Abstract
An accurate RP-HPLC method with a UV detector was developed for analyzing tinidazole in pharmaceutical formulations. Zorbax SB C8 column (150 mm × 4.6 mm, 3.5 ?m) was used with a mobile phase of Water and Isopropyl alcohol. Detection at 310?nm, with a 10?min run time and column temperature maintained at 30°C. Tinidazole calibration curve showed good linearity (22.5–135.0??g/mL, r = 0. 9998). Method validated as suitable for drug determination. The retention time of tinidazole was 2.2 minutes. Validation of the Method for the study included specificity, linearity, precision, and accuracy. This method is suitable for determining drug content in six different tablets. The new method uses green analytical HPLC to analyze drug formulations for identification and quantitative results. It utilizes an efficient column, reduces mobile phase usage, and replaces hazardous solvents with eco-friendly alternatives like water-isopropyl mixtures. The analytical method was developed and validated as per ICH guidelines.
Keywords
HPLC, Method validation, Tinidazole tablet dosage form.
Introduction
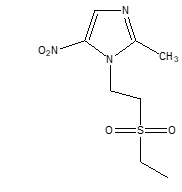
Tinidazole, a synthetic imidazole derivative, is commonly used to treat protozoal infections like trichomoniasis, giardiasis, and amoebiasis. (1) It is effective against organisms like Trichomonas vaginalis and Entamoeba histolytica. Although it is the preferred treatment for intestinal amoebiasis, its current oral tablet form leads to systemic side effects. (2) There is a strong demand for a controlled delivery system to target the colon with maximum tinidazole. (3) The Chemical Formula of Tinidazole is C8H13N3O4S. Molecular weight is 247.273 g/mol
(figure 1)
MECHANISM OF ACTION:
Tinidazole undergoes significant human metabolism before excretion, involving oxidation, hydroxylation, and conjugation. (4) It is the primary drug-related compound in plasma post-treatment, mainly metabolized by CYP3A4 without inhibiting other enzyme activities. (5,6)
Tinidazole has a plasma half-life of 12-14 hours, excreted by the liver and kidneys. 20-25% in urine, 12% in feces. (7) Tinidazole is an antiprotozoal and antibacterial agent. It generates a nitro-radical that may be responsible for its antiprotozoal activity. It also causes DNA damage in bacterial and mammalian cells. The mechanism against Giardia and Entamoeba species is unclear. (8) Various analytical methods have been developed for determining Tinidazole, including HPTLC, UV, Micellar HPLC, UV derivative, RP-HPLC, and hydrolysis studies. (9-17) Existing RP-HPLC methods often use volatile buffers and high volumes of organic solvents. The goal of this study is to develop a more environmentally friendly method using green solvents, in line with ICH guidelines. This method will be easier to use and have a reduced impact on society.
MATERIALS AND METHODS
Chemicals and Reagents
Analytical reagent-grade chemicals were used without purification. Tinidazole drug substances were obtained commercially and drug products from marked samples. HPLC grade Isopropyl alcohol was sourced from Finar Chemicals Ltd.
Instrumentation and chromatographic conditions
An HPLC system with Waters 2695 and Empower 3 software was used for analysis, including a quaternary gradient pump, autosampler, UV and PDA detector, and column oven. The signals were monitored and integrated using Waters Empower-3 software. Zorbax SB C8 (150 mm x 4.6 mm x 3.5 ?) column was utilized for chromatographic analysis. Separation was achieved with a mobile phase of Isopropyl alcohol and water (20:80) v/v at a flow rate of 1. 0 ml/min for 5 minutes. UV detector at 310 nm wavelength was used, with a column temperature of 30 °C and injection volume of 10 ?l. The mobile phase was filtered through a 0. 45 mm filter before use.
Preparation of solutions
Preparation of mobile phase
Mix water and Isopropyl Alcohol in the ratio of (80:20 v/v). and degassed.
Diluent/ Blank:
Mix water and Isopropyl Alcohol in the ratio of (80:20 v/v).
Preparation of the standard solution
Accurately Weighed and transferred 30 mg of of Tinidazole working standard into a cleaned and dried 20 mL volumetric flask, a small portion of diluent was added and sonicated to dissolve completely all components and marked with diluent. Further, 3 mL of the above-prepared stock solution was precisely transferred to a clean and dried volumetric flask of 50 mL capacity, marked with the diluent. (90 ppm Tinidazole)
Preparation of sample solution (Assay of Dosage form)
Weighed accurately 10 tablets and determined the average weight. Transfer it to a mortar and pestle and crush it to form a fine powder. Accurately weighed and transferred crushed powder equivalent to 45 mg Tinidazole to a dry and clean volumetric flack of 500 ml capacity. volumetric flask. Add to it about 350 mL of diluent, sonicate it for up to 30 mins to dissolve all the components completely, and make volume up to the mark with the diluent and mix well. Allow to cool at room temperature for a few minutes and filter through a 0.45-micron Injection filter. (90 ppm of Tinidazole System suitability test parameters
System Suitability Test (SST) assesses parameters such as tailing factor, plate count, and retention time of active ingredients. A Zorbax SB C8 column (150 mm x 4. 6 mm x 3. 5 ?) was used, with the column equilibrated using mobile phase for 30 min at a flow rate of 1.0mL/min. Chromatographic separation involved injecting 10. 0 ?l of the solution with a mobile phase ratio of isopropyl alcohol and water (20:80) at a flow rate of 1.0mL/min, yielding results on tailing factor, retention time, and plate numbers. and details of the developed method are shown in Table 1.
Forced degradation study
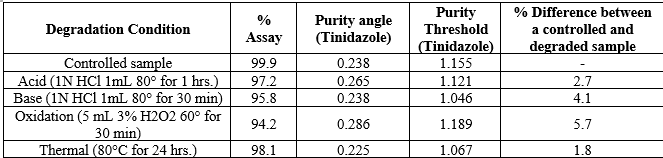
A forced degradation study tests the stability of a chemical or pharmaceutical product under harsh conditions like hydrolysis, oxidation, humidity, temperature, and photolytic stress. This study explores the degradation pathways of drug substances and products. In this specific case, the API, placebo, and sample underwent acid, base, oxidation, thermal, and photolytic conditions to detect any peaks linked to declared excipients. The API and sample were treated with 1N Hydrochloric acid at 80°C for 1 hour for acidic degradation. For base degradation, the sample was exposed to 1N Sodium Hydroxide at 80°C for 30 minutes. Under oxidizing conditions, the sample was treated with 3% Hydrogen peroxide at 60°C for 30 minutes. Thermal degradation involved exposing the sample to 80°C for 24 hours, while photolytic degradation was carried out in a photostability chamber with significant light exposure.
Method validation
The method was validated based on specificity, linearity, sensitivity, accuracy, precision, and reproducibility as per the International Conference on Harmonization guidelines. (18) Specificity was confirmed by comparing chromatograms of various samples to ensure no interfering peaks. Linearity was demonstrated using tinidazole working solutions at different concentrations. Sensitivity was determined by calculating the limit of detection and the limit of quantification. Accuracy was evaluated by comparing the percent analyte recovery at various concentration levels. Precision was assessed through various parameters such as repeatability of injection, intraday and interday repeatability, and reproducibility. Injection repeatability and percent relative standard deviation were calculated for peak area measurements. Intraday and interday variations were also studied. These validation parameters ensure the reliability and robustness of the method for analyzing tinidazole compounds in pharmaceutical formulation.
RESULT AND DISCUSSION
The purpose of method validation was to ensure that the method is fit for its purpose as mentioned in ICH guidelines. The above method was validated to establish the performance characteristics of a method (expressed in terms of analytical parameters) to meet the requirements for the intended application of the method. Fluconazole and Tinidazole showed maximum absorbance at 210 nm.
Specificity:
No interference is observed in blank, standard, and sample chromatograms compared with each other. The peak purity of the standard and sample solution met the acceptance criteria i.e. purity angle is less than the purity threshold. The blank chromatogram of the assay preparation of Tinidazole is shown in Figure 2. The specificity parameter of the developed method is accomplished by separating the injection of the standard and sample. Spectral purities of the Tinidazole peaks were evaluated for the interference of the tablet excipient. Figures 3 show the chromatogram of standard preparation, Figures 4 show the chromatogram of sample preparation.
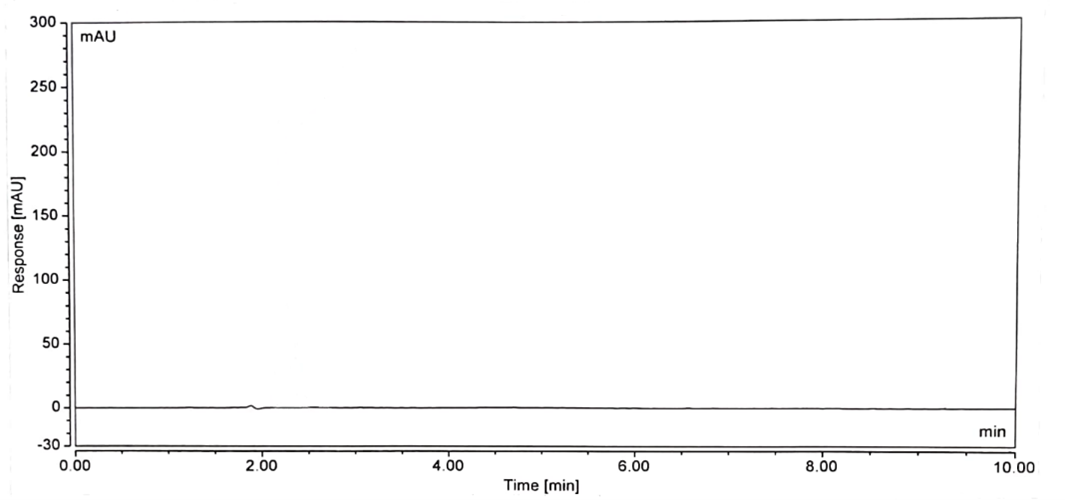
Figure 2: Blank Chromatogram associated assay of Tinidazole
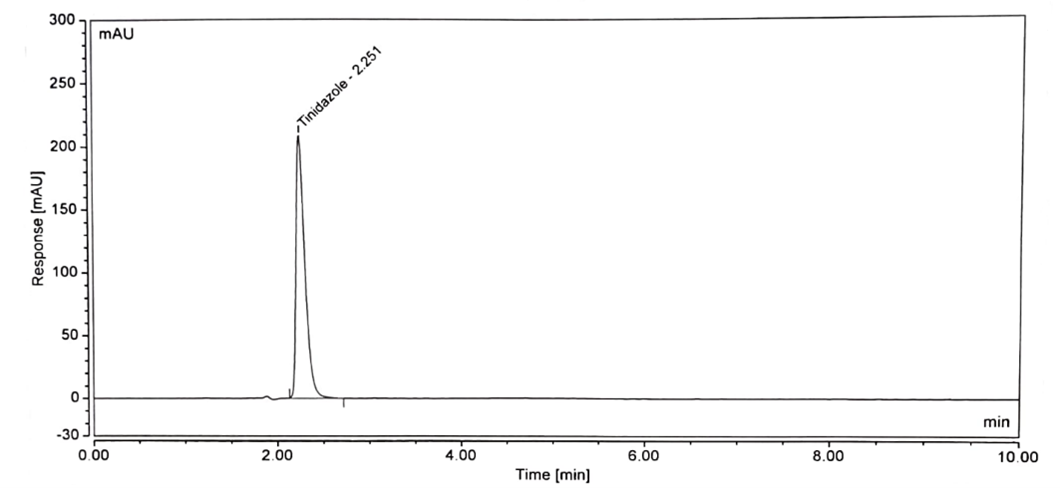
Figure 3: Standard Chromatogram of Tinidazole
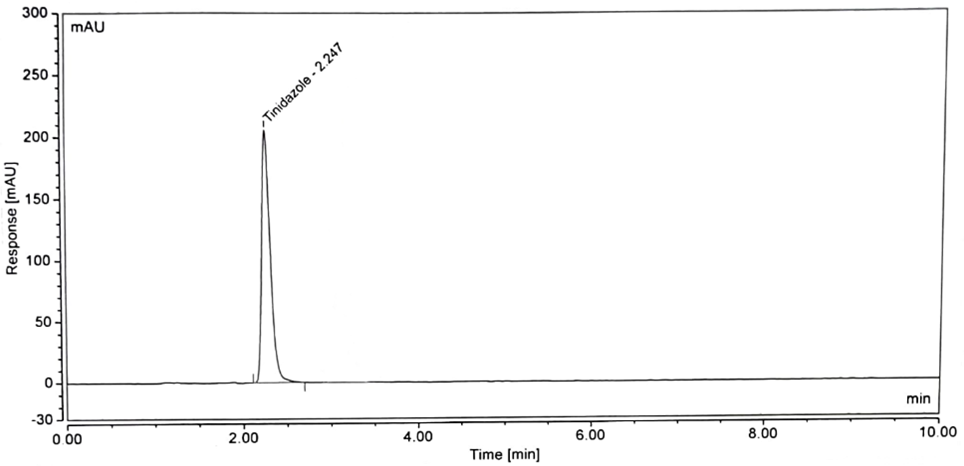
Figure 4: Sample Chromatogram of Tinidazole
Forced Degradation
No interference is observed in blank, untreated samples and stress sample chromatograms compared with each other. The peak purity of the standard and sample solution met the acceptance criteria i.e. purity angle is less than the purity threshold. For acidic degradation, the samples were treated with 1 mL of 5 N Hydrochloric acid at 80° for 1 hour. For base degradation, the sample was with 1 mL of 1 N Sodium Hydroxide at 80° for 30 min. For degradation under oxidizing conditions, the sample was treated with 3% Hydrogen peroxide 60° for 30 minutes. For thermal degradation, the sample was exposed in the oven at 80°C or 24 hours. For photolytic degradation, the sample was exposed in a photostability chamber to light providing an overall illumination of not less than 1.2 million lux hours. The observed results of forced degradation as shown in
Table 2.
Linearity
Six concentrations for Tinidazole were prepared and the linearity graph was plotted using concentration versus peak area as shown in Figure 5.
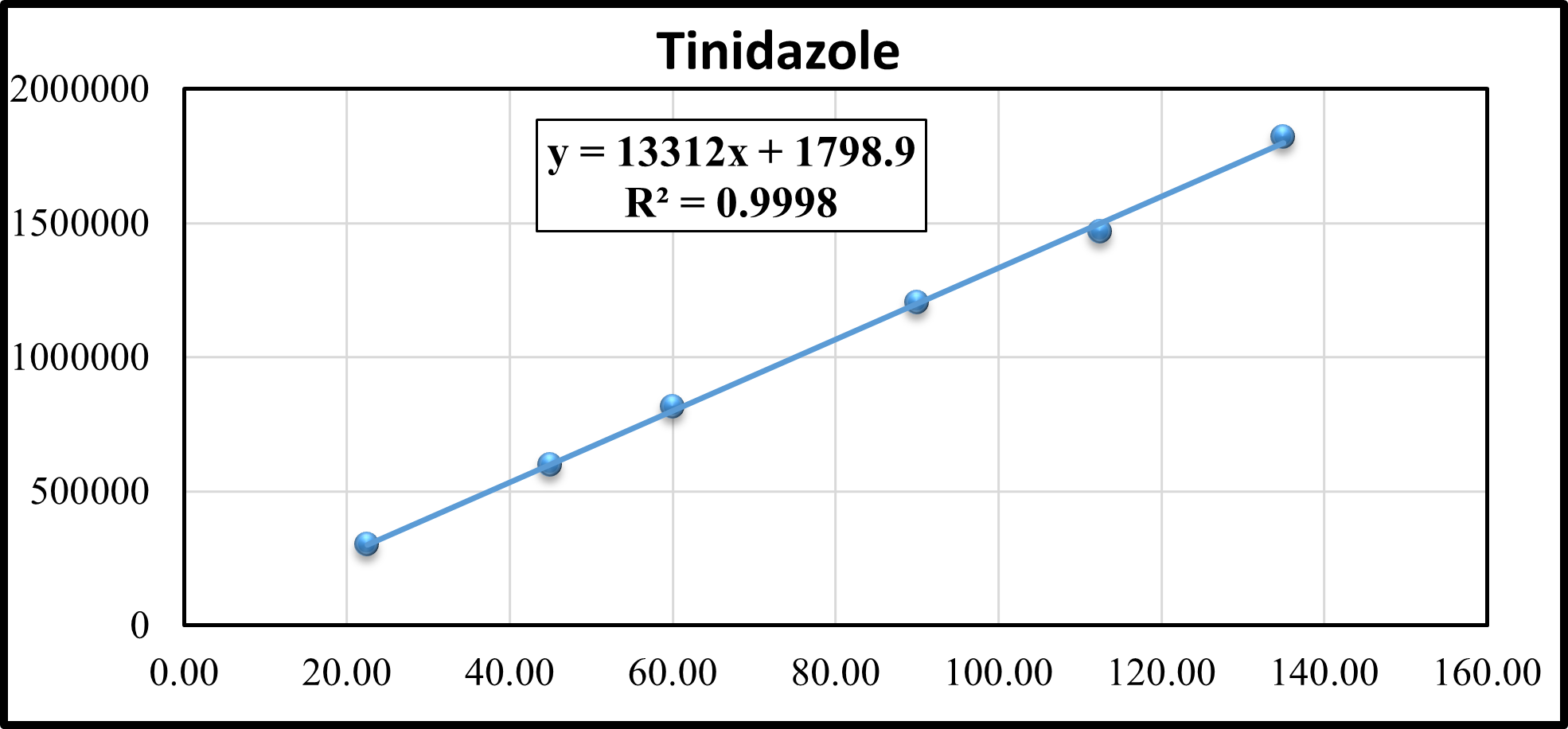
Figure 5: Linearity plot of Tinidazole
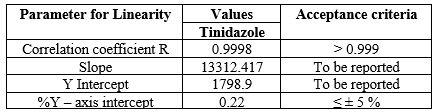
The correlation coefficient is 1.000 The analytical method was considered to be linear in the range of 22.5 – 135 ?g/ml for Tinidazole. The linearity data of Tinidazole is shown in Table 3.
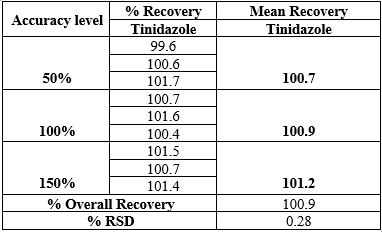
Accuracy
The method was considered to be accurate as the % individual recovery and the % mean recovery was within the acceptance criteria of 98 – 102%. The percentage recovery of Tinidazole was tabulated in Table 4.
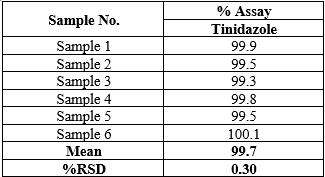
Precision
Method precision was determined by analyzing six independent assays of the sample and finding out the % RSD was well within the acceptance limit of ? 2%. Method precision result was shown in Table 5.
Intermediate Precision
Intermediate precision was determined by comparison of percentage assay of Tinidazole by different analysts and different days. Table 6

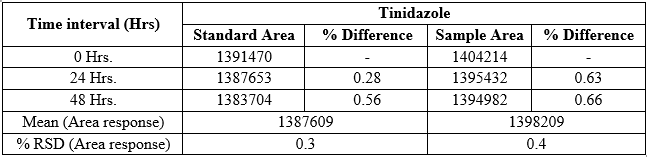
Solution stability
The stability of the standard and sample solutions was examined by analyzing the solutions stored at room temperature. The % RSD for each of the analyte peak areas was calculated and was found to be below 2.0% or the % difference between the initial and final area should not be more than 2.0. The observed solution stability data was associated with Tinidazole as shown in Table 7.
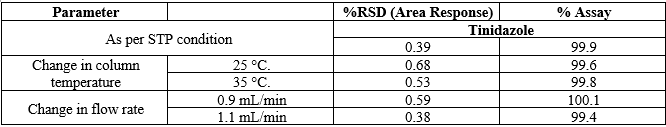
Robustness
The method was found to be robust as system suitability criteria were achieved for all the robustness parameters tested. The effect of small deliberate variations of analytical parameters is investigated to understand and establish the suitability of the method in the face of variability arising from the routine analysis. The results are shown in Table 8.
DISCUSSION
This study focused on determining co-formulated tinidazole in pharmaceutical compounds. Tinidazole was found to be soluble in organic solvents like methanol, Isopropyl alcohol, and acetonitrile. Using a mobile phase as an extraction reagent resulted in minimal solvent usage and better separation. Various mobile phase systems were tested, with a mixture of Isopropyl alcohol and water in a ratio of 20:80 under an isocratic elution system selected as the optimal mobile phase for baseline separation, symmetrical peaks, and shorter retention time. A UV detector set at 310 nm for tinidazole, with elution completed in less than 10 minutes. The method was validated following ICH guidelines for specificity, linearity, sensitivity, accuracy, precision, and reproducibility, showing that the method accurately identifies and determines each analyte without interference, within desired ranges.
CONCLUSION
The RP-HPLC method has been developed for determining Tinidazole in pharmaceutical drugs and dosage forms. This user-friendly, simple, and rapid method uses an isocratic reverse-phase high-performance liquid chromatographic (HPLC) approach. The method has been validated for its accuracy, accuracy, speed, and reproducibility. It is highly advantageous for routine analysis of Tinidazole in pharmaceutical tablet dosage forms due to its simplicity and speed.
ACKNOWLEDGMENT
One of the authors, Mr. Pranay P. More thankful to Chemclues Life Science Pvt. Ltd. for providing lab facilities, Instruments, and API during the phase of work.
AUTHOR INFORMATION
Corresponding Author:
Pranay Pravin More, Department of Chemistry, Maharshi Dayanand College of Arts, Science and Commerce, Parel, Mumbai.
E-mail: more.pranay1989@gmail.com
First Author name: Dr. Mrunalini D. Kulkarni
Email: mrunalresearch@gmail.com
CONFLICTS OF INTEREST
The authors announce no competing financial interest.
REFERENCE
- Sawyer PR, Brogden RN, Pinder RM, Speight TM, and Avery GS. Tinidazole: a review of its antiprotozoal activity and therapeutic efficacy. Drugs. 1976;11(6):423-40.
- Samuel L Stanley. Trends in Parasitology 2001;17(6):280-285.
- Naikwade SR, Kulkarni PP, Jathar SR and Bajaj AN. DARU J of Pharmaceutical Sciences. 2008;16(3):119-127.
- Monika Bakshi, Saranjit Singh, HPLC and LC–MS studies on stress degradation behaviour of tinidazole and development of a validated specific stability-indicating HPLC assay method, JBPA, Volume 34, Issue 1, 2004, Pages 11-18,
- Li Qun Ouyang, Hai Long Wu, Ya Juan Liu, Jian Yue Wang, Yong Jie Yu, Hong Yan Zou, Ru Qin Yu, Simultaneous determination of metronidazole and tinidazole in plasma by using HPLC-DAD coupled with second-order calibration, CCL, Volume 21, Issue 10, 2010, Pages 1223-1226,
- Joseph-Horne T, Hollomon DW: Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett. Apr 15, (1997), 149(2):141-9.
- Pharmacokinetics and Pharmacodynamics of the Nitroimidazole Antimicrobials. Clinical Pharmacokinetics, May 1999;36(5),353-373(21).
- Carmine AA, Brogden RN, Heel RC et al. Tinidazole in anaerobic infections: a review of its antibacterial activity, pharmacological properties, and therapeutic efficacy. Drugs. 1982;24:85-117.
- Amira H. Kamal & Samah F. El-Malla (2018) Factor Space Analysis and Dual Wavelength Spectrophotometric Methods for Simultaneous Determination of Norfloxacin and Tinidazole in Tablet, Analytical Chemistry Letters, 8:3, 393-404
- Pant, M., Dadare, K., Khatri, N. (2012). Application of UV spectrophotometric methods for simultaneous estimation of norfloxacin and tinidazole in bulk and tablet dosage forms. Der Pharma Chemica. 4: 1041-1046.
- Mohammad, M., Zawilla, N., El-Anwar, F., Aly, S. (2007). Stability indicating methods for the determination of norfloxacin in mixture with tinidazole. Chem. Pharm. Bull. 55: 1-6.
- Walash, M., Sharaf El Din, M., El-Enany, N., Eid, M., Shalan, S. (2011). Micellar liquid chromatographic method for the simultaneous determination of norfloxacin and tinidazole in pharmaceutical dosage forms and human plasma. Latin American J. Pharm. 30: 25-32.
- L. SINGH, AND S. NANDA Method for Determination of Tinidazole using Direct UV-Visible Spectrophotometry and Differential Spectrophotometry in Pure and Tablet Dosage Forms East and Central African Journal of Pharmaceutical Sciences Vol. 14 (2011) 75-80
- Salomies H Determination of Tinidazole in dosage forms by quantitative HPTLC. J Planar Chromatogr 5: (1992), 291-293.
- Patel RB, Patel AA, Gandhi TP, Patel PR, Patel VC New methods for the estimation of Tinidazole. Indian Drugs 18: (1980) 76-78.
- Salomies H, Salo JP An HPLC study of tinidazole hydrolysis. Chomatographia 36: (1993) 79-82.
- Bakshi M, Singh S HPLC and LC-MS studies on stress degradation behavior of tinidazole and development of a validated specific stability indicating HPLC assay method. J Pharm Biomed Anal 34, (2004), 11-18.
- ICH Harmonized Tripartite Guideline, 2005, Validation of Analytical Procedures: Text and Methodology Q2 (R1), International Conference on Harmonization, Geneva: Switzerland.


 Pranay Pravin More*
Pranay Pravin More*
 Mrunalini D. Kulkarni
Mrunalini D. Kulkarni












 10.5281/zenodo.13972052
10.5281/zenodo.13972052