Abstract
Research across neuroscience indicates that anxiety disorders stem from malfunctions in brain circuits that regulate emotional responses to threats. Viewing anxiety disorders as disturbances in emotional response regulation offers a useful framework, enabling a broader understanding of anxiety. It also opens doors to developing treatments encompassing psychological, behavioral, and pharmacological approaches.[1] These circuits entail signaling from the amygdala about potential threats and top-down control mechanisms from the prefrontal cortex, indicating the emotional significance of stimuli. Studying the factors influencing these mechanisms could lead to more effective cognitive-behavioral strategies for managing anxiety disorders. The amygdala's circuits primarily consist of inhibitory GABAergic interneurons, and the neurotransmitter GABA (?-aminobutyric acid) significantly influences anxiety modulation, both in normal and pathological conditions.[2] The GABA-A receptor, featuring allosteric sites, enables precise regulation of neuronal inhibition in the amygdala and serves as the target for major anxiolytic drugs. Changes in endogenous modulators of these allosteric sites, along with variations in the GABA-A receptor's subunit composition, may contribute to reduced neuronal inhibition in pathological anxiety. Furthermore, neurosteroids synthesized in the brain act as allosteric modulators of the GABA-A receptor. As their synthesis is influenced by stress and anxiety-inducing stimuli, targeting the neurosteroid-GABA-A receptor axis appears promising for anxiety modulation.[3]
Keywords
Anxiety, Amygdala, Prefrontal Cortex, ?-Aminobutyric Acid, Neurosteroids
Introduction
Aim:
To gaining a deeper understanding of the neurobiological networks linked to anxiety and their disruption in anxiety disorders is essential. This knowledge is not only necessary to comprehend the visible clinical symptoms but also to develop tailored treatments. Effective treatment should focus on retraining and restoring the malfunctioning neurobiological circuits in anxiety disorders. This review is dedicated to explaining the molecular and neurobiological processes associated with anxiety disorders, with a specific emphasis on those related to GABAergic neurotransmission, which involves ?-aminobutyric acid. Numerous brain regions participate in recognizing and managing negative emotional stimuli and generating corresponding cognitive, behavioral, or somatic responses. However, a specific set of limbic structures, particularly the amygdala situated in the median temporal lobes, plays a pivotal role in regulating negative emotions. The primary neural circuits associated with anxiety have been elucidated primarily through research on experimental animals, although not all of these pathways have been definitively demonstrated in the human brain. In humans, bilateral amygdala lesions have been linked to difficulties in recognizing facial expressions conveying fear and other negative emotions. Conversely, electrical stimulation of the amygdala induces feelings of fear and anxiety. With the advent of functional imaging, numerous studies have explored the activation of various brain regions in response to negative emotional stimuli and in the context of anxiety disorders. A consistent finding in these investigations is the activation of the amygdala. Moreover, individuals with anxiety disorders tend to exhibit more pronounced amygdala activation in response to specific stimuli compared to non-anxious individuals.[11] Importantly, successful cognitive-behavioral therapy for anxiety disorders leads to a reduction in this hyperactivation of the amygdala. The amygdala comprises distinct nuclei, but for the purposes of discussing anxiety-related brain circuitry, two groups of nuclei are of particular interest: the basolateral amygdala complex (BLA) and the centromedial amygdala complex, especially the central nucleus (CeA). The BLA receives input regarding potentially negative emotional signals from the thalamus and sensory association cortex and activates the CeA directly through excitatory glutamatergic pathways. Additionally, it activates a relay of inhibitory GABAergic interneurons, known as the intercalated neurons, situated between the BLA and the CeA, exerting an inhibitory influence on the latter. The CeA serves as the principal output pathway from the amygdala, with inhibitory GABAergic neurons projecting to the hypothalamus and brainstem, giving rise to somatic manifestations of anxiety. Projections to other basal forebrain nuclei, such as the ventrotegmental area and the locus ceruleus, may contribute to the dysphoria associated with anxiety. Furthermore, neurons from the BLA activate cells in the adjacent bed nucleus of the stria terminalis, which project to the same areas as the CeA and serve a similar role .[12] In addition to the amygdala's role in anxiety regulation, forebrain areas like the medial prefrontal cortex (PFC) and anterior cingulate cortex play crucial roles. These cortical regions receive and send excitatory glutamatergic projections to and from the BLA and are concurrently activated with the amygdala during the presentation of emotional stimuli. It has been proposed that the medial PFC regulates the experience or expression of anxiety by modulating neuronal activity in the BLA, with more dorsal cortical areas responsible for conscious, voluntary control of anxiety and more ventral areas responsible for implicit, subconscious control. This "top-down" control leads to the inhibition of amygdala output.[13] Neuroimaging studies have revealed that the medial PFC is hypoactive in certain anxiety disorders, particularly post-traumatic stress disorder and generalized anxiety disorder. Interestingly, in individuals making voluntary efforts to control their emotional reactions to negative stimuli, both the lateral and medial PFC are strongly activated. Anxious individuals often require higher levels of PFC activation than non-anxious individuals to successfully reduce negative emotions. Furthermore, a neuroimaging study has shown that activation of the anterior cingulate cortex is associated with an anxiolytic response to a placebo in individuals reacting to negatively charged cues, with no change in amygdala activation. The strength of anterior cingulate cortex activation correlates strongly with the robustness of the placebo effect. Understanding how these cortical-subcortical regulatory mechanisms function and how they can be leveraged is critical for the development of more effective anxiety interventions.[14]
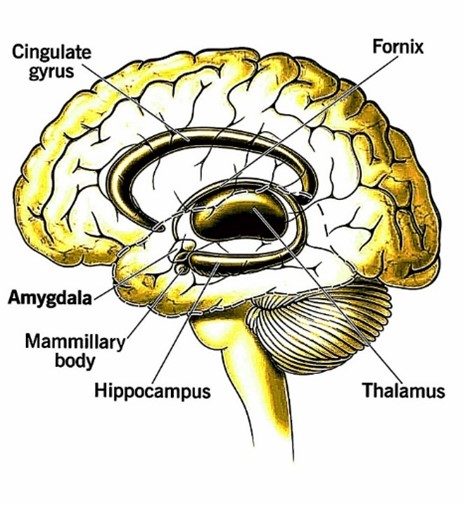
Fig. 1. Amygdala
The amygdala, a small but important part of our brain, helps us process emotions and connects them to our memories, learning, and senses. If it doesn't work properly, it can cause emotional problems and other symptoms.
The Involvement of GABA in the Amygdala:
GABAergic neurotransmission within the amygdala holds significant promise for the regulation of anxiety-related responses. A range of studies in animal models have supplied compelling evidence regarding the pivotal role played by GABAergic neurotransmission within the amygdala in modulating anxiety-related behaviors.[15] To illustrate, the introduction of GABA or GABA receptor agonists into the amygdala has consistently resulted in the reduction of fear and anxiety measures across various animal species, whereas the administration of GABA antagonists tends to induce anxiogenic effects. Moreover, the targeted inhibition of the GABA-producing enzyme, glutamic acid decarboxylase, in the amygdala has been observed to eliminate the anxiolytic response to benzodiazepines. In human studies, the administration of benzodiazepines has been shown to mitigate amygdala activation in the presence of negative emotional stimuli. [16] As mentioned above, the GABAergic intercalated neurons that are entirely contained within the amygdala play a critical role in regulating the activation of the CeA by the BLA. Furthermore, they represent a central target for the excitatory glutamatergic projections from the prefrontal cortex (PFC) to the amygdala. These intercalated neurons establish numerous monosynaptic connections among various groups of intercalated neurons. Notably, these neurons are equipped with a slowly deactivating potassium conductance, enabling them to adapt their firing patterns in response to shifts in overall amygdala activity. Consequently, these GABAergic neurons occupy a strategic position in modulating the processing of anxiety-related information within the amygdala.[17]
Neurotransmitters play a significant role in regulating anxiety levels
The manifestation of anxiety is a complex process involving the coordinated interaction of multiple brain pathways that incorporate various neurotransmitters. These neurotransmitters operate in a network influenced by both local and distant synaptic connections. Among these, the inhibitory neurotransmitter GABA has traditionally been recognized as a central player in anxiety regulation, targeted by medications like benzodiazepines for treating anxiety disorders. However, it is essential to acknowledge that GABA is not the sole neurotransmitter of significance in modulating anxiety responses within the amygdala. Various other neurotransmitters, including serotonin, opioid peptides, endocannabinoids, neuropeptide Y, oxytocin, and corticotropin-releasing hormone, have also been implicated. While these neurotransmitter pathways are crucial, this review will not delve into them in detail.[18]

Fig. 2. GABAergic Neurotransmission
GABAergic transmission is crucial for regulating anxiety by inhibiting brain regions associated with fear and stress, like the amygdala. Additionally, disruptions in GABAergic function are linked to mood disorders such as depression and bipolar disorder. Its impact on mood regulation involves interactions with neurotransmitter systems like serotonin and dopamine.
GABA, receptors for GABA, and brain structures associated with GABA
GABA serves as the principal inhibitory neurotransmitter in the central nervous system (CNS), with an estimated one-third of CNS neurons utilizing GABA as their primary neurotransmitter. This GABAergic inhibition is vital for maintaining a delicate balance between neuronal excitation and inhibition, enabling precise temporal and spatial control of transsynaptic signaling, modulation of neuronal excitability over time, and the regulation of rhythmic "pacemaker" activities in various brain regions.[19] Most GABA-containing neurons function as interneurons, governing the excitability of local circuits within specific brain regions. However, it's noteworthy that some major projection pathways, such as those originating in the thalamus and cortex, also employ GABA as their neurotransmitter.[20] Neuronal inhibition through GABA is mediated by two distinct classes of GABA receptors. Ionotropic GABAA receptors, which are fast-acting, serve as ligand-gated chloride channels, leading to rapid neuronal inhibition. Conversely, metabotropic GABAB receptors are indirectly linked to either calcium or potassium channels through G-proteins, producing slow and sustained inhibitory responses. The precise roles of these receptors in neurological and psychological function are not yet fully characterized, although baclofen, a compound that mimics GABA's action at GABAB receptors, has exhibited potent myorelaxant properties and has been proposed for potential use in treating alcohol dependence.[21] Activation of GABAA receptors results in an immediate and significant increase in chloride conductance across the cell membrane. This renders the neuron incapable of generating an action potential, leading to "phasic" inhibition of the neuron. Additionally, low (submicromolar) concentrations of GABA in the extracellular space can persistently activate extrasynaptic GABAA receptors, inducing a persistent or "tonic" inhibitory state, which makes the neuron less responsive to excitatory stimuli.[22]

Fig. 3. GABA Receptors
- The functions of GABAB receptors (GABABRs) in modulating synaptic transmission are diverse. They are found both pre-synaptically, at GABAergic and glutamatergic synapses, and post-synaptically.
- Pre-synaptically, GABABRs inhibit vesicle fusion and neurotransmitter release by blocking calcium channels. Post-synaptically, they influence the function of GIRK channels and NMDA receptors.
- Activation of post-synaptic GABABRs results in slow inhibitory postsynaptic potentials (IPSP) mediated by GIRK channels, leading to shunting inhibition. This process can hinder dendritic calcium spike propagation.
- In summary, GABABRs play a multifaceted role in synaptic transmission modulation, affecting both pre- and post-synaptic mechanisms.
The structure and functioning of GABAA receptors
The GABAA receptor is a complex structure comprised of five protein subunits that span the neuronal membrane, forming a cylindrical shape with dimensions of 11 nm in length and 8 nm in diameter. When activated by GABA, these protein subunits undergo a conformational change, resulting in the transient formation of a pore along the cylinder's axis. This pore allows chloride ions to flow across the neuronal membrane from one side to the other.[23] These protein subunits can be categorized into different families based on their amino acid sequences. Each family has a limited number of variants, with slight variations in amino acid composition. The majority of GABAA receptors found in the central nervous system (CNS) consist of two ? subunits, two ? subunits, and a ? subunit. There are other subunit families like ?, ?, and ? that can substitute for the ? subunit in certain cell types. Additionally, there is the ? subunit, which can replace the ? subunit, and the ? subunit, primarily expressed in the retina, forming homo-oligomeric GABAA receptors.[24] The ? subunits play a pivotal role in conferring sensitivity to GABA and determining the receptor's pharmacological specificity for various allosteric modulators, including benzodiazepines. GABA binds within the interface between the ? and ? subunits. Because there are two pairs of ? and ? subunits on each receptor, two GABA molecules bind to each receptor, promoting positive cooperativity in chloride conductance activation, resulting in high sensitivity.[25] Benzodiazepines and related drugs bind within the interface between the ? and ? subunits, enhancing the probability of channel opening when GABA is present. This mechanism allows benzodiazepines to facilitate GABAergic inhibition. The affinity of benzodiazepines for the GABAA receptor is influenced by the specific ? and ? subunit present. Different brain regions have GABAA receptors with varying subunit structures, leading to variations in their sensitivity to allosteric modulators.[26] Furthermore, different ? subunits exhibit anatomically specific expression patterns within the CNS. Neuronal circuits with distinct functions express GABAA receptors with specific ? subunit compositions. This differentiation occurs between brain regions, subcellular compartments (e.g., presynaptic vs. postsynaptic, somatic vs. dendritic), and even between individual synapses on the same neuron. This structural diversity suggests that various ? subunits may underlie different behavioral functions and serve as potential targets for specific therapeutic interventions.[27] Efforts have been made to develop medications with selectivity for these receptors to enhance effectiveness and reduce undesirable side effects like sedation or dependence. For example, the hypnotic drug zolpidem's relative specificity for ?1-containing GABAA receptors may explain its reduced myorelaxant effects. Animal experiments involving targeted gene deletion and point mutations in GABAA receptor genes have indicated that GABAA receptors containing the ?2 subunit play a crucial role in anxiety-related manifestations. Additionally, ?2-containing GABAA receptors are the predominant subtype in the CeA (central amygdala).[28]
Modulation of GABAA Receptors by Neurosteroids
An increasing body of evidence suggests that neuroactive steroids play a significant role in regulating neuronal function and behavioral processes as endogenous modulators. Disturbances in the levels of these neuroactive steroids within the body are believed to contribute to the pathophysiology of anxiety disorders. Neurosteroids can influence neuronal excitability by interacting with GABAA receptors and the N-methyl-D-aspartate subtype of excitatory amino acid (glutamate) receptors.[29] Specific steroids can act as positive allosteric modulators of the GABAA receptor, either directly activating the receptor or facilitating channel opening. These effects depend on the steroid type and their interactions with specific receptor subunits. Interestingly, at higher doses, some of these steroids may paradoxically inhibit GABA-mediated responses, although the underlying mechanism for this effect remains unclear.[30]
These steroid sites can be targeted by various steroids secreted by the adrenal gland, those synthesized within the central nervous system (CNS), and synthetic derivatives. For instance, alfaxolone, used in veterinary medicine as an anesthetic, is a synthetic example. In addition, certain adrenal steroids can be converted into neuroactive derivatives within the CNS. For instance, progesterone, which is inactive at the GABAA receptor, can be converted into the neuroactive 3?,5?-tetrahydroprogesterone.[31] In terms of understanding the role of GABA receptors in anxiety, locally produced neurosteroids are vital, as their synthesis within glial cells is upregulated in response to stress. This makes them an intrinsic modulatory system for fine-tuning inhibition within the CNS in reaction to anxiety-inducing stimuli. Notable neurosteroids include allopregnanolone and 3?,5?-tetrahydrodeoxycorticosterone, along with adrenal steroids such as pregnenolone and dehydroepiandrosterone, all of which are synthesized by cytochrome P450 enzymes in various types of brain cells.[32] Allopregnanolone, for instance, functions as a potent positive allosteric modulator of GABAA receptors, enhancing and fine-tuning the activity of GABA at these receptors. When administered to experimental animals, it exhibits anxiolytic, antidepressant, and anticonvulsant effects, reminiscent of those seen with barbiturates and benzodiazepines. Interestingly, the synthesis of neurosteroids in the CNS can be boosted by selective serotonin reuptake inhibitors (SSRIs), establishing a connection between these anxiety disorder treatments and the GABAergic system.[33] The production of neurosteroids varies across different brain regions and is influenced by factors such as stress, changes in the estrous cycle, pregnancy, and alcohol consumption. These fluctuations in neurosteroid levels, and their subsequent impact on the sensitivity of GABAA receptors, have been implicated in the development of conditions like premenstrual dysphoric disorder.[34] Numerous studies have explored neurosteroid concentrations in individuals with anxiety disorders, although the results have not consistently aligned. For example, individuals with generalized anxiety disorder and generalized social phobia have been found to have lower serum concentrations of pregnenolone, while those with panic disorder exhibit elevated progesterone levels correlated with increased state anxiety.[35] In a study involving healthy volunteers, the administration of cholecystokinin tetrapeptide to induce experimental panic attacks resulted in elevated levels of adrenocorticotropic hormone, cortisol, and 3?,5?-tetrahydrodeoxycorticosterone. This suggests a link between neurosteroids and stress response. Additionally, in animal experiments, the direct application of low concentrations of various neurosteroids like pregnenolone, dehydroepiandrosterone, and progesterone demonstrated anxiolytic properties. Notably, 3?,5?-tetrahydrodeoxycorticosterone was found to mitigate stress-induced increases in plasma adrenocorticotropic hormone and corticosterone levels in rats.[36] Furthermore, the administration of progesterone in humans has been shown to induce sedative and anxiolytic effects. These findings make neurosteroids an attractive target for the development of novel anxiolytic or antidepressant medications.[37] Functional brain imaging studies in humans have revealed that progesterone and its metabolites may influence anxiety states by modulating neural activity in the amygdala. Importantly, neuroactive steroids appear to have a lower potential for abuse compared to benzodiazepines. This reduced risk of abuse, coupled with no interactions with ethanol, could offer an advantage over currently available benzodiazepine-based anxiolytics.[38] Additionally, their rapid onset of action could make them superior to selective serotonin reuptake inhibitors (SSRIs). The ideal approach would involve identifying agents that can leverage this molecular pathway, delivering robust anxiolytic effects while maintaining a clear distinction from sedation and ataxia, and exhibiting good oral bioavailability.[39] However, neurosteroids themselves may not be the most suitable candidates for drug development. Firstly, they are extensively metabolized both peripherally and within the central nervous system. Secondly, there is a risk of undesirable endocrine side effects in the periphery. For instance, in a double-blind, crossover study, the administration of pregnenolone for four weeks did not produce significant effects on mood, memory, self-rated sleep quality, or subjective well-being in healthy volunteers.[40] On the other hand, small molecules that selectively enhance neurosteroid production within the CNS or mimic neurosteroid actions at GABAA receptors appear to be more promising. One such example is etifoxine, a structurally distinct small molecule that exhibits anxiolytic effects in both animal and human studies.[41] Randomized clinical trials comparing etifoxine to buspirone, sulpiride, or lorazepam have demonstrated its efficacy in patients with adjustment disorders. Furthermore, in comparison to lorazepam, etifoxine has relatively mild sedative effects and is associated with minimal memory impairment and interference with neuropsychological activity.[42]
The impact of Etifoxine on the GABAA receptor and its relationship with neurosteroids
Two separate yet interrelated molecular mechanisms have been attributed to etifoxine. One involves its direct influence on the GABAA receptor, while the other pertains to its facilitation of the synthesis of 3?,5?-neurosteroids. These actions are believed to work in concert, enhancing inhibitory GABA neurotransmission.[43] When it comes to the GABAA receptor, etifoxine functions as a positive allosteric modulator, increasing chloride conductance in response to GABA stimulation. Notably, etifoxine's binding site on the GABAA receptor is distinct from the binding sites associated with benzodiazepines and neurosteroids.[44] Recent electrophysiological investigations, utilizing recombinant GABAA receptors with well-defined subunit compositions, have pinpointed the critical role of the ? subunit in etifoxine's action. Specifically, when homomeric GABAA receptors containing only ? subunits are considered, etifoxine reduces the baseline chloride flux through these receptor ion channels.[45] In contrast, when heteromeric receptors consisting of either ? and ? subunits only or ? and ? subunits only are examined, etifoxine consistently enhances the chloride currents induced by GABA. This suggests that the ? subunit is both necessary and sufficient for etifoxine's positive allosteric effect.[46] Additionally, it's worth noting that etifoxine exhibits its most prominent activity in receptors containing the ?2 or ?3 isoforms, as opposed to the ?1 isoform. Interestingly, the presence of different ? subunits does not seem to influence etifoxine's activity. Notably, etifoxine's efficacy remains unaffected even when there is a point mutation (arginine to serine) at position 289 of the ? subunit, in contrast to loreclezole, an anticonvulsant drug targeting the ? subunit, which is rendered ineffective by a similar mutation.[47]
The second molecular target of etifoxine pertains to its interaction with the synthetic machinery responsible for neurosteroids within mitochondria. Specifically, it binds to the 18 kDa mitochondrial translocator protein (TSPO), which serves as a transporter for steroids within the inner layer of mitochondria. This interaction is believed to represent a critical, rate-limiting step in the synthesis of neurosteroids.[48] Upon administering etifoxine to rats, there is an observable dose-dependent increase in the concentrations of various neurosteroids, including pregnenolone, progesterone, 5?-dihydroprogesterone, and allopregnanolone, in both plasma and cerebrospinal fluid. It's noteworthy that this effect persists even in adrenalectomized and castrated animals, indicating that the heightened levels of neurosteroids are a result of the stimulation of neurosteroid synthesis within the central nervous system.[49] The significance of this effect on the anxiolytic properties of etifoxine becomes apparent when we consider that these effects are mitigated when finasteride, an inhibitor of 5?-reductase (a pivotal enzyme in neurosteroid synthesis), is administered. This suggests a close connection between the augmentation of neurosteroid levels and the anxiolytic impact of etifoxine.[50] The role of neurosteroids in the way etifoxine operates gains additional validation from research conducted in the context of neuropathic pain. Neurosteroids have shown considerable efficacy in addressing this condition. Notably, studies have demonstrated that etifoxine can mitigate neuropathic pain. This analgesic effect of etifoxine is obstructed by the 5?-reductase inhibitor finasteride, indicating a connection with neurosteroids.[51] It's important to acknowledge that etifoxine may possess alternative mechanisms of action that do not rely on GABA. For instance, it may be associated with mechanisms linked to the neurotrophic factor GDNF. However, it's worth noting that these GABA-independent mechanisms remain relatively poorly understood at present.[52]
CONCLUSION:
Anxiety disorders do not result from inherent brain defects but rather stem from the malfunction in brain circuits that regulate emotional responses to potential threats. This can be illustrated as an imbalance between heightened activity in the amygdala signaling potential threats and impaired top-down control mechanisms from the prefrontal cortex, which inaccurately assess the emotional significance of stimuli. Neurophysiologically, anxiety involves inhibitory networks primarily composed of GABAergic interneurons. The GABAA receptor, with its allosteric sites, allows precise regulation of neuronal inhibition. Various drugs like benzodiazepines, barbiturates, neurosteroids, some anesthetics, and alcohol act on these sites and influence anxiety. Changes in the levels of endogenous modulators, such as neurosteroids, and alterations in GABAA receptor subunit composition may contribute to reduced neuronal inhibition in pathological anxiety. To modulate anxiety specific to the type of anxious stimuli, targeting the neurosteroid–GABAA receptor axis becomes a potential approach. Molecules like etifoxine, which regulate neurosteroid production and interact with GABAA receptors, are of particular interest as they may reset the sensitivity of amygdala's interneuronal regulatory circuits.
REFRENCE:
- LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two-system framework. American journal of psychiatry. 2016 Nov 1;173(11):1083-93.
- Ferguson BR, Gao WJ. PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Frontiers in neural circuits. 2018 May 16;12:37.
- Chen X, van Gerven J, Cohen A, Jacobs G. Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety. Acta Pharmacologica Sinica. 2019 May;40(5):571-82.
- Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatric disease and treatment. 2015 Jan 17:165-75.
- Grills AE, Seligman L, Ollendick T. Anxiety disorders in children and adolescents. The Wiley handbook of anxiety disorders. 2014 Mar 24:424-56.
- Perrotta G. Anxiety disorders: definitions, contexts, neural correlates and strategic therapy. J Neur Neurosci. 2019;6(1):042.
- Rockhill C, Kodish I, DiBattisto C, Macias M, Varley C, Ryan S. Anxiety disorders in children and adolescents. Current problems in pediatric and adolescent health care. 2010 Apr 1;40(4):66-99.
- Schneier FR, Vidair HB, Vogel LR, Muskin PR. Anxiety, obsessive-compulsive and stress disorders. Psychiatry. 2014 May 5:168-203.
- Stein DJ, Craske MA, Friedman MJ, Phillips KA. Anxiety disorders, obsessive-compulsive and related disorders, trauma-and stressor-related disorders, and dissociative disorders in DSM-5. American Journal of Psychiatry. 2014 Jun;171(6):611-3.
- Bernard MM, Luc M, Carrier JD, Fournier L, Duhoux A, Côté E, Lessard O, Gibeault C, Bocti C, Roberge P. Patterns of benzodiazepines use in primary care adults with anxiety disorders. Heliyon. 2018 Jul 1;4(7).
- Kim J, Gorman J. The psychobiology of anxiety. Clinical Neuroscience Research. 2005 May 1;4(5-6):335-47.
- Chen X, van Gerven J, Cohen A, Jacobs G. Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety. Acta Pharmacologica Sinica. 2019 May;40(5):571-82.
- Meisner OC, Nair A, Chang SW. Amygdala connectivity and implications for social cognition and disorders. Handbook of clinical neurology. 2022 Jan 1;187:381-403.
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Behavioral neurobiology of anxiety and its treatment. 2010:251-77.
- Yilmazer-Hanke DM, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behavioural brain research. 2003 Oct 17;145(1-2):145-59.
- Hand KS. GABAA/benzodiazepine receptor distribution and subunit mRNA expression in human temporal lobe epilepsy. University of London, School of Pharmacy (United Kingdom); 1998.
- Palomares-Castillo E, Hernández-Pérez OR, Pérez-Carrera D, Crespo-Ramírez M, Fuxe K, de la Mora MP. The intercalated paracapsular islands as a module for integration of signals regulating anxiety in the amygdala. Brain research. 2012 Oct 2;1476:211-34.
- Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, Iosifescu DV. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Frontiers in psychiatry. 2020 Dec 23;11:595584.
- Jelitai M, Madarasz E. The role of GABA in the early neuronal development. International Review of Neurobiology. 2005 Jan 1;71:27-62.
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition?. Nature Reviews Neuroscience. 2002 Sep 1;3(9):715-27.
- REY JA. Mechanisms of Action.
- Cellot G, Cherubini E. Functional role of ambient GABA in refining neuronal circuits early in postnatal development. Frontiers in neural circuits. 2013 Aug 13;7:136.
- Delto CF. Structural and Biochemical Characterization of the GABA (A) Receptor Interacting Protein Muskelin (Doctoral dissertation, Universität Würzburg).
- Ghit A, Assal D, Al-Shami AS, Hussein DE. GABAA receptors: structure, function, pharmacology, and related disorders. Journal of Genetic Engineering and Biotechnology. 2021 Aug 21;19(1):123.
- Olsen RW. GABAA receptor: Positive and negative allosteric modulators. Neuropharmacology. 2018 Jul 1;136:10-22.
- Atack JR. The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert opinion on investigational drugs. 2005 May 1;14(5):601-18.
- Fritschy JM, Panzanelli P. Extrasynaptic GABA A receptors: subunit composition, distribution, and regulation. Extrasynaptic GABAA Receptors. 2014:15-32.
- Engin E, Liu J, Rudolph U. ?2-containing GABAA receptors: A target for the development of novel treatment strategies for CNS disorders. Pharmacology & therapeutics. 2012 Nov 1;136(2):142-52.
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Critical Reviews™ in Neurobiology. 2003;15(3&4).
- Kramer PF, Twedell EL, Shin JH, Zhang R, Khaliq ZM. Axonal mechanisms mediating ?-aminobutyric acid receptor type A (GABA-A) inhibition of striatal dopamine release. Elife. 2020 Sep 1;9:e55729.
- Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3?, 5?-tetrahydroprogesterone (allopregnanolone) availability?. Biological psychiatry. 1998 Nov 1;44(9):865-73.
- Finn DA, Jimenez VA. Dynamic adaptation in neurosteroid networks in response to alcohol. The Neuropharmacology of Alcohol. 2018:55-78.
- Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABAA receptor interactions: a focus on stress. Frontiers in neuroscience. 2011 Dec 5;5:131.
- N-Wihlbäck AC, Sundström-Poromaa I, Bäckström T. Action by and sensitivity to neuroactive steroids in menstrual cycle related CNS disorders. Psychopharmacology. 2006 Jun;186:388-401.
- Perkins EC, Newport DJ. Neurosteroids in the pathophysiology and treatment of mood and anxiety disorders. Current Treatment Options in Psychiatry. 2018 Dec;5:377-400.
- Eser D, Schüle C, Baghai TC, Romeo E, Rupprecht R. Neuroactive steroids in depression and anxiety disorders: clinical studies. Neuroendocrinology. 2007 Mar 5;84(4):244-54.
- Zorumski CF, Paul SM, Covey DF, Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiology of stress. 2019 Nov 1;11:100196.
- Schüle C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs?. Neuroscience. 2011 Sep 15;191:55-77.
- Altamura AC, Moliterno D, Paletta S, Maffini M, Mauri MC, Bareggi S. Understanding the pharmacokinetics of anxiolytic drugs. Expert opinion on drug metabolism & toxicology. 2013 Apr 1;9(4):423-40.
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcoholism: Clinical and Experimental Research. 1998 Aug;22(5):998-1040.
- do Rego JL, Vaudry D, Vaudry H. The non-benzodiazepine anxiolytic drug etifoxine causes a rapid, receptor-independent stimulation of neurosteroid biosynthesis. PLoS One. 2015 Mar 18;10(3):e0120473.
- Nuss P, Ferreri F, Bourin M. An update on the anxiolytic and neuroprotective properties of etifoxine: from brain GABA modulation to a whole-body mode of action. Neuropsychiatric Disease and Treatment. 2019 Jul 3:1781-95.
- Ugale RR, Sharma AN, Kokare DM, Hirani K, Subhedar NK, Chopde CT. Neurosteroid allopregnanolone mediates anxiolytic effect of etifoxine in rats. Brain research. 2007 Dec 12;1184:193-201.
- Poisbeau P, Gazzo G, Calvel L. Anxiolytics targeting GABAA receptors: Insights on etifoxine. The World Journal of Biological Psychiatry. 2018 Jun 22;19(sup1):S36-45.
- Brown AR. Endogenous neurosteroid actions at GABAA receptors during neuronal development (Doctoral dissertation, University of Dundee).
- Hamon A, Morel A, Hue B, Verleye M, Gillardin JM. The modulatory effects of the anxiolytic etifoxine on GABAA receptors are mediated by the ? subunit. Neuropharmacology. 2003 Sep 1;45(3):293-303.
- Madsen U, Bräuner?Osborne H, Greenwood JR, Johansen TN, Krogsgaard?Larsen P, Liljefors T, Nielsen M, Frølund B. GABA and glutamate receptor ligands and their therapeutic potential in CNS disorders. Drug discovery handbook. 2005 Jun 24:797-907.
- VON TSPO CH. CHARACTERIZATION OF TSPO 18 KDA.
- Liere P, Pianos A, Oudinet JP, Schumacher M, Akwa Y. Differential effects of the 18-kDa translocator protein (TSPO) ligand etifoxine on steroidogenesis in rat brain, plasma and steroidogenic glands: Pharmacodynamic studies. Psychoneuroendocrinology. 2017 Sep 1;83:122-34.
- Verleye M, Akwa Y, Liere P, Ladurelle N, Pianos A, Eychenne B, Schumacher M, Gillardin JM. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacology Biochemistry and Behavior. 2005 Dec 1;82(4):712-20.
- Poisbeau P, Keller AF, Aouad M, Kamoun N, Groyer G, Schumacher M. Analgesic strategies aimed at stimulating the endogenous production of allopregnanolone. Frontiers in cellular neuroscience. 2014 Jun 17;8:174.
- Kaur S, Singh S, Arora A, Ram P, Kumar S, Kumar P, Abed SN. Pharmacology of GABA and its receptors. Frontiers in pharmacology of neurotransmitters. 2020:241-92.


 Ayush Kumar* 6
Ayush Kumar* 6
 Ankita Singh 2
Ankita Singh 2
 Anupama Kumari 3
Anupama Kumari 3
 Arnab Roy 3
Arnab Roy 3
 Sajid Ansari 4
Sajid Ansari 4
 Subham Kumar Lohani 4
Subham Kumar Lohani 4
 Nahida Khatun 4
Nahida Khatun 4
 Priyanka Kumari 5
Priyanka Kumari 5
 Suman Kumari 5
Suman Kumari 5
 Divya Roshni Panna 5
Divya Roshni Panna 5
 Manoj Kanti Ghosh 4
Manoj Kanti Ghosh 4
 K. Rajeswar Dutt 1
K. Rajeswar Dutt 1



 10.5281/zenodo.11141718
10.5281/zenodo.11141718