Abstract
Objective: To evaluate the antipsychotic activity of ethanolic extract of Benincasa hispida peel using experimental animal models. Methods: The fresh fruits of Benincasa hispida were collected, and washed, and the peels were dried before being ground into a fine powder. The ethanolic extract was obtained by the maceration process. Two doses of Benincasa hispida peel extract (200 mg/kg and 400 mg/kg body weight) were administered for 21 days in rats for the pole-climbing avoidance test, and for 15 days in mice for the ketamine-induced stereotypic behaviour model. In the pole-climbing test, Haloperidol (1 mg/kg, i.p.) served as the standard antipsychotic drug, while in the ketamine-induced stereotypy model, Olanzapine (5 mg/kg, i.p.) was used as the standard. Results: The ethanolic peel extract of Benincasa hispida (EPBH) exhibited significant (P<0.05, P<0.01) dose-dependent inhibition of conditioned avoidance response (CAR) in rats, demonstrated by the increased time spent on the grid floor of the chamber. In the ketamine-induced stereotypic behaviour model, EPBH administered for 15 consecutive days at two different doses significantly (P<0.05, P<0.01) reduced stereotypic behaviours such as falling, weaving, head-bobbing, and turning in mice. The results indicate that Benincasa hispida ethanolic peel extract exerts antipsychotic activity in both models, with the higher dose of 400 mg/kg showing the most pronounced effects. Conclusion: The present study highlights the significant antipsychotic potential of Benincasa hispida peel extract, particularly at the higher dose of 400 mg/kg. While the exact mechanisms underlying its antipsychotic effects remain unclear, these findings warrant further research to isolate and identify the active compounds responsible for its therapeutic effects.
Keywords
Antipsychotic activity, Benincasa hispida, Ethanolic extract, Pole-climbing avoidance, Stereotypic behaviour.
Introduction
Psychosis essentially signifies a severe mental disruption, marked by confusion and diminished or inability to distinguish personal identity from reality [1]. Schizophrenia (SCZ), the most intricate and chronic spectrum of psychosis, affects approximately 1-1.5% of the global population. It is a multifactorial neurodevelopmental disorder that impacts various cognitive, motor, and behaviour domains, characterized by positive (e.g., hallucinations and delusions), negative (e.g., anhedonia and social withdrawal), and cognitive (e.g., executive and memory dysfunction) [2]. Schizophrenia has been linked to several bio-pathophysiological hypotheses, notably neurotransmitter dysregulation, redox disruption, neuroinflammation, and mitochondrial dysfunction. The homeostatic derangements in dopamine (DA), glutamate (GLU), gamma-aminobutyric acid (GABA), serotonin (5-hydroxytryptamine (5-HT), norepinephrine (NE) and acetylcholine (Ach) neurotransmitters are the clinical implications of the disorder [1]. Among these, dopamine a critical role in regulating motor functions, motivation, and cognition, and any dysregulation is particularly implicated in schizophrenia symptoms. Hyperactivity in the mesolimbic dopamine pathway is associated with positive symptoms, while hypoactivity in the mesocortical dopamine pathways is linked to negative and cognitive symptoms [1]. Although antipsychotic medications, primarily target D2, and help alleviate symptoms there is a risk of numerous adverse effects. Antipsychotic medications are not curative and do not abolish chronic thought disorders. Still, they frequently decline the power of hallucinations and delusions and allow the person with schizophrenia to function more effectively in supportive and structured environments [3]. While typical APs (haloperidol, fluphenazine, perphenazine) are high affinity for the D2 receptor and are a clinically relevant liability to induce movement disorders, atypical antipsychotics act on both D2 receptor and 5-HT2A receptor), providing better efficacy with reduced motor side effects [4].
Given the limitations of conventional antipsychotics, there is growing interest in alternative treatments with fewer adverse effects. Herbal medicines offer a safer alternative with minimal side effects. The WHO estimates that 70-80% of the global population relies on plant-based medicines for primary healthcare with the potential to manage chronic conditions like, including psychiatric disorders [5]. Traditional medicine systems, such as Ayurveda, offer promising therapies for central nervous system (CNS) disorders [6]. Notably, certain plant species, such as Rauwolfia (from which reserpine was isolated) have been instrumental in revolutionizing the treatment of schizophrenia.[7] Benincasa hispida is a member of the Cucurbitaceae family, commonly known as ash gourd, winter melon, wax gourd, and white pumpkin with a history in traditional medicine, especially in South and Southeast Asia [9],[10]. Traditionally, it has been used as an anabolic, anthelminthic, brain tonic, carminative, diuretic, memory enhancer, refrigerant, restorative, and rejuvenator and to treat a wide range of ailments, including gastrointestinal issues, respiratory and heart diseases, diabetes, and urinary disorders. And also, to treat mental illnesses and nervous disorders such as epilepsy, paranoia, and insanity[11]. Benincasa exocarpium (BE), the fruit peel of ash gourd, has been used in traditional Chinese medicine to prevent and treat metabolic diseases such as hyperglycemia and obesity12. Plants like Brassica oleracea, Ocuimum sanctum, Alstonia Scholaris Bacopa Monniera, Lonchocarpus cyanescens contain flavonoids and phenols as key phytoconstituents which play a significant role in the management of psychosis [13]. The plant is rich in flavonoids, phenols, and alkaloids and is in the treatment of chronic diseases [14]. This study aims to explore the antipsychotic potential of Benincasa hispida peel extract in animal models.
MATERIALS AND METHODS:
Chemicals such as Haloperidol, Ketamine, and Olanzapine of the pure analytical grade will be procured from E. Merck (India) Ltd, Mumbai and all other chemicals and reagents of the pure analytical grade will be procured from local suppliers. Haloperidol and Olanzapine were used as a standard drug for comparison in behaviour models.
Experimental animals
Healthy albino rats (150-250g) and mice (20-25 g) both of either sex were used for the study. The animals are maintained under standard conditions (temperature 22 ± 2°C, relative humidity 60±5%, and 12 h light/dark cycle) and have free access to a standard pellet diet and water ad libitum. The Institutional Animal Ethics Committee reviewed and approved the experimental protocol (Approval no: SCP/IAEC/F150/P212/2023). All the procedures were performed in accordance with the Institutional Animal Ethics Committee constituted as per the direction of the Committee for Control and Supervision of Experiments on Animals (CCSEA).
Collection and authentication of the fruit
The fresh fruits of Benincasa hispida used for the study were collected in March locally. The plant was authenticated by taxonomist Dr. Siddaraju M. N, Department of Botany, University College Mangalore, Dakshina Kannada, Karnataka, India.
Preparation of Ethanolic Extract of Benincasa hispida Peel
The fresh fruits of Benincasa hispida were washed and the peel was shade-dried at room temperature and powdered. The 150g of dried powdered material was macerated with 1.5L of ethanol at room temperature. The extract was then filtered and evaporated under a rotatory vacuum evaporator at 400C. The dried extract was then stored at 40C until further use [16]. The dried extract was subjected to a qualitative test for the identification of different phytoconstituents.
Dose selection
A dose of 200mg/kg and 400mg/kg body weight will be taken as per the previous work[17].
Experimental design:
Pole climbing avoidance in rats
The Cook and Weidley method utilized a pole-climbing apparatus within a dimly lit, soundproof box. The chamber (25×25×40 cm) had a grid floor delivering mild electric shocks and a wooden pole (2.5 cm diameter) as a shock-free zone. A speaker atop the chamber provided a conditioned stimulus (CS). During training, a sound cue (CS) preceded a 1.5 mA electric foot shock (unconditioned stimulus, US) by 20 seconds, teaching rats to climb the pole to avoid the shock.
Experimental Design
The Wistar albino rats (180–250g) of either sex were randomly divided into four groups of six animals each. The different groups were assigned as follows.
Group I: Animals served as control and received only vehicles for 21 consecutive days.
Group II: Animals were administered Haloperidol (1mg/kg, i.p.) 30 minutes before the test. Group III: Animals were treated with 200mg/kg p.o. of Benincasa hispida peel extract for 21 consecutive days.
Group IV: Animals were treated with 400mg/kg p.o. of Benincasa hispida peel extract for 21 consecutive days.
Conditioned avoidance responses (CARs) were assessed, with classical antipsychotics reducing CARs without affecting unconditioned escape responses. Data were expressed as the percentage of animals failing to climb and their latency to climb within 30 seconds [18].
Ketamine-induced stereotypic behaviour in mice
Ketamine-induced stereotypy, a behavioral model for assessing antipsychotic activity, mimics schizophrenia-like symptoms. Ketamine, an NMDA receptor antagonist, blocks GABAergic neurons, leading to glutamatergic hypoactivity and elevated dopamine in limbic and subcortical brain regions. This causes hallucinations in humans and stereotypic behaviors such as falling, weaving, head-bobbing, and turning in mice.
Experimental Design
Mice of either sex weighing between 20-30g were divided into 5 groups of 6 animals each.
Group I: Animals serve as control and will receive only vehicle for 15 consecutive days.
Group II: Animals will be administered Ketamine (50mg/kg i.p.) for 15 consecutive days.
Group III: Animals of the standard group will receive Olanzapine (5mg/kg) p.o. after 30 minutes ketamine will be given for 15 consecutive days.
Group IV: Animals will be treated with a dose of 200mg/kg p.o. of Benincasa hispida peel extract and after 30 minutes Ketamine will be given for 15 consecutive days.
Group V: Animals will be treated with a dose of 400mg/kg p.o. of Benincasa hispida peel extract and after 30 minutes Ketamine will be given for 15 consecutive days.
In the experiment, mice were acclimatized in plastic cages (37 × 24 × 30 cm) for 30 minutes. Ketamine (50 mg/kg, i.p.) induced stereotypic behaviors, which were quantified every 15 minutes for 60 minutes by counting occurrences of falling, head-bobbing (pacing with rearing), weaving (side-to-side neck movement), and turning [19].
Statistical Analysis
All the data was expressed as Mean±SEM. The statistical significance between groups was compared using one-way ANOVA, followed by Dunnet’s multiple comparison test to evaluate the significance of the difference between the control, standard, and test groups. p-value ? 0.01 was considered statistically significant. The analysis was conducted using GraphPad Prism software.
RESULTS
Extraction
The yield of ethanolic peel extract of Benincasa hispida was found to be 7.4%. This indicates the proportion of moderate extractable components isolated using ethanol as a solvent.
Phytochemical analysis
The phytochemical analysis is the pivotal aspect of research and delves into the examination of plant-derived compounds. The phytochemical analysis of the Benincasa hispida peel extract identified that the extract consists of alkaloids, phenols, flavonoids, tannins, carbohydrates, steroids, terpenoids, and amino acids. The presence of such phytochemicals helps to understand the underlying pharmacological activities of the plant and paves the way for the development of novel drugs and treatments.
Cook’s Pole Climbing Avoidance in Rats
The rats treated with standard antipsychotic drugs failed to climb on the pole after the conditioned stimulus (CS) but did climb in response to the unconditioned stimulus (US) on the aversion of the foot shock through the grid floor.
Table No. 1: Effect of EPBH in Conditioned Avoidance Response
|
Group
|
Treatment
|
Latency of pole climbing (sec)
|
Number of times/ events the rat escaped
|
% Reduction in no. of times escaped
|
|
I
|
Control
|
2.1±0 .307 .307
|
14± 0.563 0.563
|
0
|
|
II
|
Haloperidol
(1mg/kg, i.p.)
|
8.5±0.224
|
8± 0.224*** 0.224***
|
42.8
|
|
III
|
EPBH (200mg/kg)
|
3.5±0.314
|
11± 0.211* 0.211*
|
28.5
|
|
IV
|
EPBH (400mg/kg)
|
6.0± 0.447 0.447
|
10± 0.167** 0.167**
|
35.7
|
|
All the Results are expressed as mean ± SEM; (n = 6). Statistical significance was determined using one-way ANOVA followed by Dunnett’s test. ***p<0>**p<0>*p<0>
|
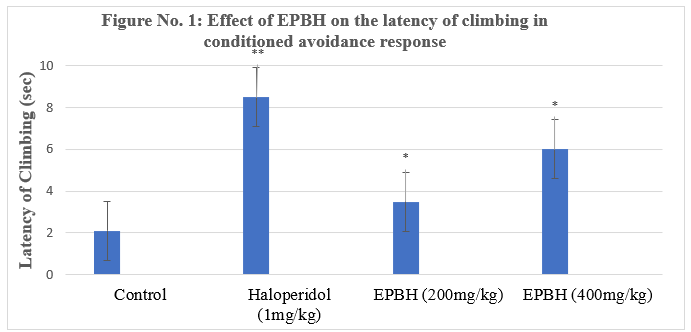
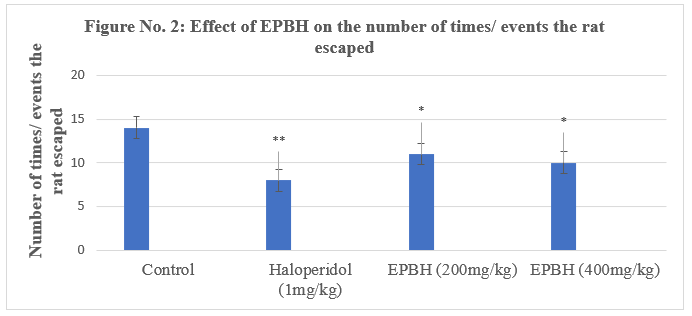
In the pole climbing avoidance test, all the treated animals exhibited a significant decrease in avoidance response compared to the control group. The standard haloperidol demonstrated 42.8% a significant reduction in the avoidance response. The animals treated with two doses of EPBH (200mg/ kg and 400mg/ kg) showed a dose-dependent, significant decrease in avoidance response (28.5%; P<0>
Ketamine-induced Stereotypic Behaviour in Mice
The present model evaluated the effect of treatment on ketamine-induced stereotypic behavior in mice. Administration of ketamine (50 mg/kg, i.p.) induced stereotypic behaviors such as falling, weaving, head-bobbing, and turning.
Table No. 2: Effect of EPBH in Ketamine-Induced Falling Behaviour
|
Group
|
Treatment
|
Falling Behaviour
|
|
15min
|
30min
|
45min
|
60min
|
|
I
|
Control
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
|
II
|
Ketamine (50mg/ kg)
|
13.1± 0.401# 0.401#
|
10.3± 0.760# 0.760#
|
8.1± 0.543# 0.543#
|
5.3± 0.667# 0.667#
|
|
III
|
Ketamine (50mg/kg) + Olanzapine (5mg/ kg)
|
3.6± 0.333*** 0.333***
|
2.8± 0.307*** 0.307***
|
1.8± 0.307*** 0.307***
|
1.3± 0.211*** 0.211***
|
|
IV
|
Ketamine (50mg/kg)+EPBH (200mg/kg)
|
|
11.3± 0.211* 0.211*
|
| |
| |
| |
|
9.3± 0.333* 0.333*
|
7.16± 0.167* 0.167*
|
3.66± 0.333* 0.333*
|
|
V
|
Ketamine (50mg/kg)+EPBH (400mg/kg)
|
7.3± 0.333** 0.333**
|
5.5± 0.224** 0.224**
|
4.0± 0.258** 0.258**
|
1.8± 0.307** 0.307**
|
|
All the Results are expressed as mean ± SEM (n = 6). Data were analyzed using one way ANOVA by Dunnett’s test. ***p<0>**p<0>*p<0>#p<0>
|
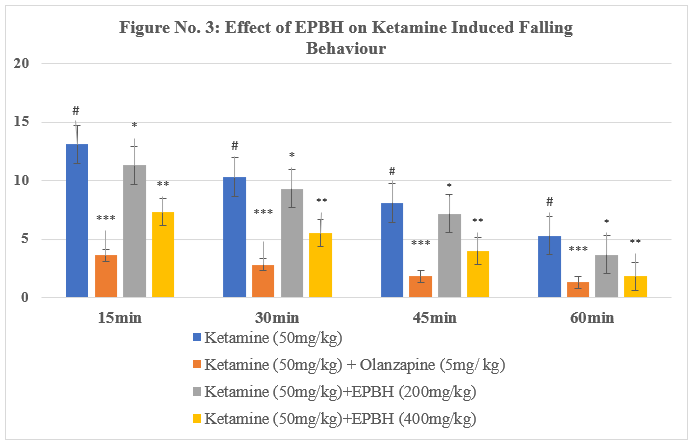
Table No. 3: Effect of EPBH in Ketamine Induced Weaving Behaviour
|
Group
|
Treatment
|
Weaving Behaviour
|
|
15min
|
30min
|
45min
|
60min
|
|
I
|
Control
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
|
II
|
Ketamine (50mg/ kg)
|
5.16± 0.307# 0.307#
|
5.18± 0.29# 0.29#
|
3.8± 0.401# 0.401#
|
3.16± 0.211# 0.211#
|
|
III
|
Ketamine (50mg/kg) + Olanzapine (5mg/ kg)
|
1.5± 0.224*** 0.224***
|
1.6± 0.333*** 0.333***
|
1.3± 0.333*** 0.333***
|
0.6± 0.222*** 0.222***
|
|
IV
|
Ketamine (50mg/kg) + EPBH (200mg/kg)
|
3.5± 0.224* 0.224*
|
3.1± 0.307* 0.307*
|
2.16± 0.167* 0.167*
|
1.16± 0.116* 0.116*
|
|
V
|
Ketamine (50mg/kg) + EPBH (400mg/kg)
|
2.6± 0.333** 0.333**
|
2.5± 0.224** 0.224**
|
1.5± 0.222** 0.222**
|
1.0± 0.21** 0.21**
|
|
All the Results are expressed as mean ± SEM (n = 6). Data were analyzed using one way ANOVA by Dunnett’s test. ***p<0>**p<0>*p<0>#p<0>
|
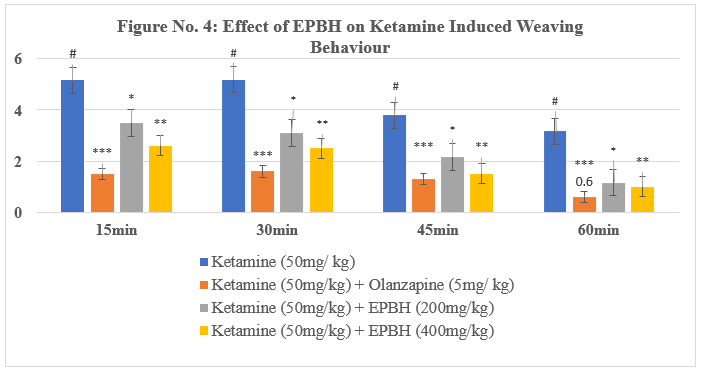
Table No. 4: Effect of EPBH in Ketamine Induced Head-bobbing Behaviour
|
Group
|
Treatment
|
Head-bobbing Behaviour
|
|
15min
|
30min
|
45min
|
60min
|
|
I
|
Control
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
|
II
|
Ketamine (50mg/ kg)
|
4.8± 0.167# 0.167#
|
5.1± 0.166# 0.166#
|
3.6± 0.211# 0.211#
|
3.1± 0.116# 0.116#
|
|
III
|
Ketamine (50mg/kg) + Olanzapine (5mg/ kg)
|
1.5± 0.224*** 0.224***
|
1.6± 0.333*** 0.333***
|
1.0± 0.258*** 0.258***
|
0.6± 0.211*** 0.211***
|
|
IV
|
Ketamine (50mg/kg) + EPBH (200mg/kg)
|
3.8± 0.307* 0.307*
|
3.5± 0.224* 0.224*
|
2.3± 0.211* 0.211*
|
1.3± 0.192* 0.192*
|
|
V
|
Ketamine (50mg/kg) + EPBH (400mg/kg)
|
2.3± 0.211** 0.211**
|
2.5± 0.224** 0.224**
|
1.3± 0.211** 0.211**
|
0.8± 0.167** 0.167**
|
|
All the Results are expressed as mean ± SEM, (n = 6). Data were analyzed using one way ANOVA by Dunnett’s test. ***p<0>**p<0>*p<0>#p<0>
|
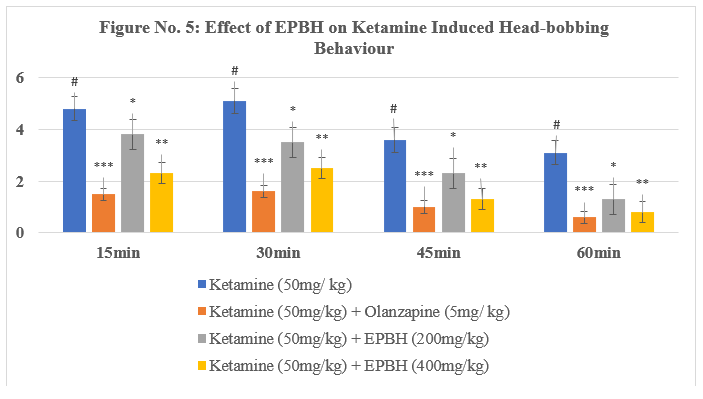
Table No. 5: Effect of EPBH in Ketamine Induced Ketamine Induced Turning Behaviour
|
Group
|
Treatment
|
Turning Behaviour
|
|
15min
|
30min
|
45min
|
60min
|
|
I
|
Control
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
0± 0 0
|
|
II
|
Control+ Ketamine (50mg/ kg)
|
20.1±  0.11# 0.11#
|
19.1± 0.167# 0.167#
|
15.5± 0.224# 0.224#
|
10.1± 0.116# 0.116#
|
|
III
|
Ketamine +(50mg/kg) + Olanzapine (5mg/ kg)
|
10.3± 0.167*** 0.167***
|
7.6± 0.211*** 0.211***
|
5.8± 0.307*** 0.307***
|
4.3± 0.211*** 0.211***
|
|
IV
|
Ketamine (50mg/kg) + EPBH (200mg/kg)
|
15.8± 0.167* 0.167*
|
11.6± 0.211* 0.211*
|
9.6± 0.224* 0.224*
|
6.8± 0.166* 0.166*
|
|
V
|
Ketamine (50mg/kg) + EPBH (400mg/kg)
|
10.3± 0.234* 0.234*
|
8.6± 0.117* 0.117*
|
5.3± 0.422** 0.422**
|
3.8± 0.166** 0.166**
|
|
All the Results are expressed as mean ± SEM, (n = 6). Data were analyzed using one way ANOVA by Dunnett’s test. ***p<0>**p<0>*p<0>#p<0>
|
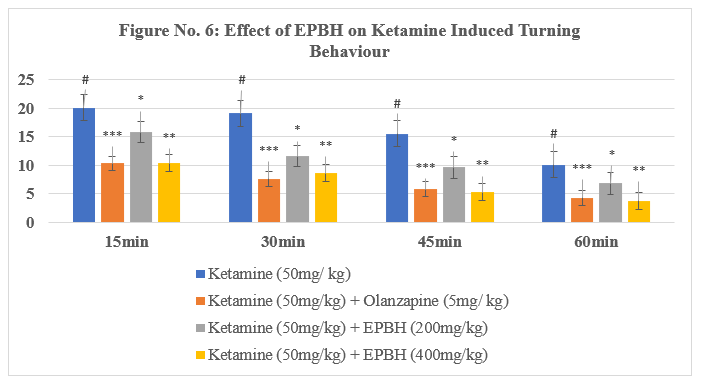
The treatment with the antipsychotic drug olanzapine (5 mg/kg, i.p.) significantly reversed stereotypic behaviors, demonstrating its therapeutic potential in reducing psychotomimetic effects induced by ketamine. The animals treated with two doses of EPBH (200mg/ kg and 400mg/ kg) showed a dose-dependent, significant decrease in stereotypic behaviours (P<0>
DISCUSSION
Schizophrenia is a chronic and debilitating illness, characterized by chronic psychotic symptoms and psycho-social impairment that attacks the most advanced functions of the human brain. It is a multifactorial disorder affecting millions worldwide. Schizophrenia is frequently a chronic and disabling disorder, characterized by heterogenous positive and negative symptoms. Diagnosis of schizophrenia requires at least two or more symptoms, and at least one of the two symptoms must be positive symptoms. The occurrence of these symptoms reflects the interplay of neurotransmitters. The disorder is strongly related to the interaction of dopamine and other neurotransmitters in specific brain regions, including the frontal, temporal, and mesostriatal areas [20]. The present study explored the antipsychotic potential of ethanolic peel extract of Benincasa hispida (EBHP) in both the conditioned avoidance response (CAR) and ketamine-induced stereotypic behavior models, two widely accepted paradigms for assessing the antipsychotic efficacy of compounds to schizophrenia. The conditioned avoidance response model is primarily used to assess compounds for antipsychotic potential by evaluating their ability to modulate dopamine-driven behaviors associated with positive symptoms of schizophrenia.21 In this study, the administration of EBHP for 21 days led to a dose-dependent suppression of conditioned avoidance behavior, with a significant increase in the time spent on the grid floor and delayed latency to climb the pole without affecting the escape response. These findings indicate that ethanolic peel extract of Benincasa hispida possesses antipsychotic properties, likely mediated by its ability to inhibit dopaminergic signaling in brain regions responsible for hyperactivity and avoidance responses. This inhibition of conditioned avoidance response (CAR) is consistent with the effects of established antipsychotic drugs such as haloperidol, which similarly reduces avoidance behavior by blocking dopamine D2 receptors [22]. The ability of ethanolic peel extract of Benincasa hispida (EBHP) to suppress conditioned avoidance at doses that do not impair escape behavior is particularly promising, suggesting its selective influence on the dopaminergic pathways involved in positive psychotic symptoms. Furthermore, in the ketamine-induced stereotypic behavior model, ethanolic peel extract of Benincasa hispida (EBHP) showed significant inhibition of stereotypy in mice, characterized by reductions in turning, weaving, head-bobbing, and falling behaviors. These behaviors are considered analogs of the hyperactivity, disorganized movement, and social withdrawal seen in schizophrenia. Ketamine, as an NMDA receptor antagonist, induces these behaviors by disrupting glutamatergic transmission and resulting in dopamine overactivity, a mechanism that mimics the positive and some negative symptoms of schizophrenia. The inhibitory effect of EBHP on ketamine-induced hyperlocomotion suggests that the extract may act on both glutamatergic and dopaminergic pathways, making it a potential candidate for managing the symptoms of schizophrenia [23, 24]. The phytochemical analysis of EBHP revealed the presence of key constituents such as flavonoids, phenols, and alkaloids, which may account for its observed antipsychotic activity. Flavonoids and phenols are known for their antioxidant and neuroprotective properties, which may help mitigate oxidative stress and neuroinflammation, both of which are implicated in schizophrenia pathophysiology. Additionally, alkaloids present in the extract may exert their effects by modulating acetylcholine levels, enhancing GABAergic transmission, and acting as NMDA receptor antagonists, all of which are relevant to the neurochemical imbalances observed in schizophrenia [25]. The dopaminergic and serotonergic pathways likely play a significant role in the antipsychotic effects of EBHP. The suppression of hyperactive behaviours, including the inhibition of CAR and stereotypic actions, suggests that EBHP may reduce the overactivity of dopaminergic neurons in the mesolimbic pathway, a hallmark of positive psychotic symptoms. The potential involvement of serotonergic modulation is also supported by the extract's ability to counteract ketamine-induced hyperlocomotion, which may reflect its interaction with 5-HT receptors, similar to atypical antipsychotics like olanzapine. While the results of this study are promising, further research is needed to elucidate the specific mechanisms of action underlying the antipsychotic effects of EBHP. Future studies should investigate the precise receptor interactions, dose-response relationships, and long-term safety profile of the extract. It would provide a more comprehensive understanding of its therapeutic potential.
CONCLUSION:
In conclusion, the ethanolic extract of Benincasa hispida peels demonstrated significant inhibition of both conditioned avoidance and ketamine-induced stereotypic behaviour models. The phytochemical constituents of the extract, including flavonoids, phenols, and alkaloids, may contribute to its antipsychotic effects through the modulation of dopaminergic, serotonergic, and glutamatergic pathways. These findings suggest that EBHP holds promise as a potential natural therapeutic agent for the management of schizophrenia. However, additional studies are warranted to confirm these preliminary findings and to explore the full scope of its pharmacological actions.
ACKNOWLEDGMENT
The authors are thankful to the Management of Srinivas College of Pharmacy, for providing the necessary facilities to carry out research work
REFERENCES
- Omeiza NA, Bakre A, Ben-Azu B, Sowunmi AA, Abdulrahim HA, Chimezie J et.al. Mechanisms underpinning Carpolobia lutea G. Don ethanol extract's neurorestorative and antipsychotic-like activities in an NMDA receptor antagonist model of schizophrenia. J Ethnopharmacol. 2023; 30(301):1157-67.
- Farah FH. Schizophrenia: An overview. Asian J of pharmaceutics.2018; 12(2):77–85.
- Kaur H, Kaur R, Rani V, Sharma K. Antipsychotic drugs. Advances in Neuropharmacology: Drugs and Therapeutics. 2020;297-316.
- De Bartolomeis A, Barone A, Begni V, Riva MA. Present and future antipsychotic drugs: A systematic review of the putative mechanisms of action for efficacy and a critical appraisal under a translational perspective. Pharmocol Res. 2022;1(176):1060-78.
- Ambikar DB, Mohanta GP. Effect of dried fruit extract of Benincasa hispida on brain behaviour in laboratory animals. Cell Tissue Res. 2013;13(1):12-9.
- Zhang M, Lei J, Wang Y, Zhang J, Liu D. Ethnopharmacology, phytochemistry and pharmacology of Benincasae Exocarpium: A review. Chin Herb Med. 2022;15(1):15-26.
- Fusar?Poli P, Estradé A, Stanghellini G, Venables J, Onwumere J, Messas G, et al. The lived experience of psychosis: a bottom?up review co?written by experts by experience and academics. World Psychiatry. 2022 ;21(2):168-88.
- Pahari, N., Mahanti, B., Banerjee, M., Mandal, K., Bhowmick, B., & Roy, A. A concise review on Benincasa hispida (Thunb.) Cogn. Plant. Int. J. Health Sci. Int. J. Health Sci 6(6), 844–53.
- Wang Q, Zhao C, Zhang Q, Zhou X, Yan Z, Sun Y, et al. Synergistic Effect of Benincasa hispida Peel Extract and KI on the Corrosion Inhibition of Mild Steel in HCl. Sustainability. 2023;15(14):11370.
- Islam MT, Quispe C, El-Kersh DM, Shill MC, Bhardwaj K, Bhardwaj P, et al. A literature-based update on Benincasa hispida (Thunb.) Cogn.: traditional uses, nutraceutical, and phytopharmacological profiles. Oxid Med Cell. Longev. 2021;1(1):2-14.
- Gupta P, Chikkala S, Kundu P. Ash gourd and its applications in the food, pharmacological and biomedical industries. Int. J. Veg. Sci. 2021;27(1):44-53.
- Das A, Chakraborty S. Kushmanda (Benincasa hispida): an amazing food in Ayurveda. World J pharm res. 2020;9(15):753-60.
- Otimenyin SO, Ior LD. Medicinal plants used in the management of psychosis. In complementary Therapies. IntechOpen, 2021:1(6):130-40.
- Shakya A, Chaudhary SK, Bhat HR, Ghosh SK. Acute and sub-chronic toxicity studies of Benincasa hispida (Thunb.) cogniaux fruit extract in rodents. Regul. Toxicol. Pharmacol. 2020; 118(1):10475-8.
- Singh J, Singh V, Shukla S, Rai AK. Phenolic content and antioxidant capacity of selected cucurbit fruits extracted with different solvents. J Nutr Food Sci. 2016;6(6):1-5.
- Ayyar P, Ravinder JR. Animal models for the evaluation of antipsychotic agents. Fundam Clin Pharmacol. 2023;37(3):447-60.
- Shakya A, Chaudhary SK, Bhat HR, Ghosh SK. Acute and sub-chronic toxicity studies of Benincasa hispida (Thunb.) cogniaux fruit extract in rodents. Regul. Toxicol. Pharmacol. 2020;118(1):10475-8
- Sharma K, Parle M, Yadav M. Evaluation of antipsychotic effect of methanolic extract of Ocimum sanctum leaves on laboratory animals. J Appl Pharm Sci. 2016;6(5):171-7.
- Rodrigues T, Bressan GN, Krum BN, Soares FA, Fachinetto R. Influence of the dose of ketamine used on schizophrenia-like symptoms in mice: A correlation study with TH, GAD67, and PPAR-?. Pharmacol. Biochem. Behav. 2023;233(1):1736-58.
- Pannu A, Parle M. Anti-psychotic activity of Pyrus Communis juice. Int J of Pharmacy and Pharmaceutical Sci. 2017; 9(4):113-20.
- Seeman P. A dopaminergic D2 receptor out of control in psychosis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013; 46:146-52.
- Wadenberg ML. Conditioned avoidance response in the development of new antipsychotics. Curr. Pharm. Des. 2010;16(3):358-70.
- Yadav M, Parle M, Dhingra MS. Protective effect of Brassica oleracea juice against Ketamine-induced stereotypic behaviour in mice. J Med Plan Stu. 2017;5(1):200-4.
- Yadav M, Parle M, Lamba D, Kumar S, Dahiya M, Joshi K. Neuroprotective Effect of Dark Chocolate on Ketamine-Induced Animal Model Of Psychosis Through The Reversal Of Behavioral, Biochemical And Histopathological Changes. J. Adv. Zool. 2024;45(2):365.
- Saleem A, Akhtar MF. Alternative therapy of psychosis: Potential phytochemicals and drug targets in the management of schizophrenia. Front. Pharmacol. 2022;17(13):8956-68


 Josna Priya Lewis*
Josna Priya Lewis*












 10.5281/zenodo.14276123
10.5281/zenodo.14276123