Abstract
This study aimed to evaluate the concentration of azadirachtin and the fatty acid constants of neem leaf oil for its potential as a bio-insecticide, cosmetics, and nutritional supplements. The objective is to quantify azadirachtin using High-Performance Liquid Chromatography (HPLC) and assess the oil’s phytochemical properties. HPLC analysis, using a reverse-phase column with a methanol mobile phase (60:40), revealed the highest azadirachtin concentration in semi-dried leaves (20.12 µg/mL), followed by fresh (11.7µg/mL) and dried leaves (5.85 µg/mL). Analytical tests determined the acid value (4 mg NaOH/g oil), saponification value (140 mg NaOH/g oil), iodine value (15 mg I?/100g oil), RM value (27.5 mg NaOH/g oil), and Polenske value (50 mg NaOH/g oil). These results highlight neem leaf oil as a promising source of bioactive compounds, suitable for pharmaceutical and industrial use. The study confirms the efficacy of HPLC in quantifying azadirachtin and emphasizes neem's diverse applications.
Keywords
Azadirachtin, HPLC, Phytochemicals, Iodine value, Saponification value.
Introduction
Azadirachta indica, commonly known as Neem, is a medicinal plant belonging to the family Meliaceae. It is native to India and other parts of South Asia and has been used since ancient times for its healing properties, particularly for treating wounds and skin diseases. Various parts of the tree, including its leaves, seeds, and fruits, have been used for medicinal purposes [1].
The neem tree contains several bioactive compounds, one of the most abundant being azadirachtin. Azadirachtin is a limonoid, specifically a tetranortriterpenoid, and has a chemical formula C??H??O?? and a molecular weight of 720.72 g/mol [1]. Azadirachtin is primarily extracted in large quantities from the kernel of neem seeds, it is present in a large concentration [2].
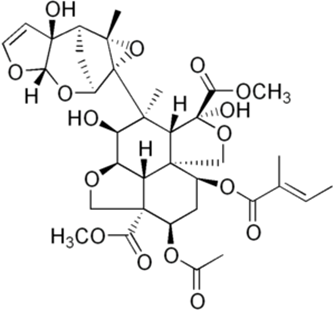
Fig 1: Structure of Azadirachtin
It is best known for its pesticidal activity, making it widely used in agriculture due to its biodegradability and minimal toxicity to humans. In addition to its pesticidal properties, azadirachtin exhibits antimicrobial, antimalarial, and anticancer activities [3]. In addition to azadirachtin, neem seeds also contain significant amounts of fatty acids, which contribute to the medicinal and pesticidal properties of the tree [4].
Fatty acids are essential components of lipids and play critical roles in energy storage, cell membrane structure, and signaling. The primary fatty acids found in neem oil include linoleic acid, oleic acid, palmitic acid, and stearic acid, along with smaller amounts of arachidic acid and behenic acid [4].
These fatty acids hold significant commercial value, being used in the production of soaps, cosmetics, and detergents. For instance, sodium and potassium salts derived from fatty acids are key ingredients in soaps, while fatty acids in skincare products help maintain skin health and function. Neem oil, rich in both azadirachtin and fatty acids, has a typical aromatic odor that repels insects and is also helpful in treating chronic skin conditions such as psoriasis, eczema, and ringworm. Furthermore, neem oil is widely used in cosmetics to rejuvenate and improve skin appearance [5]. While previous research has predominantly focused on the availability of azadirachtin and fatty acids in neem seed oil, the current study shifts its focus to the leaves of the neem tree, which are abundantly available year-round. The aim is to evaluate and determine the concentration of azadirachtin and fatty acids from neem leaves. The rationale behind choosing leaves over seeds is their constant availability, making them a more sustainable and accessible resource for further research and practical applications.
Methodology
Collection and Preparation of neem leaves
Neem leaves, free from pests and diseases are collected. The leaves are thoroughly washed with water, followed by drying at room temperature, the leaves are categorized into three groups: dried, semi-dried, and fresh leaves. The leaves are shed-dried for a week. Subsequently, all three categories of neem leaves were subjected to fine grinding using a mechanical mixer blender to achieve a uniform powdered consistency. This powder was then used for subsequent analysis [6]. Extraction of Azadirachtin from neem leaves Aza is extracted using Soxhlet extraction. 20g of fine powder of neem leaves is placed in a thimble in the Soxhlet apparatus, and 100 ml of methanol is added to the round bottom flask. The heating mantle is kept on for an hour, allowing about 8 cycles of continuous vaporization, condensation, percolation, and siphoning to be repeated to get the concentrated extract. Visually, the extracts appeared as dark green to brownish solutions, with the intensity of color correlating to the concentration of active compounds in the extract. The extracts were then stored for further chemical analysis [6 &7].
Evaluation of Azadirachtin from neem leaves by HPLC
The Azadirachtin is determined using the HPLC. Chromatographic separation is carried out in the RP column, with a detection wavelength set at 214nm. The mobile phase consists of Methanol: Water (60:40), and the flow rate is set at 1ml/min [7]. Evaluation of Fatty acid from neem leaves by chemical method For the fatty acid determination, the oil is extracted by the process of solvent evaporation using a boiling water bath. For the determination of fatty acid constants, the following tests are performed: Acid number, iodine number, saponification number, Richert Meissal number, and Polenske number. The oil appeared as a brownish liquid, characteristic of neem oil, with a distinct herbal aroma [9].
Acid value determination: The acid value is determined by dissolving the oil in neutralized ethanol, followed by titration with 0.1 N NaOH in the presence of a phenolphthalein indicator until a stable pink endpoint is reached, as described in the literature [8]. The acid value is calculated using the formula:
Acid value=(Mol wt of NaOH ×Normality of NaOH ×Volume of titrant)/(Weight of sample (Ws)
The iodine value of the oil is determined using standard titration methods as described in the literature [8, 9]. A known amount of oil is reacted with Wij’s solution, followed by titration with sodium thiosulfate after the addition of potassium iodide and a starch indicator. A blank titration is also conducted for comparison. It is calculated by using the formula:
Iodine value=(12.69×(Vb-Vs)×N)/( (Ws)
Where,
Vb =Volume of sodium thiosulfate used in the blank titration (mL)
Vs = Volume of sodium thiosulfate used in the sample titration (ml)
N = Normality of sodium thiosulphate solution
Ws = Weight of sample
The saponification value is determined by heating the oil with excess alcoholic KOH, followed by titration of the remaining KOH with HCl, using phenolphthalein as an indicator, as outlined in the literature [8]. The saponification value is then calculated based on the amount of KOH consumed by using the formula given below:
Saponification value=(28.05×(A-B)×N)/( (Ws)
Where,
WS = Sample weight
A = Titration volume of blank
B = Titration volume of sample
N = Normality of HCl solution
- Reichert-Meissl (RM) value
The RM value is determined by saponifying the oil with sodium hydroxide, followed by acidification with sulfuric acid. The volatile fatty acids are distilled, collected, and titrated with sodium hydroxide, as described in the literature [10]. It is calculated as given below:
Reichert-Meissl value = (A – B) × N × 11
Where,
A = Volume, in ml, of NaOH solution required for the test,
B = Volume, in ml, of NaOH solution required for blank,
N = Normality of NaOH solution
The Polensky value assesses the quality of fatty acids in oils by measuring unsaturation. To determine it, 0.5g of oil sample is dissolved in a non-polar solvent like hexane. A 0.1 N NaOH solution was used to titrate the oil solution until a stable endpoint is reached, indicated by a color change with phenolphthalein [10]. The Polensky value is calculated using the formula:
Polensky value = 10 ×V×N
Where,
A = Volume, in ml, of NaOH solution required for the test
B = Volume, in ml, of NaOH solution required for blank
N = Normality of NaOH solution
RESULTS
The current study of Evaluation and determination of Azadirachtin and fatty acid constants from Azadirachta indica depicts the following result :
HPLC Analysis
The HPLC analysis for the Azadirachtin standard is conducted using an RP column with a mobile phase Methanol: Water (60:40) at a flow rate of 1 mL/min, a detection wavelength of 214 nm, and a column temperature of 37°C. The standard’s retention time is 2.88 minutes, with a peak area of 33.7721 mAUmin. In the samples, Sample 1 (dried leaves) showed a retention time of 3.327 minutes and a peak area of 197.78 mAUmin, indicating an Azadirachtin concentration of 5.85 µg/mL. Sample 2 (semi-dried leaves) had a retention time of 3.253 minutes and a peak area of 679.7476 mAUmin, with an Azadirachtin concentration of 20.12 µg/mL. Sample 3 (fresh leaves) showed a retention time of 2.305 minutes and a peak area of 395.5559 mAUmin, indicating a concentration of 11.71 µg/mL. This data highlights differences in Azadirachtin concentration and retention times among the samples analyzed.
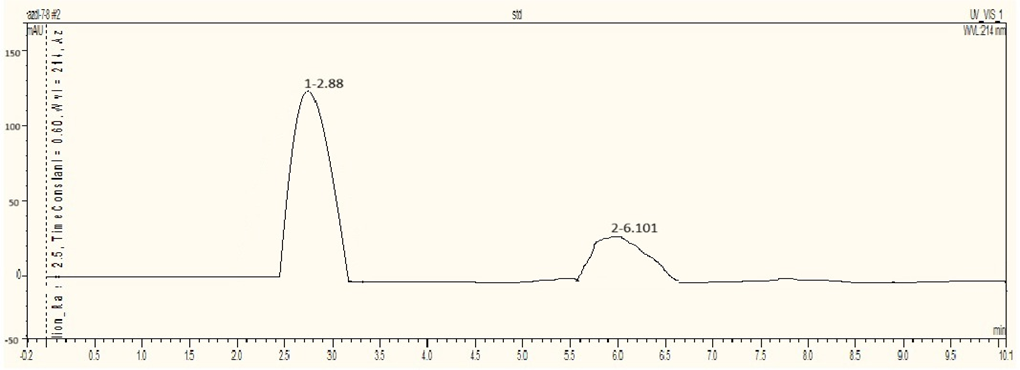
Fig 2: Chromatogram for Azadirachtin Standard
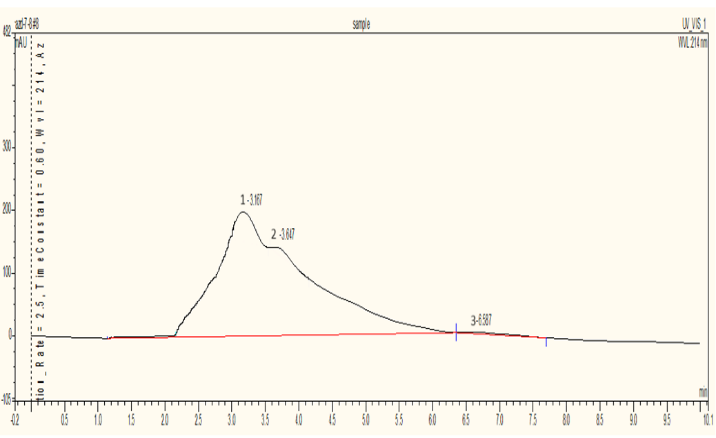
Fig. 3: Chromatogram for Sample 1 (Fully dried neem leaves)
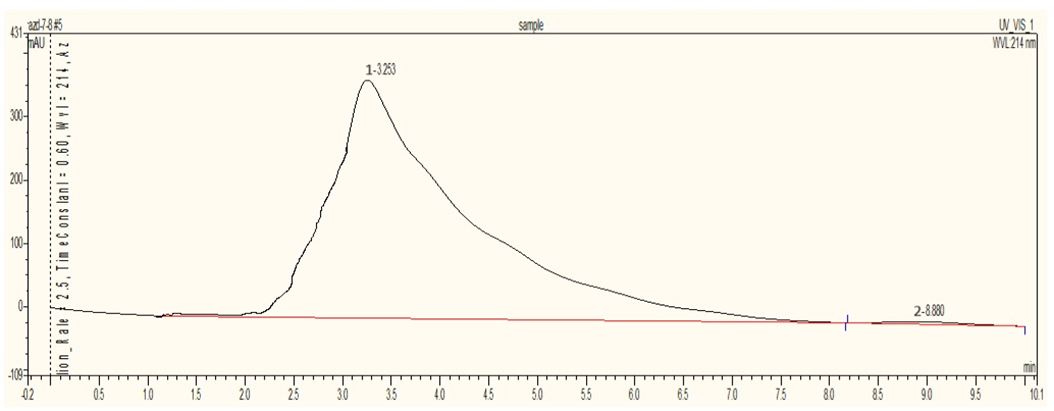
Fig. 4: Chromatogram of Sample 2 (Semi-dried neem leaves)
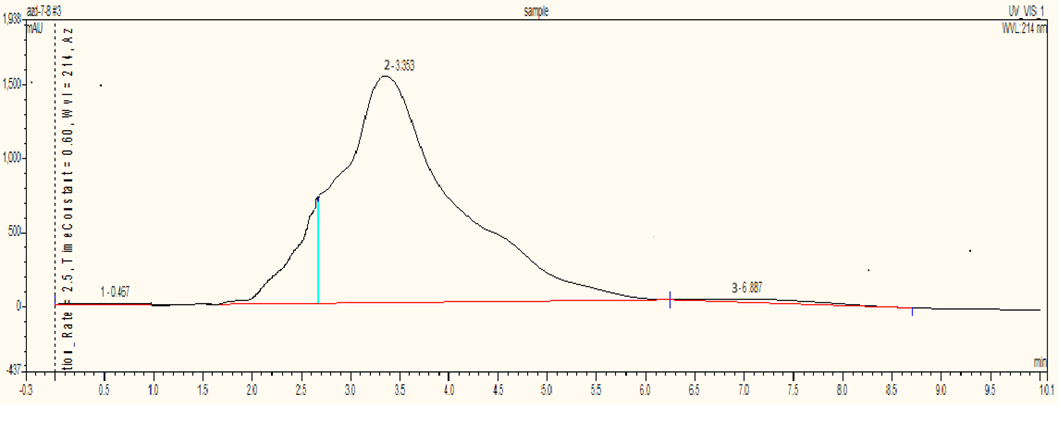
Fig. 4: Chromatogram of Sample 3 (Fresh neem leaves)
Determination of fatty acid constants from neem leaf oil
The fatty acid present in the neem oil is subjected to different analytical methods for the determination of the physiochemical properties of oil. In this analysis we obtain the following value: an acid of 4mg NaOH/g oil indicates a low level of free fatty acid, suggesting it is less prone to degradation. The saponification value of 140mg NaOH/g oil indicates short-chain and medium-chain fatty acids. The iodine value of 15g I2/100g oil indicates a low degree of unsaturation which makes the oil more stable and less prone to oxidation. RM no. 27.5mg NaOH/g oil suggests that short-chain fatty acids are present moderately. Polansky no. 50mgNaOH/g oil which indicates a significant amount of unsaturation.
DISCUSSION
In the current research of Evaluation and determination of Azadirachtin and fatty acid constants from Azadirachta indica, HPLC analysis confirmed that semi-dried neem leaves yielded the highest concentration of azadirachtin (9.3 µg/ml), compared to fresh (4.7 µg/ml) and fully dried leaves (3.7 µg/ml) when compared to the standard peak of Aza. This supports earlier studies, such as [2], observed variations in AZA concentrations depending on the drying method of neem seeds. Similarly, [1] emphasized optimizing extraction conditions to preserve azadirachtin's bioactivity. The purity of azadirachtin in the sample may be compromised due to co-existing compounds such as polyphenols and terpenoids etc., which interfere with the accurate quantification of azadirachtin. Their study [7] compared different extraction methods (cold press and Soxhlet) and highlighted how the choice of technique impacts the yield of azadirachtin. While their study focused on neem seed extraction, our findings with neem leaves suggest that optimizing pre-extraction conditions, such as drying, can significantly enhance the efficiency of extraction, even with different parts of the neem plant. In line with the physicochemical analysis of neem oil, our study showed a high acid value of 4 mg NaOH/g oil, indicating a notable presence of free fatty acids. This aligns with findings from [5], who also reported significant free fatty acid content in neem oil, which can result from hydrolysis during extraction. A low iodine value of 15 g I?/100 g oil suggests that the oil contains predominantly saturated fatty acids, making it stable and resistant to oxidative degradation—a characteristic desirable for pharmaceutical and cosmetic applications [14]. The moderate saponification value (140 mg NaOH/g oil) and Reichert-Meissl value (27.5 mg NaOH/g oil) further indicate the presence of short to medium-chain fatty acids, which are associated with good soap-making properties, as also highlighted by [12]. Our study also recorded a high Polenske value (50 mg NaOH/g oil), suggesting the presence of volatile fatty acids, which can contribute to the antimicrobial properties of neem oil. This is consistent with [4], who found that neem oil's fatty acid composition enhances its antibacterial activity, making it promising for medicinal and cosmetic formulations. Comparing our findings to those of [6], who investigated the simultaneous extraction of oil and azadirachtin, the semi-dried leaves method in our study proved to be a superior approach for maximizing azadirachtin yield without compromising the physicochemical properties of neem oil. This makes the semi-drying process highly efficient for both pharmaceutical and biopesticide applications, where a balance between high azadirachtin concentration and oil stability is required.
CONCLUSION
In the current research of Evaluation and determination of Azadirachtin and fatty acid constants from Azadirachta indica, HPLC analysis confirmed that semi-dried neem leaves yield the highest concentration of azadirachtin, making partial drying the optimal method for maximizing its extraction. Excessive drying diminishes azadirachtin content, critical for enhancing the potency and cost-effectiveness of neem-based biopesticides, pharmaceuticals, and cosmetics. Neem oil analysis further revealed a high acid value, indicating significant free fatty acid content, alongside a low iodine value, reflecting the predominance of stable, saturated fatty acids. The moderate saponification, Reichert-Meissl, and Polensky values suggest short to medium-chain fatty acids and volatile compounds. These unique properties underscore neem oil’s immense potential in pharmaceuticals, cosmetics, and food industries, positioning it as a valuable, multifunctional natural resource.
REFERENCES
- Fernandes, S., Santos, N., & Morgado, J. (2020). Chemistry, bioactivities, extraction, and analysis of azadirachtin: State-of-the-art. Fitoterapia, 134, 104732. https://doi.org/10.1016/j.fitote.2019.02.006
- Dubhashi, S., Pranay, V., Singaiah, M., Satwik, J., Prasad, V. V. L. N., & Diwan, P. V. (2013). Studies on extraction and HPLC analysis of azadirachtin from kernels of neem seeds. Journal of Advanced Pharmacy Education & Research, 3(1), 57-60.
- Alzohairy, M. A. (2020). Therapeutic roles of Azadirachta indica (Neem) and their active constituents in disease prevention and treatment. Evidence-Based Complementary and Alternative Medicine, 7382506. https://doi.org/10.1155/2016/7382506.
- Sandanasamy, J., Nour, A. H., Tajuddin, S. N., & Nour, A. H. (2013). Fatty acid composition and antibacterial activity of neem (Azadirachta indica) seed oil. The Open Conference Proceedings Journal, 4, 43-48.
- Yahaya, S., Sani, A., Murtala, Y., Momoh, H., & Mohammed, S. (2022). Determination of fatty acids and physicochemical properties of neem (Azadirachta indica L.) seed oil extracts. Dutse Journal of Pure and Applied Sciences, 8(2), 149-160. https://dx.doi.org/10.4314/dujopas.v8i1a.16
- Subramanian, S., Salleh, A., & Bachmann, R. (2020). Simultaneous extraction and separation of oil and azadirachtin from seeds and leaves of Azadirachta indica using binary solvent extraction. Natural Product Sciences, 25(2), 150-158. https://doi.org/10.20307/nps.2019.25.2.150
- Esparza-Díaz, G., Villanueva-Jiménez, J., López Collado, J., & Rodríguez-Lagunes, D. (2010). Azadirachtin extraction using cold press and Soxhlet methods. Biopesticides International, 6(1), 45–51.
- Nnam, R. E., Okoro, O. I., Oko, A. N., & Oji, C. O. (2021). Crop protectant from pest by neem (Azadirachta indica) oil bioinsecticide. Central Asian Journal of Theoretical and Applied Sciences, 2(2), 63-76. ISSN 2660-5317.
- Raghuramulu, N., Nair, K. M., & Kalyanasundaram, S. (Eds.). (2003). A manual of laboratory techniques. National Institute of Nutrition.
- Aggarwal, M. (2018). Experiment-27: Determination of Reichert Meissl (RM) value and Polenske value (PV) in oils and fats. Indira Gandhi National Open University (IGNOU). http://egyankosh.ac.in//handle/123456789/43431.
- Hundie, K., Abdissa, D., & Bayu, A. (2022). Extraction, optimization, and characterization of neem seed oil via Box-Behnken design approach. Journal of the Turkish Chemical Society Section A: Chemistry, 9(2), 513-526. https://doi.org/10.18596/jotcsa.1039997
- Das, P., Sharma, N., Puzari, A., et al. (2021). Synthesis and characterization of neem (Azadirachta indica) seed oil-based alkyd resins for efficient anticorrosive coating application. Polymer Bulletin, 78, 457–479. https://doi.org/10.1007/s00289-020-03120-8
- Sarkar, S., Singh, R. P., & Bhattacharya, G. (2021). Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: An update on molecular approach. 3 Biotech, 11(4), 178. https://doi.org/10.1007/s13205-021-02745
- Hamadou, B., Djomdi, F., Falama, R. Z., Cedric, D., Guillaume, P., Pascal, D., & Philippe, M. (2020). Influence of physicochemical characteristics of neem seeds (Azadirachta indica A. Juss) on biodiesel production. Biomolecules, 10(4), 616. https://doi.org/10.3390/biom10040616
- Islas, J. F., Acosta, E., G-Buentello, Z., Delgado-Gallegos, J. L., Moreno-Treviño, M. G., Escalante, B., & Moreno-Cuevas, J. E. (2020). An overview of neem (Azadirachta indica) and its potential impact on health. Journal of Functional Foods, 74, 104171. https://doi.org/10.1016/j.jff.2020.104171
- Anya, A. U., Chioma, N. N., & Obinna, O. (2012). Optimized reduction of free fatty acid content on neem seed oil, for biodiesel production. Journal of Basic and Applied Chemistry, 2(4), 21-28.
- Tesfaye, B., & Tefera, T. (2017). Extraction of essential oil from neem seed by using Soxhlet extraction methods. International Journal of Advanced Engineering, Management and Science (IJAEMS), 3(6), 646. https://doi.org/10.24001/ijaems.3.6.5
- Tesfaye, B., Tefera, T., Misikir, O., & Tsegaye, G. (2018). Extraction and comparison of essential oil from neem seed by using Soxhlet extraction and simple distillation methods. International Journal of Engineering Technologies and Management Research, 5(9), 74–81. https://doi.org/10.29121/ijetmr.v5.i9.2018.291
- Ayoola, A. A., Efeovbokhan, V. C., Bafuwa, O. T., & David, O. T. (n.d.). A search for alternative solvent to hexane during neem oil extraction. Chemical Engineering Department, Covenant University, Ota, Nigeria. https://doi.org/10.24001/ijaems.
- Liauw, M. Y., Natan, F. A., Widiyanti, P., Ikasari, D., Indraswati, N., & Soetaredjo, F. E. (2008). Extraction of neem oil (Azadirachta indica A. Juss) using n-hexane and ethanol: Studies of oil quality, kinetic and thermodynamic. ARPN Journal of Engineering and Applied Sciences, 3(3), 49-54.
- Hiremath, S. I., Menasinakai, A. S., Gharge, S., Koganole, P., & Mali, N. (2023). Quality control and standardization of azadirachtin in herbal medicines by spectroscopic and chromatographic techniques. International Journal of Creative Research Thoughts (IJCRT), 11(2), 210–220. https://doi.org/10.13140/RG.2.2.31278.27207?
- Huang, H. P., & Morgan, E. D. (1990). Analysis of azadirachtin by supercritical fluid chromatography. Journal of Chromatography A, 519, 137-143. https://doi.org/10.1016/S0021-9673(01)84011-4
-
- Ambrosina, P., Fresa, R., Fogliano, V., Monti, S. M., & Ritieni, A. (1999). Extraction of azadirachtin A from neem seed kernels by supercritical fluid and its evaluation by HPLC and LC/MS. Journal of Agricultural and Food Chemistry, 47(12), 5252-5256. https://doi.org/10.1021/jf9905368
- Forim, M. R., da Silva, M. F. G. F., Cass, Q. B., Fernandes, J. B., & Vieira, P. C. (2010). Simultaneous quantification of azadirachtin and 3-tigloylazadirachtol in Brazilian seeds and oil of Azadirachta indica: Application to quality control and marketing. Analyst, 135(9), 2334–2340. https://doi.org/10.1039/c0ay00008f
- Kilani-Morakchi, S., Morakchi-Goudjil, H., & Sifi, K. (2021). Azadirachtin-based insecticide: Overview, risk assessments, and future directions. Frontiers in Agronomy, 3, 676208. https://doi.org/10.3389/fagro.2021.676208


 Dr. Raja Kumar Parabathina*
Dr. Raja Kumar Parabathina*
 Nidhi Dubey
Nidhi Dubey





 10.5281/zenodo.14328527
10.5281/zenodo.14328527