Abstract
This study investigated the phytochemical composition and therapeutic potential of onion peel (Allium cepa L.) extract, focusing on its anti-inflammatory and antibacterial properties. The onion peel was subjected to extraction and fractionation using petroleum ether, diethyl ether, and ethyl acetate. The resultant extracts were analysed by thin-layer chromatography (TLC) and subsequently confirmed using infrared (IR) spectroscopy, mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy, leading to the identification of key bioactive compounds such as quercetin, isorhamnetin, and kaempferol. The anti-inflammatory activity of the extract was evaluated in a carrageenan-induced paw oedema model in Wistar albino rats. The ethanolic extract demonstrated a dose-dependent reduction in paw thickness, with significant effects observed at 200 mg/kg and 400 mg/kg doses, comparable to those of the standard drug indomethacin. The antibacterial potential was assessed against gram-positive (Staphylococcus aureus and Bacillus subtilis) and gram-negative (Proteus vulgaris and Escherichia coli) bacteria at concentrations ranging from 100 to 800 µg/µl, using ciprofloxacin as a reference. The maximum inhibition zone was observed at 800 µg/µl. These findings underscore the significant anti-inflammatory and antimicrobial properties of onion peel extract and confirm the presence of key flavonoids responsible for its bioactivity. These results suggest the potential of onion peel waste as a valuable source of natural therapeutic agents, warranting further pharmaceutical and biomedical research.
Keywords
Anti-Inflammatory, Anti-Bacterial, Onion Peel, Bioactive Compounds.
Introduction
For millennia, natural products have played a crucial role in healthcare and disease prevention. Ancient civilizations, including those in India, China, and North Africa, have documented the use of natural resources to treat various ailments. The global transition towards processed food consumption, insufficient physical activity, sedentary lifestyles, and exposure to polluted environments has resulted in an increase in disorders such as obesity, cardiovascular diseases, diabetes, neurodegenerative conditions, and cancers. Oxidative stress is considered a primary factor in these health issues. Studies have demonstrated that plant-based bioactive phytonutrients can protect against damage caused by excessive free radical formation and oxidative stress. Onion peel has emerged as an excellent source of fructo-oligosaccharides, dietary fibers, polyphenols, and antioxidants[1]. The chemical composition of onions is influenced by factors such as environment, cultivar type, agricultural practices, ripening stage, and preservation duration. Notably, onion peel contains significantly higher levels of flavonoids than the edible portion. The yellow and purple colours of onions are attributed to two main subgroups of flavonoids, anthocyanins, and quercetin, along with their derivatives[2]. The outer layer of onions is particularly rich in major flavonoids, including quercetin di-glucoside, quercetin, quercetin aglycone, kaempferol, and quercetin 4-O-glucoside[3]. Extensive research on onion peel/waste and its extracts has revealed that they are a rich source of phytoconstituents with diverse therapeutic potential and pharmacological activities[4, 5]. A deeper understanding of these biological properties could facilitate further research and potential applications of onion waste in biomedical and pharmaceutical industries. This study aimed to evaluate the anti-inflammatory and antibacterial activities of onion peel extract and analyse its bioactive compounds.
MATERIALS AND METHODS
Dried onion peels were collected from different onion vendors at Perundurai, Erode, Tamil N?du.
Method Of Extraction
The scales were pulverized in laboratory mill and sieved. Particles smaller than 0.2 mm was used to experiments for extraction. Extraction was done by 2 hours heating under reflux condenser with 96 percent ethanol. (Proportion of solvent to material was:50 ml of solvent per 1 g of powder). Extracts were filtered under vacuum, and 20 microliters of clear supernatants were taken to analysis. Solvent was evaporated in rotary evaporator under vacuum in 40°C. The residue was collected[6, 7].
Fractionitation Of Extract by Solvent-Solvent Extraction Method
The ethanolic extract was dissolved in water and the aqueous suspension of ethanolic extract was sequentially extracted with petroleum ether, diethyl ether and ethyl acetate solvents (solvents are chosen based on their order of polarity i.e., non-polar to polar) using separatory funnel. 2g of the ethanolic extract was dissolved in 20 ml of water. Then the aqueous suspension of ethanolic extract was extracted with 40 ml of petroleum ether for 30 minutes which was repeated for at least three times. The petroleum ether phase(FS1) was collected and evaporated to dryness under vacuum. As in the similar way, the aqueous suspension of ethanolic extract was sequentially extracted with diethyl ether and ethyl acetate and evaporated to dryness to give the corresponding diethyl ether(FS2) and ethyl acetate(FS3) components respectively. The samples of respective solvents are taken for further analysis[8, 9].
Fractionation Scheme for Solvent-Solvent Extraction
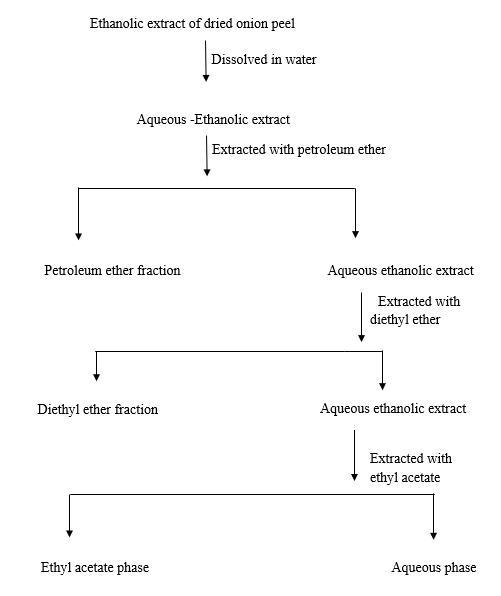
All the three samples were undergone confirmatory tests and TLC. The given below table mention the solvent system for identification of flavonoids in TLC.
Table:1 Solvent system for identification of flavonoids in TLC.
|
Stationary phase
|
Silica gel G
|
|
Mobile phase
|
Chloroform: methanol: formic acid (6:4:1)
|
|
Chamber saturation
|
30 mins
|
|
Visualization
|
Solution of methanolic ferric chloride solution
|
After performing TLC, the respective fractionated samples were undergone IR, MASS and NMR spectral analysis to confirm the major compound present in samples.
Evaluation Of Anti-Inflammatory Activity of Whole (Allium Cepa. L) Onion Peel Extract.
The anti-inflammatory property of onion peel (Allium Cepa. L) extract was studied against carrageenan induced acute paw edema in rats.
Animals
Experiments were performed on Wister albino rat (300-350 gm) housed at 22 ± 2o C under a 12-h light/12-dark cycle with free access to food and water. Experimental animals were obtained from the animal house of NCP, Erode(Institutional Animal ethical committee no: NCP/IAEC/UG-2022-23-06). Throughout the experiment period, the animals were housed in large spacious propylene cage boxes. The animals were with standard laboratory diet and water.
Chemicals
Carrageenan, Standard drug- Indomethacin.
Procedure
24 Male Wister rats were weighed and taken for the study. Rats were divided into four groups (n = 6), each receiving normal (control), Indomethacin (20 mg/kg p.o.) (reference standard), and 200 and 400 mg/kg of ethanolic extract of onion peel respectively, which are administered before 30 mins eliciting paw edema. Carrageenan (0.1 ml of 1%) was injected into the sub plantar tissue of the right hind-paw of each rat to provide acute inflammation. The volume of the carrageenan injected into the foot was measured at 0, 30, 60, 120, and 180 minutes using a vernier[10, 11].
Evaluation Of Anti-Bacterial Activity of Whole Onion Peel (Allium cepa. L) Extract
Minimum Inhibitory Concentration
The onion peel extract was serially diluted to concentrations ranging from 800 to 25 µg/100 µL (800, 400, 200, 100, 50, and 25). These dilutions were individually added to the appropriate microbial growth medium in separate test tubes. Subsequently, each test tube was inoculated with the bacterial strains. The tubes were incubated overnight, after which the turbidity of the medium was assessed to evaluate bacterial growth.
Anti-Bacterial Activity
Antibacterial activity screening was performed for the ethanolic extract of onion peel using around two-gram positive (Staphylococcus aureus, Bacillus subtillis) and two-gram negative bacterial strains(Proteus vulgaris,Escherichia coli). Various concentration of extract was used (100µg/µl, 200µg/µl), 400µg/µl, 800 µg/µl) to test the antibacterial activity against ciprofloxacin(10 µg/ml ) as the standard drug and ethanol as a control.
Procedure
The medium was prepared by heating a specified quantity of dehydrated medium in purified water using a water bath and subsequently distributing it into 100 ml conical flasks. These flasks were sealed with cotton plugs and sterilized in an autoclave at 121°C (15 lb.) for 45 min. Sterile universals were administered to 25 ml of the agar medium. Flat-bottomed Petri dishes were positioned on a level surface to ensure uniform thickness of the medium layer. Each universal was combined with 0.2 ml of various bacterial strains and nutrient agar medium in sterile petri dishes and then allowed to solidify. Using a cork-borer (6 mm), four wells were created in each agar plate containing the bacterial culture. Test solutions (100, 200, 400 and 800 µg/µl)) were prepared using ethanol as the solvent. Under aseptic conditions, 0.2 ml of each solution was introduced into the wells using a dropping pipette and labelled. The plates were maintained at room temperature for 3-5 hours to facilitate diffusion of the solution. For antibacterial activity testing, the Petri dishes were incubated at 37°C for 24 h. The diameter of the inhibition zone (mm) surrounding each well was then measured. The results were compared to those of ciprofloxacin (10 µg/ml) for antibacterial activity assessment[12, 13].
RESULTS AND DISCUSSION
Confirmatory Test
The confirmatory tests such lead acetate and sodium hydroxide test shows positive response for the fractionated samples which indicates the presence of flavonoids[14].
Thin Layer Chromatography
From the TLC, Rf value of the fragmented samples were calculated and found to be 0.87,0.89,0.84 respectively.
Spectroscopic Analysis Of Fractionated Samples (Fs1-3) From Dried Onion Peel Extract
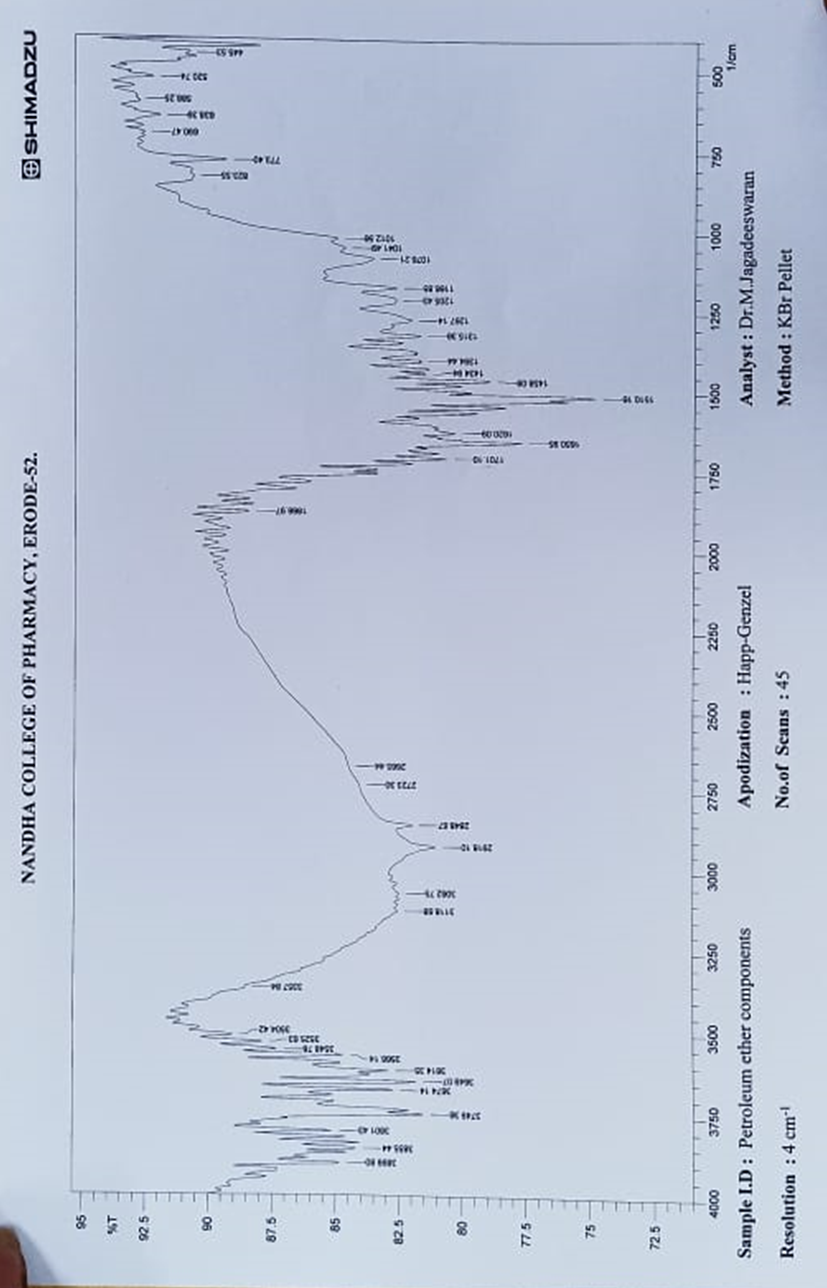
Figure:1 IR Spectrum of Fractionated Sample (FS1)
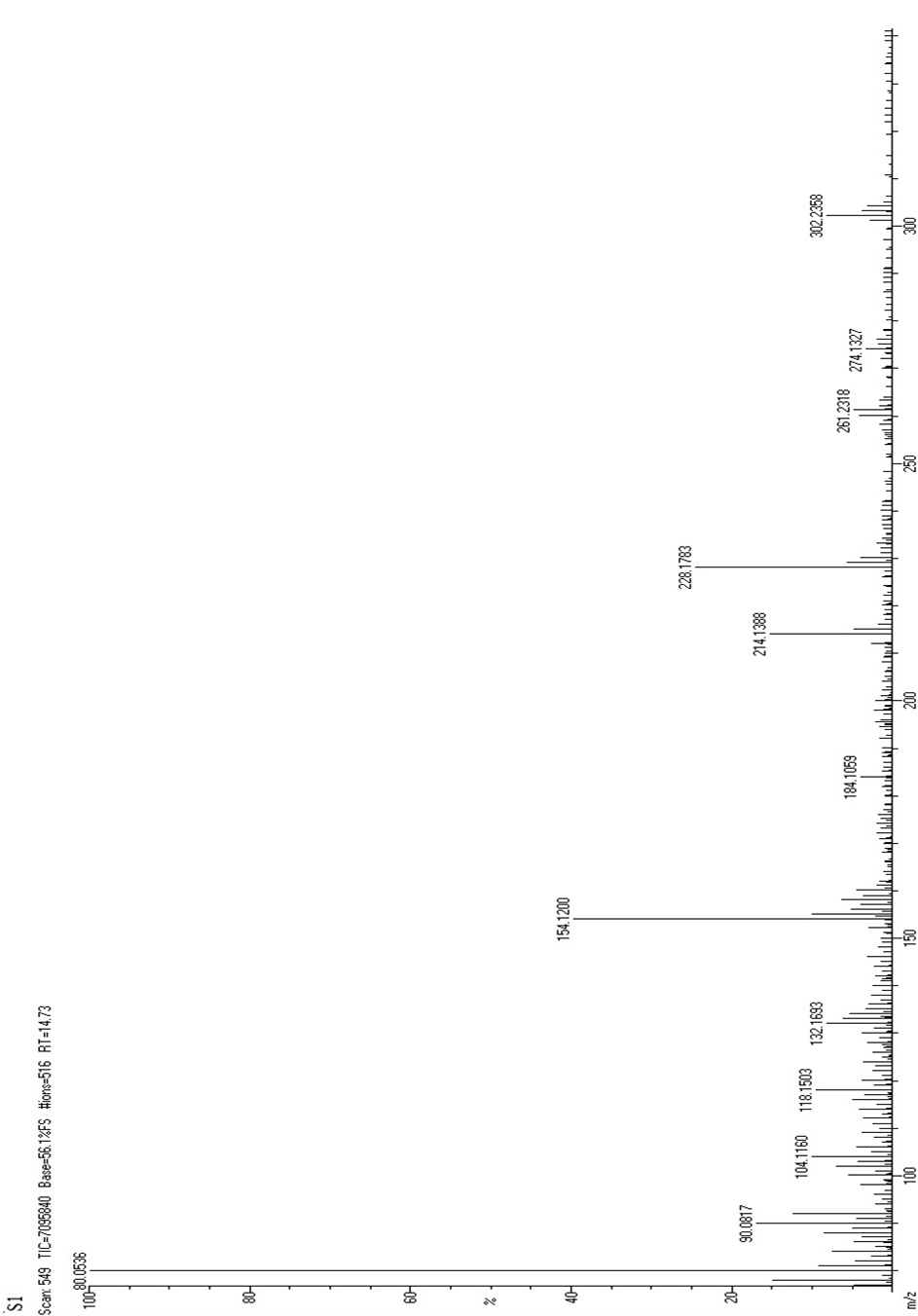
Figure:2 Mass Spectrum of Fractionated Sample (FS1)
The proposed fragmentation pattern of fractionated sample (FS1) is as follows:


Figure:3 NMR Spectra of Fractionated Sample (FS1)
Spectral Data of Fractionated Sample (FS1)
FT-IR analysis revealed peaks at 3504 cm-1and 1078 cm -1,1166 cm -1,1205 cm -1,1267 cm-1 which were consistent with the presence of hydroxyl groups. The peak at 1650 cm-1 was attributed to the stretching vibration of a carbonyl group. Several others peaks were observed at 1620 cm -1, 1510 cm -1, and 1458 cm-1 which were consistent with the presence of a phenyl ring skeleton. The peaks observed at 1701 cm-1 also sug gested the presence of oxygen containing heterocycle. Mass M/Z=302.23 (C15H10O7), 275.23(C14H11O6), 261.20 (C13H9O6), 231.22(C13H11O4)
216.18(C12H8O4), 152.10 (C7H4O4), 134.13 (C8H6O2). NMR (ppm)=7.14(Aromatic CH), 3.85(-OH group), 7.10(C4H8O2). Hence the sample FS1 is predicted to contain Quercetin.
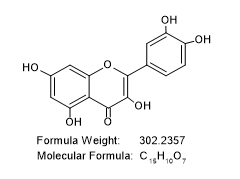
IUPAC NAME: 2-(3,4-dihydroxy phenyl)-3,5,7-trihhydroxy chromen-4-one
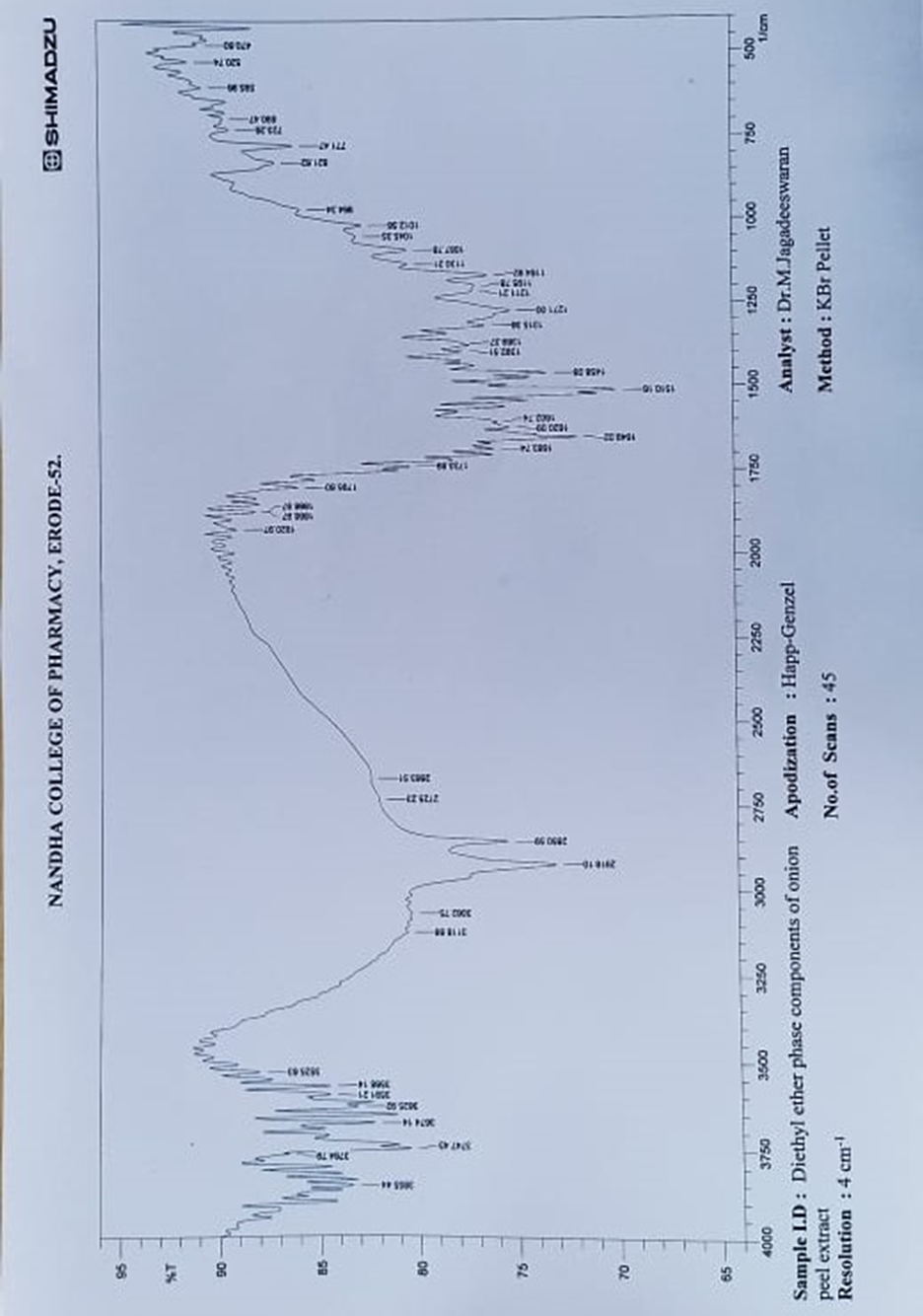
Figure;4 IR Spectrum of Fractionated Sample (FS2)
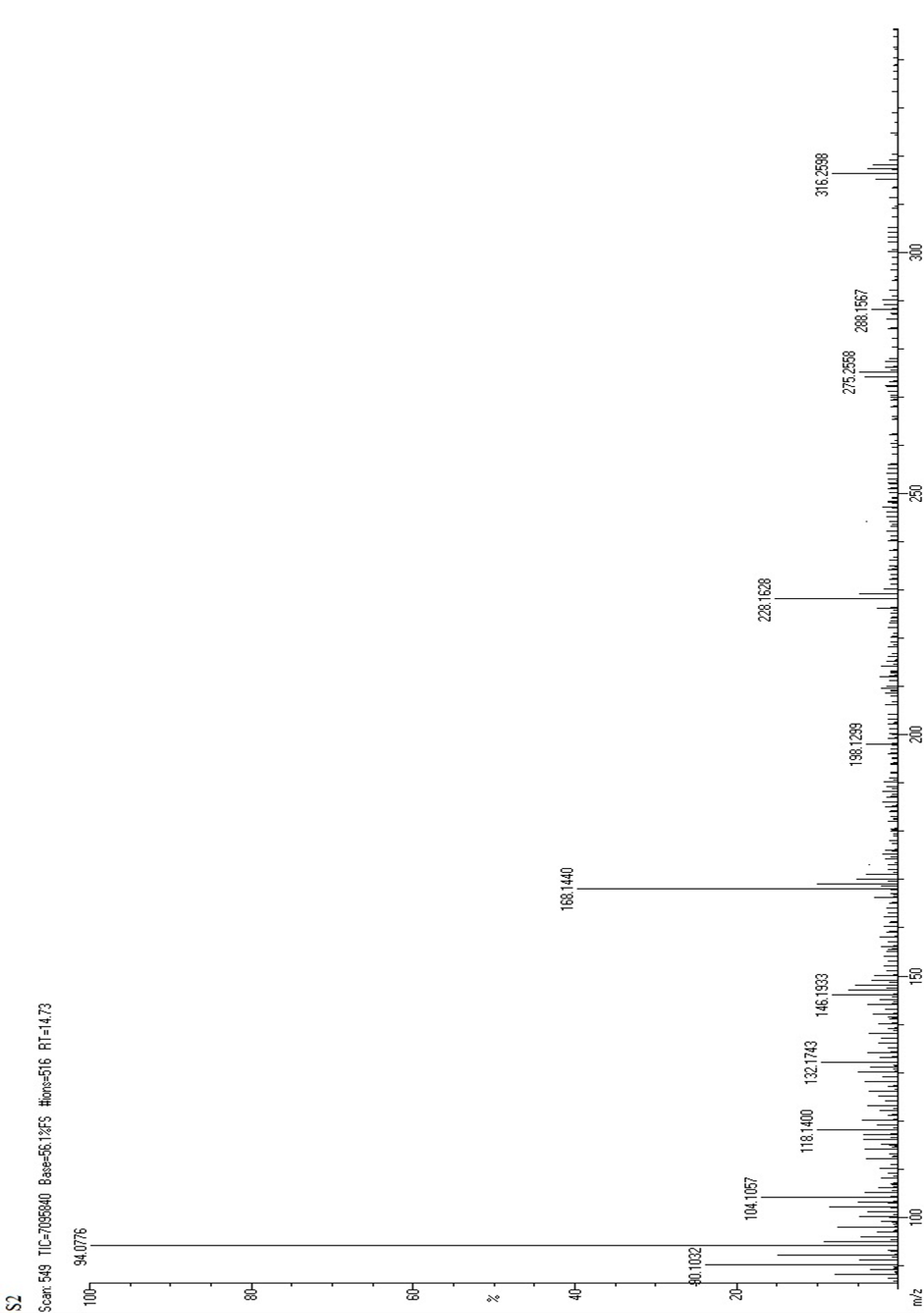
Figure:5 Mass Spectrum of Fractionated Sample (FS2)
The proposed molecular fragmentation process of fractionated sample (FS2) is given as follows

Molecular weight=316.26228


Figure:6 NMR Spectrum of Fractionated Sample (FS2)
Spectral Data of Fractionated Sample (FS2)
FT-IR analysis revealed peaks in range of 1250-1050 cm -1which were consistent with the presence of hydroxyl groups. The peak at 1649 cm-1 was attributed to the stretching vibration of a carbonyl group. The strong peak at 2850 cm -1indicates the presence of methoxy group. Several other peaks were observed at 1620,1510 and 1458 cm -1, which were consistent with the presence of a phenyl ring skeleton. The peaks observed at 1733 cm -1 also suggested the presence of oxygen containing heterocycle. Mass m\z =316.26 (C16H12O7), 288.25 (C15H12O6), 274.26 (C15H14O5), 244.24 (C14H12O4), 228.24 (C14H12O3), 172.13 (C7H8O5), 148.15 (C9H8O2). NMR (ppm)= 7.14 (Aromatic CH), 7.10 (C4H8O2), 3.85 (S,CH-OH), 2.4-4.4 (S, OCH3). Hence, the fractionated sample (FS2) was predicted to be contain Isorhamnetin.

Molecular weight=316.26228
IUPAC: 3,5,7-trihydroxy-2-(4 hydroxy-3-methoxy phenyl) chromen -4-one

Figure:7 IR Spectrum of Fractionated Sample (FS3)
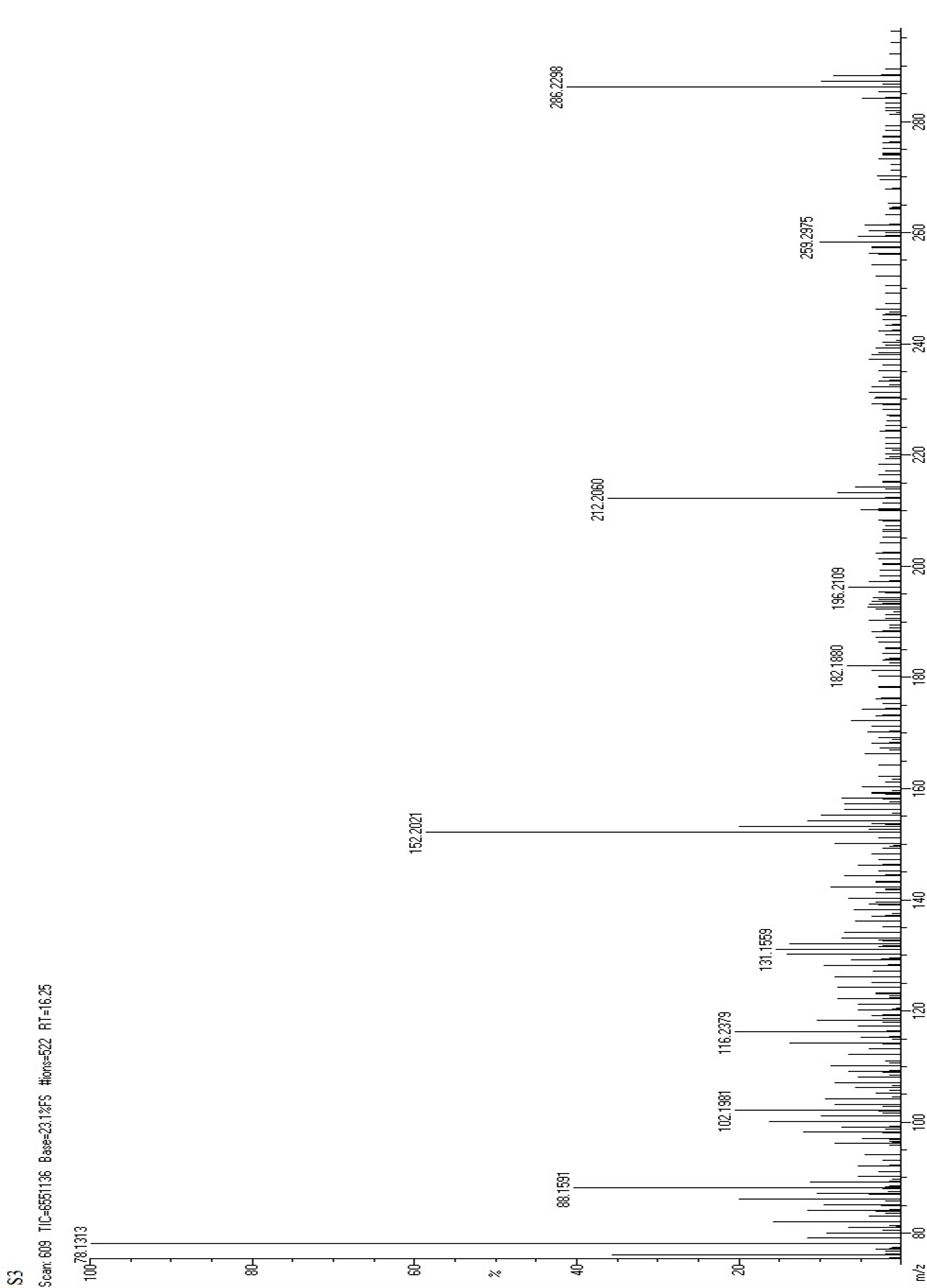
Figure:8 Mass Spectrum of Fractionated Sample (FS3)
The proposed fragmentation pattern of fractionated sample (FS3) is given as follows


Figure:9 NMR Spectrum of Fractionated Sample (FS3)
Spectral Data of Fractionated Sample (FS3)
FT-IR analysis revealed peaks in range of 1250-1050 cm-1 which were consistent with the presence of hydroxyl groups. The peak at 1650 cm-1 was attributed to the stretching vibration of a carbonyl group. Several other peaks were observed at 1580 cm -1,1510 cm -1 and 1458 cm -1, which were consistent with the presence of a phenyl ring skeleton. The peaks observed at 1733 cm -1 also suggested the presence of oxygen containing heterocycle.Mass m\z =286.24 (C15H10O6), 258.22 (C14H10O5), 214.21 (C13H10O3), 198.21 (C13H10O2), 152.10(C7H4O4), 118.13 (C8H6O). NMR (ppm)=7.14 (C6H6), 7.10 (C4H8O2), 3.85 (S, CH-OH). Hence, the fractionated sample(FS3) was predicted to be contain Kaempferol.
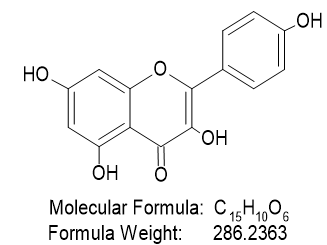
Iupac Name :3,5,7-trihydroxy-2-(4-hydroxyphenyl) chromen-4-one
Anti-Inflammatory Activity
Table:2 Anti-Inflammatory Activity of Ethanolic Extract of Onion Peel
|
S No
|
Dose
|
After The Drug Administration
|
|
|
|
0 Mins
|
15 Mins
|
30 Mins
|
60 Mins
|
120 Mins
|
180 Mins
|
|
Control
|
Normal Saline
|
5.89±0.011
|
5.93±0.020
|
6.01±0.023
|
6.12±0.029
|
6.08±1.478
|
5.98±0.015
|
|
Group 1
|
20 mg/kg
|
5.90±0.023
|
5.96±0.020
|
5.46±0.020
|
4.89±0.026
|
4.50±0.023
|
3.89±0.026
|
|
Group 2
|
200mg/kg
|
5.76±0.026
|
5.89±0.020
|
5.76±0.023
|
5.633±0.023
|
4.93±0.020
|
4.73±0.017
|
|
Group 3
|
400mg\kg
|
5.89±0.020
|
5.81±0.014
|
5.71±0.017
|
5.31±0.020
|
4.92±0.020
|
4.68±0.020
|
The data represented as mean ± SEM. The standard drug indomethacin 20 mg/kg produce significant reduction in paw thickness and treatment with ethanolic extract of onion peel reduced the carrageenan induced paw edema in dose dependent manner.

Figure 10: The above figure illustrates the anti-inflammatory activity of onion peel extract.
Anti-Microbial Activity
Minimum Inhibitory Concentartion
The onion peel extract did not show any visible growth at the lowest concentration of 200µg/ 100 µl.
Anti-Bacterial Activity
The ethanolic extract of onion peel on gram positive and gram negative organisms at a concentration range of 100, 200, 400, 800 µg/µl and standard (ciprofloxacin) 10 µg/ml showed significant inhibition in concentration dependent manner. In this study,ethanolic extract of onion peel showed maximum inhibition at 800 µg/µl against all organisms which is compared with standard ciprofloxacin drug.
Table:3 Zone of inhibition for gram positive and gram-negative bacteria against different concentration of the extract of onion peel (in mm)
|
Bacteria
|
100 µg/µl
|
200 µg/µl
|
400 µg/µl
|
800 µg/µl
|
Standard drug
µg/ml
|
|
Staphylococcus aureus
|
NO
|
17
|
24
|
28
|
26
|
|
Bacillus susbtilis
|
NO
|
16
|
21
|
24
|
23
|
|
Proteus vulgaris
|
NO
|
14
|
20
|
26
|
27
|
|
Escherichia coli
|
NO
|
12
|
18
|
21
|
17
|
Gram Positive Organisms
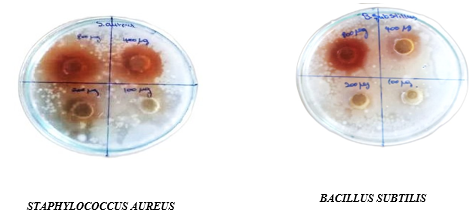
Gram Negative Organisms

CONCLUSION
This investigation comprehensively elucidated the phytochemical composition and therapeutic potential of onion peel extract, emphasizing its significant anti-inflammatory and antibacterial properties. Through rigorous spectral analysis, the identification of primary flavonoids, including quercetin, isorhamnetin, and kaempferol, underscored the bioactive profile of the extract. The therapeutic efficacy of the extract was demonstrated by its anti-inflammatory effects in animal models and potent antibacterial activity against both gram-positive and gram-negative bacteria. These findings support the use of onion peel waste as a valuable source of natural bioactive compounds, presenting opportunities for further research and potential pharmaceutical applications.
Consent For Publication
Not applicable.
Funding
None.
Conflict Of Interest
The authors declare no conflict of interest, financial or otherwise.
Ethics Approval
The animal experimentation conducted in this study adhered to ethical guidelines and was approved by the Institutional Animal Ethics Committee of Nandha College of Pharmacy (Approval NO: NCP/IAEC/UG-2022-23-06). All animal-related procedures complied with institutional and national standards for laboratory animal care and use. The researchers implemented measures to minimize animal distress and utilized the minimum number of animals necessary to achieve the scientific objectives of the study. Appropriate housing, nutrition, and veterinary care were provided throughout the research period. The study strictly adhered to 3Rs principles (Replacement, Reduction, and Refinement) to ensure ethical and humane treatment of the animals involved.
ACKNOWLEDGEMENT
We are thankful to the management of Nandha College of Pharmacy for providing us valuable support and guidance to perform this study. This study is a part of B. pharm project work under The Tamil Nadu Dr. M.G.R Medical University, Chennai.
REFRENCES
- Shabir I, Pandey VK, Dar AH, et al (2022) Nutritional Profile, Phytochemical Compounds, Biological Activities, and Utilization of Onion Peel for Food Applications: A Review. Sustainability (Switzerland). https://doi.org/10.3390/su141911958
- HERRMANN K (1976) Flavanols and flavones in food plants: a review†. Int J Food Sci Technol 11:433–448
- Fossen T, Pedersen AT, Andersen ØM (1998) Flavonoids from red onion (Allium cepa). Phytochemistry 47:281–285
- Choi E-Y, Lee H, Woo JS, et al (2015) Effect of onion peel extract on endothelial function and endothelial progenitor cells in overweight and obese individuals. Nutrition 31:1131–5
- Suh HJ, Lee JM, Cho JS, Kim YS, Chung SH (1999) Radical scavenging compounds in onion skin. Food Research International 32:659–664
- Constantin OE, Milea A ?tefania, Bolea C, Mihalcea L, Enachi E, Copolovici DM, Copolovici L, Munteanu F, Bahrim GE, Râpeanu G (2021) Onion ( Allium cepa L.) peel extracts characterization by conventional and modern methods. International Journal of Food Engineering 17:485–49
- Kumar M, Barbhai MD, Hasan M, et al (2022) Onion ( Allium cepa L.) peel: A review on the extraction of bioactive compounds, its antioxidant potential, and its application as a functional food ingredient. J Food Sci 87:4289–4311
- Mtunzi FM, Ejidike IP, Ledwaba I, Ahmed A, Pakade VE, Klink MJ, Modise SJ (2017) Solvent–solvent fractionations of Combretum erythrophyllum (Burch.) leave extract: Studies of their antibacterial, antifungal, antioxidant and cytotoxicity potentials. Asian Pac J Trop Med 10:670–679
- Truong D, Ta NTA, Pham TV, Huynh TD, Do QTG, Dinh NCG, Dang CD, Nguyen TKC, Bui AV (2021) Effects of solvent—solvent fractionation on the total terpenoid content and in vitro anti?inflammatory activity of Serevenia buxifolia bark extract. Food Sci Nutr 9:1720–1735
- Osadebe PO, Okoye FBC (2003) Anti-inflammatory effects of crude methanolic extract and fractions of Alchornea cordifolia leaves. J Ethnopharmacol 89:19–24
- Patil KR, Mahajan UB, Unger BS, Goyal SN, Belemkar S, Surana SJ, Ojha S, Patil CR (2019) Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int J Mol Sci 20:4367
- Masfria, Haro G, Mierza V (2019) Analysis Chemical Compounds and Antimicrobial Activity of Red Onion (Allium Cepa L.) Bulb Skin Extract. Rasayan Journal of Chemistry 12:1002–1010
- Ma Y-L, Zhu D-Y, Thakur K, Wang C-H, Wang H, Ren Y-F, Zhang J-G, Wei Z-J (2018) Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.). Int J Biol Macromol 111:92–10
- Khandewal KR NPP (2005) A text book of Practical Pharmacognosy. In: 27th ed. p 12..


 S. Dibenthiran*
S. Dibenthiran*
 T. Sivakumar
T. Sivakumar
 S. Indrakumar
S. Indrakumar
 S Dineshkumar
S Dineshkumar
 C. Aravind
C. Aravind
 P. Tharani
P. Tharani




















 10.5281/zenodo.15016190
10.5281/zenodo.15016190