Abstract
Several Indian plants are used in the nation's traditional herbal medicine system and are recognized to have therapeutic potential. Spermadictyon suaveolens Roxb. is a specific herb having a number of therapeutic benefits. The participation of mineral elements in the structural and functional processes of life gives them enormous significance. For the first time, the elemental profile of Spermadictyon suaveolens Roxb (ten elements to be analyzed: Cu, Na, Ca, Cr, Mn, Fe, Ni, Cd, Zn and Pb) was established in order to create a solid platform for investigating the plant's therapeutic qualities. By using the ICP-AES technique, elements from the root, stem, and leaves were examined. Many components with biological effects on humans were discovered to be present in a variety of quantities. To comprehend the fundamental contribution to the physiology and fundamental functions of human existence, the findings and references have been examined. Also, a few crucial considerations regarding heavy metals and their toxicity are presented here. These details would be used as a guide when determining the dosage of a medicine made from this plant's medicinal herbs.
Keywords
Spermadictyon suaveolens Roxb, Elemental composition, ICP-AES, Cu, Na, Ca, Cr, Mn, Fe, Ni, Cd, Zn, Pb.
Introduction
Herbal plants have been used from ages as traditional medicinal sources in India. Due to the affordability and access to procure these medications without prescription, medicinal plants are popular and its use as nutraceuticals or phytonutrient has increased tremendously all over the world like Brazil, UK, parts of Europe, North America and Australia [1]. Bioactive molecules in medicinal plants, such as flavonoids, alkaloids and polyphenols have high metal binding capabilities and elements like Fe, Mn, Co etc. in the soil that can be taken into the plant tissues by the plants [2]. When this happens, the elemental concentration may increase more than needed or reduce more than minimum need causing illnesses in human body when such plants are consumed[3].
Industrialization, urbanization, Industrial activities have all led to the increase in the concentrations of toxic elements [4]. Normally the medicinal properties of herbal medicinal plants have been advocated to the vitamin content, essential oils and other organic constituents. However, now it has been observed that increased dosage or the consecutive administration of medicinal plants leads to alarming accumulation of elements which in turn causes other health issues [5]. Elemental constituents as well as the dosage rate of these plants must be well known in-order to avoid any danger to the consumers as these herbal medicinal preparations may be carrying harmful toxins[6] [7]. Medicinal plants which form the raw materials for the medicinal products, is recommended by WHO to be tested for the presence of Heavy metals, thus regulating the level of elements present in the drug. Medicinal plants may be contaminated during the growth and processing stages from soil, water or air [8].
The present study is undertaken to detect and determine concentration level of 10 different mineral elements in Spermadictyon suaveolens Roxb. S. suaveolens is native of Tropical America [9] and found in China as well as Indo-Pak subcontinent of Asia [10]. In India, it is found in Maharashtra, Uttarakhand [11] and other parts of north and central India, belonging to the family Rubiaceae, Order Gentiales of Tracheophyta.
It is commonly known as ‘Forest Champa,’ ‘Van-Champa’, ‘Gidesa,’ ‘Jitsaya’ etc. and has a considerable distribution in tropical dry or moist deciduous forests. It is a 1-2m in height moderately branched shrub which show divaricate structure. Dark coloured elliptic leaves, lanceolate shaped which narrows down at the base with opposite decussate arrangement. Tiny, white flowers with peculiar fragrance. Triquetrous seeds, not many, surrounded by a teeth like structures. Capsules have 5 valves [10]. Hamiltonia suaveolens is used as synonym of this plant [12]. This plant is known for its medicinal properties and used for the treatment of different ailments in humans. Bark and leaves show antioxidant and antimicrobial effects and the wood is expended as fuel during cold climate [13].The roots of this plant has been used as a wound healer, treatment of diabetes, rheumatoid arthritis and bloody dysentery in veterinary medicine, while the stem and root have been used in healing diseases concerning with the bones as well as Herpes virus [14].
No work has been yet reported on elemental profile of Spermadictyon suaveolens Roxb. Therefore, this work has been conducted.
MATERIALS AND METHODS:
Sample collection:
Whole plant parts of Spermadictyon suaveolens Roxb. were collected from Karnala region, near Karnala Bird Sanctuary, Panvel, Maharastra. The plants were identified at Blatter’s herbarium, St. Xavier’s College, Mumbai. The accession number for Spermadictyon suaveolens Roxb. is 23598 of H.Santapau.
Sample preparation:
The plant was first cut and the different parts of root, stem and leaves were sorted. The surface contaminants of plant samples were removed by washing with deionized water. It was shade dried and powdered in grinder to obtain fine powder. The powder was then stored in air tight glass containers and used for further analysis. Two grams of powder from each plant part was dissolved in nitric acid and heated until the reddish-brown fumes disappeared. Perchloric acid was then added to the above solution and heated for 5 min. This was followed by addition of aqua regia and heated the volume was then made up to 25ml in a standard flask by adding de ionized water. Elements were analyzed and estimated using Inductively Coupled Plasma - Atomic Emission Spectrometer (Model: ARCOS from M/s. Spectro, Germany)[15]
RESULTS AND DISCUSSION:
The literature revealed that there are permissible limits for intakes of elements so as to prevent
health risk factors like cardiovascular diseases in humans as well as animals.[16]. The most recent Periodic Table contains 112 known elements, However, only 21 one of these are convincingly essential for human beings.[17]. The important elements that are majorly required by the human body are calcium, sodium, magnesium, phosphorus, and potassium. While all of the other remaining inorganic elements are trace elements like zinc, manganese, molybdenum, iodine, selenium, sulfur, iron, chlorine, cobalt, and copper in human body have specific biochemical characteristic role in the human tissues [18]. It has been seen that various plant parts of Spermadictyon suaveolens Roxb. are a good source of trace and major elements. The elements are present in very minute quantities in the various parts of any plant and therefore a good technique is required for its detection and study. In the present investigation we have applied one of the most sensitive ICP-AES analytical techniques.
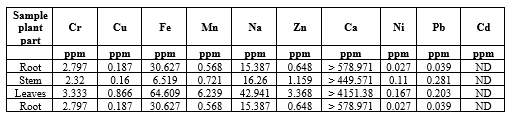
Table.1 Concentration of elements in root, stem and leaf of Spermadictyon suaveolens Roxb.
*ND means less than 0.1 ppm (parts per million)
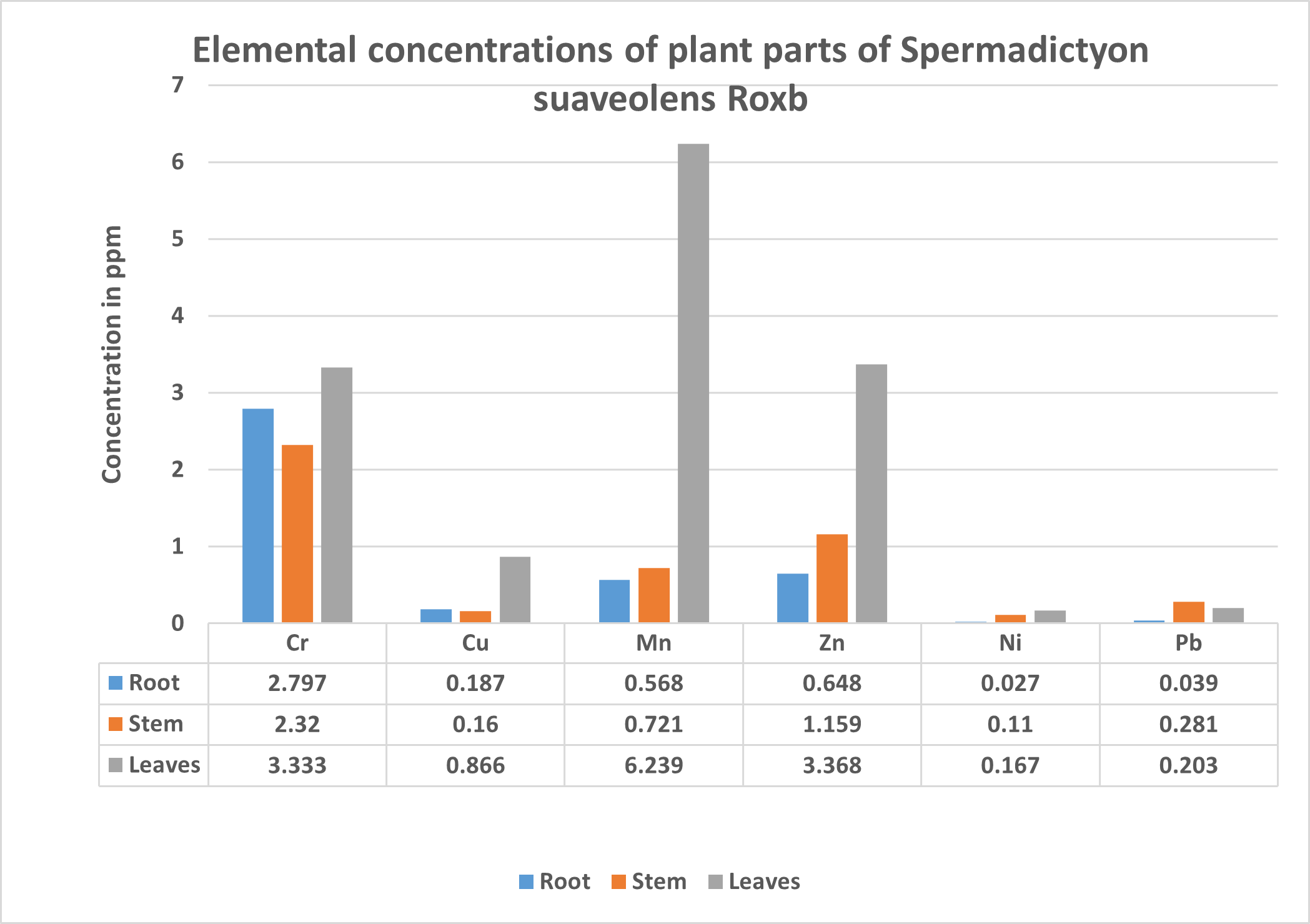
Table 2. Concentration of Cr, Cu, Mn, Zn, Ni and Pb in Leaves,stem and root of Spermadictyon suaveolens Roxb.
Chromium (Cr)
Chromium is considered as an essential trace element that can help improve insulin sensitivity and thus enhance protein, carbohydrate, and lipid metabolism [19]. However, Cr, if ingested in more than required quantities, may result in very harmful effects on the liver as well as kidneys and lung [20]. In a particular study, all the medicinal plant samples showed the concentration level of chromium was found to be in the range of 0.88-5.30 ppm and the daily dietary intake of 0.05-0.2 mg of chromium leads to general good health of the human beings [21]. The maximum limit in edible plants for chromium as set by FAO/WHO is 2 ppm [22]. For medicinal plants the WHO limits for Chromium have not yet been established [23]. It has been found the level of Cr in plants is usually in the range 0.03–1.6 mg kg?1 [24]. The Highest concentration of Chromium in the plant Spermadictyon suaveolens Roxb. was found highest in the root with 3.333 ppm followed by the leaves with 2.797 ppm, while the least was found in the stem, 2.32 ppm (Table.2).
Nickel (Ni)
Nickel has been seen to play an important role in the insulin production and is an essential element in animals. Nickel in the form of Ni2+ available in plants is believed for an enzyme functioning in nitrogen Cofactor metabolism and helps in maintenance of membrane structure, control of prolactin, nucleic acid metabolism.[25]. A dose of nickel as nickel sulfate at very low concentration 0.6 mg, have been observed to produce a positive skin reaction in some individuals.[26]. Though needed in minute quantity, it’s deficiency may results in the disorder of liver.[23] Ni deficiency may cause the allergic dermatitis called nickel itch and also is suspected to be a carcinogen which may affect the lungs as well as the nasal cavities.[27]. Animals have a nickel requirement of less than 200 ppb of diet and a basal nickel requirement of less than 100 ppb daily for adult humans.[26]. Nickel if deficient in the human body causes reduced formation of blood cells [28] whereas if overly ingested may cause allergic skin reactions, lung damage, carcinogenic effects.[29] Medicinal plant limitation of Nickel has not been assigned yet. However, the FAO/WHO limit has been 1.63 ppm in edible plants.[30] Nickel in the plant Spermadictyon suaveolens Roxb. was found highest in the leaves with 0.167 followed by the stem with 0.11 ppm, while the least was found in the root, 0.027 ppm (Table.2)
Cadmium (Cd)
The limit set by FAO/WHO for the allowed concentration in edible plants was 0.21ppm. However, the permissible limit set by WHO, China and Thailand were only 0.3 ppm. In Canada, limit of 0.3 ppm has been set for raw medicinal plant material, whereas, 0.006 ppm has been set as a limit in finished herbal products.[31]. It has been found that recurrent accumulation of Cadmium with the increasing age may cause adverse effect on the kidneys and thus the health in older age. Contrastingly it’s been studied that children show more absorption than adults as the Cadmium retention is higher in early stages of development[26]. Although generally considered as toxic metal, cadmium deficiency may cause negative health state in some animals like chicken.[28] Cadmium in the plant Spermadictyon suaveolens Roxb has been detected at very negligible amount of less than 0.1 ppm (Table.2).
Copper (Cu) Copper is an essential element however can be toxic at excessive levels causing phytotoxicity in plants at a concentration higher than 20-100 ppm DW (dry weight)[27]. Human nutrition depends on the necessity of Copper for normal Iron metabolism and the formation of red blood cells. Its inadequate intake may be a cause for Anemia,[32] fatigue and decreased number of white blood cells, on the other and a genetic disorder known as Menkes disease is caused due to Copper, involves a wide variety of fatal symptoms [33].Copper acts as a cofactor for many enzymes and is considered as the third main mineral present in human body. Other problems related to the deficiency include Leukemia, arthritis, brittle bones, muscle soreness etc [28].In plants Copper is a component of many redox and lignin-biosynthetic enzymes and its deficiency causes Chlorosis, dead spots in leaves, stunted growth, terminal buds die, necrosis in young leaves.[25]The permissible limit by FAO/WHO for edible plants for copper is 10 ppm however medicinal plant limit has not been set by the WHO, although in china and Singapore the medicinal plant limit for cu were 20 ppm and 150ppm respectively.[23] Copper in the plant Spermadictyon suaveolens Roxb has been detected at very low amount well below any permissible limit. Highest reported in leaves 0.866 ppm and then root 0.187 ppm, and least in stem 0.16 ppm (Table.2).
Manganese (Mn)
Humans, animals and plants all require Manganese (Mn) for its growth, maintenance and development, as it is an essential element. Especially in plants it is a crucial part of the respiratory process and its deficiency can cause catastrophic effect on agriculture. In humans and animals its deficiency at the initial phase of development may cause abnormalities in the skeletal muscle and other irreversible ataxia adding to fertility issues. At high levels Mn becomes poisonous causing genetic alterations leading to changes in the lungs and central nervous system.[34] Required by the plants in low concentrations we have seen that plants uptake of Mn greatly exceeds the requirement [35]. Manganese is involved in the formation of Amino Acids, activation of few enzymes and coenzymes, photolysis of water in photosynthesis and synthesis of chlorophyll, thus its deficiency in plants also whitening discoloration of the leaves and senescence[25]. Consumption of this metal in excess quantities has been shown to cause low intelligence quotient in children, reduced level of haemoglobin generation, Parkinson disease as well as abortion and stillbirth in women [36]. In humans the estimated amount for the dairy consumption of Mn is 11mg/day [27]. In Plants Manganese in the form of Mn2+ actively aids in the formation of chlorophyll and activates certain enzymes and also aids in the formation auxin and starch in plants. while its deficiency may cause Chlorosis, mottled or bronzed leaves and abnormal roots. [25] FAO/WHO (1984) set 2 ppm the limit in edible plants. Medicinal plant permissible limit for the element of Mn has not been set by WHO however it has been reported that certain medicinal plants in Egypt show the concentration of 44.6 to 339 ppm.[37] Manganese in the plant Spermadictyon suaveolens Roxb has been detected at very low concentrations, highest seen in leaves 6.239 ppm and then stem 0.721 ppm, and least in root 0.568 ppm (Table.2).
Lead (Pb)
Lead is a non-essential trace elements studied to have no particular functions neither in human body nor in plants. Although very little information is available regarding any benefits of lead in the body, it has been studied that rats which were subjected to a lead depleted diet showed retardation of growth. [26]. Increased levels of lead intake have effects like colic, anemia, headache, convulsions. Also causing chronic nephritis of the kidneys, brain damage and central nervous system disorders[27]. WHO/FAO permissible limits of lead in edible plants is 0.43 ppm. [31]. However, WHO prescribed limit of pb for medicinal plant is 10 ppm and dietary intake is 3 ppm/ week. [23] Lead in the plant Spermadictyon suaveolens Roxb. was found highest in the Stem with 0.281 ppm followed by the leaves with 0.203 ppm, while the least was found in the root, 0.039 ppm (Table.2).
Zinc (Zn)
Zinc is an essential trace element for plants as well as animals. It has been seen that, processes like normal growth, brain development, behavioral response, bone formation and wound healing in animals have been beneficially affected by the presence of Zn [27].Diabetics with Zn deficiency fail in the power of perception and may cause loss of sense of touch and smell [38]. It has been seen that, a daily diet containing approximately 1 mg/day for men and 0.7 mg/day for women is necessary [26] FAO/WHO (1984) has set the permissible limit for Zn in edible plants at 27.4 ppm, but the limit for medicinal plants have not been set up. However, the range of Zn in agricultural products should be between 15 to 200 ppm.[31]. Zn in the plant Spermadictyon suaveolens Roxb. was found highest in the leaves with 3.368 ppm followed by the stem with 1.159 ppm, while the least was found in the root, 0.648 ppm (Table.2).
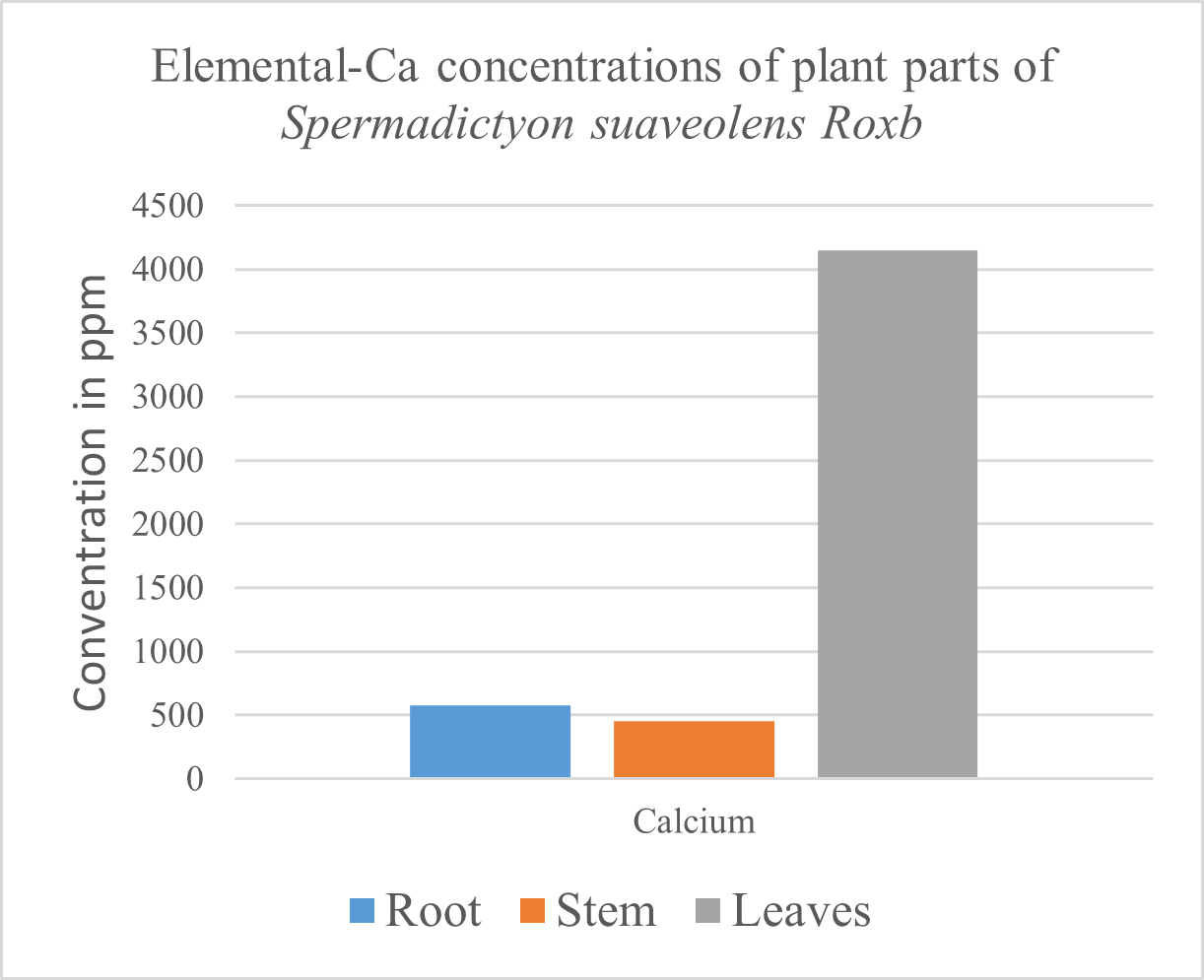
Table 3. Concentration of Ca in Leaves, stem and root of Spermadictyon suaveolens Roxb.
Calcium (Ca)
920 g to 1200 g of the body weight of an adult is made up of calcium, 99% of which is in the bones and teeth. And is required for heart, muscle, and digestive system health, builds bone, supports synthesis and function of blood cells [18]. In plants Calcium is involved in formation and stability of cell walls and membrane structure and permeability, also activates some enzymes along with regulation of responses of cells to stimuli. Calcium also helps in repair of worn-out cells and body mechanisms [26]. Deficiency of Calcium is rare in nature [39], If it occurs however, deficiency of Calcium causes the leaves to deform, death of terminal buds and reduced root growth [25]. `tip burn' of leafy vegetables, `brown heart' of leafy vegetables or `black heart' of celery, `bitter pit' of apples and `empty pod' in peanut etc.[39] In people, Increased calcium in diet can cause fecal fat content as well as gastrointestinal hormones leading to reduced food intake, thus enough Calcium may decrease fat deposition [40]. Calcium in the plant Spermadictyon suaveolens Roxb. was found highest in the leaves with > 4151.38 followed by the root with > 578.971 ppm, while the least was found in the stem, > 449.571 ppm (Table.3).
Iron (Fe)
Nutritional deficiency most commonly contributes to Iron deficiency especially in toddlers and women of child-bearing age [41].This may be because of reduced iron consumption, or because of defective absorption or excessive blood loss [42]. A normal requirement of Iron in full-term child is 0.3 mg while it is more for prematurely born infants. This requirement increases as age increases to about 1.6 mg per day for adolescence time, and slowly drops to 1.2 mg in normal people [43].Fats, proteins and carbohydrate oxidation if facilitated by Fe to control the weight as well as the diabetes in man.[27]. Low iron may cause infections of the intestine, bleeding of the nose and blockage of blood flow to the heart muscle [38] High consumption of iron may increase the risk for colorectal cancer [44]. Fe permissible limits in Plant material is 219.8±6.8[45]. FAO/WHO permissible limit for Fe is 20 ppm for edible plants but WHO (2005) have not proposed a limit for medicinal plants, also all the medicinal plants were seen to accumulate more Fe than proposed by FAO/WHO. In cattle, the permissible limit of Fe consumption is 1000 ppm by the national research Council.[31]. It has been reported that in selected medicinal plants of Egypt, the range of Fe was between 261 ppm to 1239 ppm [37]. Iron in the plant Spermadictyon suaveolens Roxb. was found highest in the leaves with 64.609 ppm followed by the root with 30.627 ppm, while the least was found in the stem 6.519 ppm (Table.4).

Table 4. Concentration of Fe and Na in Leaves, stem and root of Spermadictyon suaveolens Roxb
Sodium (Na) Sodium is an essential element, however excessive it is associated with blood pressure and sometimes heart failure in humans [8]. 2.4 gm consumption per day of Na is seen to be beneficial for humans.[46]. High concentration of sodium in the serum is called hypernatremia and may result in salt toxicity [25]. Na in plants acts as a micronutrient that helps in metabolism as well as formation of Phosphonenol pyruvate and chlorophyll synthesis [47]. Na is not an essential element in terrestrial plants for growth and development or reproduction but has been seen to be essential for C4 plants [48]. In certain plants, sodium deficiency resulted in less growth and chlorosis and necrosis of the leaves [49]. And also leads to reduction in PSII activity [50]. The United States Pharmacopeia Convention (USP) has put no concentration limits of elements Na, Fe and Zn impurities [1]. Sodium in the plant Spermadictyon suaveolens Roxb. was found well under usable limit, highest in the leaves with 42.941ppm followed by the stem with 16.26 ppm, while the least was found in the root 15.387 ppm. (Table.4)
CONCLUSION:
As inferred by the above information, the medicinal herb, Spermadictyon suaveolens Roxb. analyzed in this work is a source of biologically active elements, and may contribute in the therapeutic properties of this pant. This information highlights the knowledge of the nutritional properties as well as the mineral contents of this plant. Thus, the human body can use it as a source of macro and micro components. The trace elements contained in the medicinal plants has a significant effect on its pharmacological action. The pharmacological function of medicinal plants is significantly influenced by the trace elements they contain. In addition to the secondary metabolite constituents, this study has shown that this plant accumulates certain elements and thus may be used beneficially for human pharmacognostic practice to good effect. The development and production of new phytomedicines that may be used to control and cure a variety of ailments will be aided by these investigations, even though there is no direct evidence of the element contents in the plant's curative nature.
CONFLICT OF INTEREST:
No conflict of interest has been expressed by the authors in any part of this investigation.
AKNOWLEGEMENT:
The authors are very grateful to IIT SAIF Mumbai for assisting in the analysis for this research.
REFERENCES:
- Tschinkel PFS, Melo ESP, Pereira HS, Silva KRN, Arakaki DG, Lima N V., et al. The Hazardous Level of Heavy Metals in Different Medicinal Plants and Their Decoctions in Water: A Public Health Problem in Brazil. Biomed Res Int 2020;2020.
- Saha A, Pawar VM, Jayaraman S. Characterization of polyphenols in Terminalia arjuna bark extract. Indian J Pharm Sci 2012;74:339–47.
- Garg A, Ruchi S, Maharia R, Dutta R, Arpita D. Quantification of minor, trace and toxic elements in stems of Santalum album (L.), Mangifera indica (L.) and Tinospora cordifolia by instrumental neutron activation analysis. Journal of Plant Science and Phytopathology 2022;6:008–14.
- Dietrich M, Huling J, Krekeler MPS. Metal pollution investigation of Goldman Park, Middletown Ohio: Evidence for steel and coal pollution in a high child use setting. Science of the Total Environment 2018;618:1350–62.
- WHO. Our planet, our health. Report of the WHO Commission on health and environment. 1992.
- Yener ?. Trace Element Analysis in Some Plants Species by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Journal of the Institute of Science and Technology 2019;2019:1492–502.
- Saraf A, Samant A. Evaluation of some minerals and trace elements in Achyranthes aspera Linn. AcademiaEdu 2013;3:229–33.
- Riaz Ullah. Investigation of macro and micro-nutrients in selected medicinal plants. Afr J Pharm Pharmacol 2012;6:1829–32.
- Thakur S, Dutt HC, Singh B, Sharma YP, Tashi N, Charak RS, et al. Plant and fungi diversity of Devi Pindiyan Valley in Trikuta Hills of northwestern Himalaya, India. J Threat Taxa 2019;11:14827–44.
- Kavita M, Mokat D, Pradesh A. Pharmacognostic Studies of Drug Spermadictyon suaveolens Roxb. 2016;3:234–9.
- Rawat DS, Tiwari JK, Tiwari P, Singh H. Floristic diversity of montane zone of Western Ramganga Valley, Uttarakhand, India. J. Econ. Taxon. Bot 2016;40:104–125
- Ejaz Ahmed, Mehr P, Shah A. TAXONOMIC, PHYTOCHEMICAL AND BIOLOGICAL SCREENING OF SOME SELECTED MEDICINAL PLANTS OF LESSER HIMALAYA PAKISTAN. (Thesis) 2018;
- Muhammad Ajaib, Shazia Khalid UH nif. Spermadictyon suaveolens: A potential natural antimicrobial and antioxidant source. International Journal of Phytomedicine 2014;6:256–67.
- Papitha R, Ravi L, Selvaraj CI. Phytochemical Studies and Gc-Ms Analysis of Spermadictyon Suaveolens Roxb. Int J Pharm Pharm Sci 2017;9:143–9.
- Srilatha Srinivas K, Saraf AA. Profile of elemental composition of Oroxylum indicum L.(Vent.) collected from different geographical regions of India. Pharmacognosy Journal [Internet] 2011;3:50–4.
- Musa Özcan M. Determination of the mineral compositions of some selected oil-bearing seeds and kernels using Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES). Grasas y Aceites 2006;57:211–8.
- Bogden JD, Klevay L.M. The Essential Trace Elements and Minerals- Basic Concepts in Clinical Nutrition of the Essential Trace Elements and Minerals: A Guide for Health Professionals. 1989;3:3–9.
- Godswill AG, Somtochukwu IV, Ikechukwu AO, Kate EC. Health Benefits of Micronutrients (Vitamins and Minerals) and their Associated Deficiency Diseases: A Systematic Review. International Journal of Food Sciences 2020;3:1–32.
- Wang ZQ, Cefalu WT. Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Curr Diab Rep 2010;10:145–51.
- Zayed AM, Terry N. Chromium in the environment: Factors affecting biological remediation. Plant Soil 2003;249:139–56.
- Bakar MA, Chandra Bhattacherjy S. Assessment of Heavy Metals Concentration in Some Selected Medicinal Plants Collected from BCSIR, Chittagong Cultivation Area in Bangladesh. Hamdard Med 2012;55:26–32.
- Alphonso P, Saraf A. Chemical profile studies on the secondary metabolites of medicinally important plant Zanthoxylum rhetsa (Roxb.) DC using HPTLC. Asian Pac J Trop Biomed 2012;2:1293–8.
- Maobe M, Gatebe E, Gitu L, Rotich H. Profile of heavy metals in selected medicinal plants used for the treatment of diabetes, malaria and pneumonia in Kisii Region, Southwest Kenya. Global Journal of pharmacology 2012;6:245–51.
- Pawlisz AV, Kent RA, Schneider UA, Jefferson C. Canadian water quality guidelines for chromium. Environ Toxicol Water Qual 1997;12:123–83.
- Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants?: A review. African Journal of Food Science 2010;4:200–22.
- FAO; WHO. Trace elements in human nutrition and health World Health Organization. 1996. Available from: https://apps.who.int/iris/handle/10665/37931
- Khan SA, Khan L, Hussain I, Marwat KB, Akhtar N. Profile of Heavy Metals in Selected Medicinal Plants. J Weed Sci Res 2008;14:101–10.
- Bhat GM, Mukhdoomi MA, Shah BA, Ittoo MS. Dermatoglyphics: in health and disease - a review , Dermatoglyphics?: in health and disease - a review. 2016;2014.
- Cameron JI. Nickel. Natural Resources in US-Canadian Relations, Volume 2: Patterns and Trends in Resource Supplies and Policies 2019;2:45–92.
- FAO/WHO. Contaminants. In Codex Alimentarius, vol. XVII, Edition 1. FAO/WHO Codex Alimentarius Commision, Rome. 1984.
- Jabeen S, Shah MT, Khan S, Hayat MQ. Determination of major and trace elements in ten important folk therapeutic plants of Haripur basin, Pakistan. Journal of Medicinal Plants Research 2010;4:559–66.
- Angelova M, Asenova S, Nedkova V. Copper in the human organism. Trakia Journal of Sciences 2011;9:88–98.
- Al-fartusie FS, Mohssan SN. Indian Journal of Advances in Chemical Science Essential Trace Elements and Their Vital Roles in Human Body. Indian Journal of Advances in Chemical Science 2017;5:127–36.
- Lima PDL, Vasconcellos MC, Montenegro RC, Bahia MO, Costa ET, Antunes LMG, et al. Genotoxic effects of aluminum, iron and manganese in human cells and experimental systems: A review of the literature. Hum Exp Toxicol 2011;30:1435–44.
- Pittman JK. Managing the manganese: Molecular mechanisms of manganese transport and homeostasis. New Phytologist 2005;167:733–42.
- Siabi WK. Application of Mwacafe plant for the removal of Iron and Manganese. People-Centred Approaches to Water and Environmental Sanitation: Proceedings of the 30th WEDC Conference 2004;632–6.
- Sheded MG, Pulford ID, Hamed AI. Presence of major and trace elements in seven medicinal plants growing in the South Eastern Desert, Egypt. J Arid Environ 2006;66:210–7.
- Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. American Journal of Clinical Nutrition 2003;78(3).
- White PJ, Broadley MR. Calcium in plants. Ann Bot 2003;92:487–511.
- Park S, Kang S, Kim DS. Severe calcium deficiency increased visceral fat accumulation, down-regulating genes associated with fat oxidation, and increased insulin resistance while elevating serum parathyroid hormone in estrogen-deficient rats. Nutrition Research 2020;73:48–57.
- Cogswell ME, Looker AC, Pfeiffer CM, Cook JD, Lacher DA, Beard JL, et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003-2006. American Journal of Clinical Nutrition 2009;89:1334–42.
- Camaschella C. Iron deficiency. Blood 2019;133:30–9.
- Dadoun S. Iron deficiency. Praticien en Anesthesie Reanimation 2012;16:94–101.
- Nelson RL. Dietary iron and colorectal cancer risk. Free Radic Biol Med 1992;12:161–8.
- ALGAS ORGANICS InterLab. Certificate of Analysis. Sigma-Aldrich 2019;281:1. Available from: https://cellgenix.com/products/gmp-scgm/
- Imelouane B, Tahri M, Elbastrioui M, Aouinti F, Elbachiri A. Mineral Contents of Some Medicinal and Aromatic Plants Growing in Eastern Morocco. J Mater Environ Sci 2011;2:104–11.
- Mohammed SY. Quantitative phytochemical and elemental analysis of Guiera senegalensis leaf extract. Journal of Pharmacognosy and Phytotherapy 2013;5:204–7.
- Maathuis FJM. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J Exp Bot 2014;65:849–58.
- Brownell PF. Sodium as an Essential Micronutrient Element for Plants and its Possible Role in Metabolism. Adv Bot Res 1980;7:117–224.
- Subbarao G V., Ito O, Berry WL, Wheeler RM. Sodium - A Functional Plant Nutrient. CRC Crit Rev Plant Sci 2003;22:391–416.


 Divya Lobo P.*
Divya Lobo P.*
 Aparna Saraf
Aparna Saraf




 10.5281/zenodo.10931738
10.5281/zenodo.10931738