity of the polymers used, are evaluated. Additionally, physical properties such as melting point, bulk density, tapped density, Carr’s index, Hausner’s ratio, and angle of repose are determined to assess the material's flowability and compressibility.
Physical Characterization of Matrix Tablets (Post-compression Parameters):
Post-compressional parameters like hardness, friability, weight variation, and content uniformity are essential for ensuring the quality and performance of the matrix tablets. These characteristics are evaluated following the procedures outlined in the Indian Pharmacopoeia.
Hardness and Friability:
Tablet hardness, which reflects the force required to break a tablet under diametric compression, is measured using a Monsanto hardness tester on 10 tablets. Friability is tested using a Labline friability tester by rotating 20 tablets for 4 minutes at 25 rpm. The friability test assesses the tablet's resistance to chipping and abrasion during handling and transport.
Appearance and Thickness:
The tablets are visually inspected for color, odor, and any physical defects such as chips, cracks, or irregularities in surface texture. Tablet thickness is measured using a Vernier caliper, with 20 tablets from each batch used for this assessment. The average thickness is calculated to ensure uniformity across the batch.
Weight Variation:
Weight variation is determined by weighing 20 randomly selected tablets from each batch using a digital electronic balance (Citizen). The average weight is calculated, and each tablet's individual weight is compared against the average, with the weight variation expressed as a percentage deviation. This test ensures consistency in tablet weight, which is crucial for dose uniformity.
Drug Content (Content Uniformity):
The drug content of each batch is determined in triplicate to ensure uniform distribution of the active pharmaceutical ingredient (API). Twenty tablets are selected, weighed, and finely powdered. A precise quantity of the powder is dissolved in water, filtered, and analyzed using UV spectrophotometry (Shimadzu 1800) after appropriate dilutions. This test ensures each tablet contains the correct amount of API.
In Vitro Dissolution Study and Kinetic Modeling of Drug Release:
The release rate of venlafaxine hydrochloride from the sustained-release matrix tablets is studied over 12 hours using the USP Type II apparatus (Paddle method) at a rotation speed of 50 rpm. The dissolution medium used is 900 ml of distilled water maintained at a temperature of 37°C ± 0.5°C. Dissolution parameters are outlined in Table 3. The dissolution data are used to model the drug release kinetics, providing insight into the release mechanism and rate-controlling factors.
Table 3: Dissolution parameter:
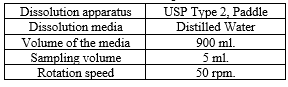
Kinetic Modeling of Drug Release:
To determine the mechanism of drug release from the hydrophilic matrix, all seven formulations of Venlafaxine Hydrochloride matrix tablets were subjected to in-vitro release studies. The data obtained from these studies were analyzed using various kinetic models to understand the drug release behavior and the rate-controlling mechanisms. The following models were applied to evaluate the release kinetics:
1.Cumulative Percent Drug Released vs. Time (Zero-Order Kinetics):
In zero-order kinetics, the rate of drug release is constant over time, meaning the same amount of drug is released per unit of time regardless of its concentration in the matrix. This model is particularly relevant for sustained-release systems, where the goal is to maintain a consistent drug release profile over an extended period. The cumulative percent of drug released is plotted against time to determine if the release follows a zero-order process.
2.Log Cumulative Percent Drug Retained vs. Time (First-Order Kinetics):
First-order kinetics describes a release process where the rate of drug release is directly proportional to the amount of drug remaining in the matrix. In this model, the log of the cumulative percent drug retained (or remaining in the matrix) is plotted against time. A linear relationship would indicate first-order kinetics, suggesting that the drug release rate slows down as the drug concentration decreases over time.
3.Log Cumulative Percent Drug Released vs. Square Root of Time (Higuchi’s Classical Diffusion Model):
Higuchi’s model is based on the assumption that drug release from the matrix occurs primarily through diffusion. This model is particularly suited for systems where the drug is dispersed uniformly within a polymer matrix. A plot of the log of cumulative percent drug released versus the square root of time is constructed to assess whether the drug release follows a diffusion-controlled mechanism. A linear plot indicates that the release is governed by Fickian diffusion.
4.Log of Cumulative Percent Drug Released vs. Log Time (Peppas Exponential Equation):
The Peppas model, or the Korsmeyer-Peppas equation, is used to analyze drug release from polymeric systems when the mechanism is not well understood or when multiple release processes (such as diffusion and erosion) may be involved. A plot of the log of cumulative percent drug released versus log time is generated to determine the release exponent (n), which provides insight into the release mechanism:
- If n=0.5n = 0.5n=0.5, the release follows Fickian diffusion.
- If 0.5
- If n=1.0n = 1.0n=1.0, it suggests zero-order kinetics, often indicative of case-II transport, where drug release is dominated by polymer relaxation or erosion.
These kinetic models help in understanding the underlying mechanisms of drug release and in optimizing the formulation for desired release characteristics. By analyzing the in-vitro release data using these models, it is possible to determine whether the drug release is diffusion-controlled, erosion-controlled, or follows a combination of mechanisms.
RESULTS AND DISCUSSION:
the formultion batches of Venlafaxine Hydrochloride sustain release matrix tablets evaluates and the resulta are mentioned below according to methods carried out.

Figure. 2: UV Spectra of Venlafaxine Hydrochloride

Figure. 3: Calibration curve of Venlafaxine Hydrochloride in Distilled Water.

Figure. 4: FTIR spectra of Venlafaxine Hydrochloride.

Figure 5: FTIR spectra of hydroxy propyl methyl cellulose, Sodium carboxy methyl cellulose and Venlafaxine Hydrochloride.
Interpretation of FTIR spectra of Venlafaxine Hydrochloride :
Interpretation of FTIR spectra has been done based on chemical structure of Venlafaxine Hydrochloride (Figure. 1) and in spectra the walength found at 3322.83= 0-H (stretch), 2586,2942= C-H (stretch), 769 = C-H (bend), 1612= C-C (stretch), 1153 = C-N (stretch) (Figure. 4& 5).
Table 4: Physical characteristics of prepared tablet blend (pre-compressional):

Table 5: Evaluation of prepared tablets

Table 6: In-vitro drug release study (Cumulative % Drug Release).

Table 7: Drug release kinetics of formulation (R-Value)

In Vitro Dissolution Study and Kinetic Modeling of Drug Release:The cumulative percent drug release data for all formulations are presented in Table 6. A plot of cumulative percentage release versus time for the sustained-release matrix tablets revealed a slow and controlled drug release profile across the formulations. The initial release rate during the first hour ranged between 13.25% and 19.97%, depending on the polymer concentration, indicating an absence of burst release. The drug release was more controlled in the later stages, particularly in formulations containing a higher proportion of polymer, which contributed to prolonged release.
The release mechanism varied across formulations:
- Formulation F1 followed a diffusion-controlled release mechanism.
- Formulation F2 exhibited a dissolution-controlled release mechanism.
- Formulations F3 to F7 showed a combination of both diffusion and dissolution mechanisms, indicating a more complex release behavior.
Formulation F1, which contained 50% HPMC as the polymer, released 60.99% of the drug within 12 hours. This release rate was not within the desirable range for a sustained-release formulation. In contrast, Formulation F2, containing 50% SCMC, demonstrated a 97.67% drug release within 10 hours, indicating a more suitable release profile. Formulations F3 through F7 utilized a combination of HPMC and SCMC at a total polymer concentration of 50%. Among these:
- Formulation F5, with 16.66% HPMC and 33.33% SCMC, released 91.24% of the drug over 12 hours.
- Formulation F6, with 33.33% HPMC and 16.66% SCMC, exhibited 98.41% drug release over 12 hours.
- Formulation F7, containing 25% HPMC and 25% SCMC, showed the most desirable release profile, achieving 99.73% drug release within 12 hours.
The study demonstrated that the drug release rate increased as the proportion of polymers decreased. Additionally, formulations with a combination of diffusion and dissolution mechanisms exhibited more controlled and sustained drug release, falling within the desirable limits for extended-release tablets. The matrix tablets swelled to varying degrees, forming gel-like structures during the dissolution process, which also influenced the release behavior based on the polymer concentrations used. To further investigate the release mechanisms, the dissolution data were fitted to different kinetic models, including zero-order, first-order, and Higuchi’s square root of time models, as well as the Peppas equation. As shown in Table 7, Formulation F1 followed the Peppas release model, suggesting a complex release mechanism involving diffusion. Meanwhile, Formulations F2 through F7 demonstrated a zero-order release pattern, indicating a constant drug release rate over time, which is ideal for sustained-release formulations.

Figure 6: Zero order release profile of all formulation.
CONCLUSION:
The results of the present study demonstrate that Hydroxypropyl Methylcellulose (HPMC) and Sodium Carboxymethyl Cellulose (SCMC) are effective as drug release retardants. The drug release from the sustained-release matrix tablets was dependent on the proportion of these polymers and was successfully extended over a 12-hour period. The release mechanism primarily followed zero-order kinetics, as evidenced by the in-vitro release data. In kinetic modeling, the release data for Formulation F7 were best fit by the zero-order model, with a high regression coefficient (R?2; = 0.9932), indicating a consistent release rate over time. This result aligns with established pharmacopoeias and standard references. Thus, Formulation F7, prepared by direct compression, was identified as the optimized or ideal formulation for sustained release. The formation of a thick gel structure due to the presence of HPMC and SCMC in the tablet matrix effectively delays the drug release, thereby ensuring prolonged drug activity. This approach enhances the sustained-release profile of Venlafaxine Hydrochloride, which can lead to improved patient compliance. Physical characterization of all formulations confirmed that they met the specifications outlined in official standards, affirming their quality and suitability for sustained-release applications.
REFERENCE:
- Patel, M., & Patel, B. (2014). "Hydroxypropyl Methylcellulose: A Review of its Use in Drug Delivery Systems." Journal of Pharmaceutical Sciences and Research, 6(6), 404-410.
- Kaur, G., & Gupta, V. (2018). "Sodium Carboxymethyl Cellulose: A Review of its Applications in Drug Delivery Systems." International Journal of Pharmaceutical Sciences and Research, 9(4), 1547-1553.
- Siepmann, J., & Siepmann, F. (2008). "Mathematical Modeling of Drug Delivery." International Journal of Pharmaceutics, 364(2), 328-343. doi:10.1016/j.ijpharm.2008.05.025.
- Peppas, N. A., & Freeman, B. E. (1980). "Mechanisms of Solute Release from Porous Hydrophilic Matrices." Journal of Applied Polymer Science, 24(7), 2883-2890. doi:10.1002/app.1980.070240706.
- Higuchi, T. (1963). "Mechanism of Sustained Action Medication: A Theoretical Analysis of the Rate of Release of Solid Drugs Dispersed in Solid Matrices." Journal of Pharmaceutical Sciences, 52(12), 1145-1149. doi:10.1002/jps.2600521205
- Korsmeyer, R. W., Gurny, R., Docler, J., & Peppas, N. A. (1983). "Mechanisms of Drug Release from Solid Matrices." International Journal of Pharmaceutics, 15(1), 25-35. doi:10.1016/0378-5173(83)90064-0
- Hammami, M., & Ghorab, M. (2019). "Hydroxypropyl Methylcellulose-Based Hydrogels for Controlled Drug Release: Preparation, Characterization, and Release Studies." Journal of Drug Delivery Science and Technology, 52, 524-536. doi:10.1016/j.jddst.2019.05.004
- Yadav, N., & Yadav, S. K. (2019). "Sodium Carboxymethyl Cellulose-Based Drug Delivery Systems: A Review." Journal of Controlled Release, 303, 284-293. doi:10.1016/j.jconrel.2019.04.031
- Ritger, P. L., & Peppas, N. A. (1987). "A Simple Equation for Describing Heterogeneous Drug Release." Journal of Controlled Release, 5(1), 23-36. doi:10.1016/0168-3659(87)90046-7
- Miller, N., & Rose, G. A. (2018). "A Comprehensive Review of Kinetic Models for Controlled Drug Delivery Systems." Pharmaceutics, 10(4), 234. doi:10.3390/pharmaceutics10040234
- Sharma, S., & Bhardwaj, T. R. (2016). "Sustained Release Matrix Tablets: Design, Development, and Evaluation." European Journal of Pharmaceutical Sciences, 88, 117-130. doi:10.1016/j.ejps.2016.03.026
- Chien, Y. W. (1992). "Novel Drug Delivery Systems." Marcel Dekker Inc.
- European Pharmacopoeia. (2017). "European Pharmacopoeia 9th Edition." European Directorate for the Quality of Medicines & HealthCare (EDQM).
- Indian Pharmacopoeia. (2018). "Indian Pharmacopoeia 2018." The Indian Pharmacopoeia Commission.
- Bhowmik, D., & Dey, S. (2020). "A Review on Matrix Tablets: Design and Recent Advances." Journal of Pharmaceutical Innovation, 15(2), 229-244. doi:10.1007/s12247-020-09417-6
- Ganguly, K., & Das, S. (2017). "Controlled and Sustained Release Drug Delivery Systems: An Overview." American Journal of Phytomedicine and Clinical Therapeutics, 4(1), 8-15.
- Khan, Y., & Kumar, V. (2020). "Hydrogel-Based Drug Delivery Systems: A Review of the Current Status and Future Perspectives." Drug Development and Industrial Pharmacy, 46(6), 951-964. doi:10.1080/03639045.2020.1752170
- Wang, H., & Wang, W. (2018). "Advanced Hydrogels for Drug Delivery Applications." Materials Science and Engineering: C, 93, 105-118. doi:10.1016/j.msec.2018.08.009.
- Bodkhe A., Zurao P., Chandewar A, Jaiswal S., Designing and evaluation of extended release tablet of Venlafaxine hydrochloride using hydrophobic matrix.Der Pharmacia Lettre. 2010; 2 (1): 329-335.
- Goyal K., Karani N., Pethe A., Formulation and Evaluation of Once-daily Sustained ReleaseVenlafaxine hydrochloride Tablet using Hydrophilic matrix. Journal of Pharmacy Research. 2009; 2(8):1287-1291.
- Wan Sia Henga Paul,Lai Wah Chana, Michael G. Easterbrookb, Xiaoman Lia., Investigation of the influence of mean HPMC particle size and number of polymer particles on the release of aspirin from swellable hydrophilic matrix tablets. J Controlled Release.2001; (76):39–49.


 Prasanna Babu Racheeti*
Prasanna Babu Racheeti*













 10.5281/zenodo.13910760
10.5281/zenodo.13910760