Abstract
Endometrial cancer is a serious condition that requires prompt medical attention and treatment. Phyllanthus amarus, commonly known as the "stonebreaker" plant, has been studied for its potential health benefits, including its anti-cancer properties. However, there's limited scientific evidence specifically linking Phyllanthus amarus to the treatment or prevention of endometrial cancer. It's important to approach the treatment of endometrial cancer under the guidance of qualified healthcare professionals. Treatment options typically include surgery, radiation therapy, chemotherapy, hormone therapy, or a combination of these approaches, depending on the stage and specific characteristics of the cancer. While some herbs and natural remedies may have supportive or complementary roles in cancer treatment, they should not be used as a substitute for conventional medical treatments. Always consult with your healthcare provider before incorporating any new treatments, including herbal remedies, into your cancer care plan. They can provide guidance tailored to your individual circumstances and ensure that any treatment decisions are safe and effective.
Keywords
Phyllanthus amarus, Extraction, Sohxlet Extraction, Endometrial cancer, Invitro testing, MTT assay
Introduction
Endometrial cancer stands as a significant health challenge, affecting women globally and demanding comprehensive approaches for treatment and management. While conventional therapies have made substantial strides, the quest for adjunctive or alternative treatments persists, with a keen eye on natural remedies like medicinal plants. Among these, Phyllanthus amarus, colloquially known as the stonebreaker plant, has emerged as a subject of interest due to its purported medicinal properties, including potential anti-cancer effects. This article delves into the botanical intricacies and medicinal potential of Phyllanthus amarus, offering insights into its classification, morphology, and related species. Furthermore, it explores the broader context of herbal medicine in the realm of endometrial cancer treatment, emphasizing the importance of evidence-based approaches and collaboration between traditional and modern medical practices. As the scientific community continues to unravel the therapeutic mechanisms of Phyllanthus amarus and its counterparts, understanding their role in complementing conventional therapies becomes paramount. By navigating the intersection of traditional wisdom and modern science, we aspire to unlock novel avenues for enhancing endometrial cancer care, ultimately empowering women with comprehensive and personalized treatment strategies. Join us on this journey as we navigate the intricate landscape of botanical medicine and its potential implications for endometrial cancer management, shedding light on the promise and challenges of integrating natural remedies into the broader spectrum of healthcare.[1,2]
PLANT DESCRIPTION
Phyllanthus amarus: The Stonebreaker Plant
Overview: Phyllanthus amarus, commonly known as the stonebreaker plant, is a small, annual herbaceous plant native to tropical regions, including parts of the Americas, Asia, and Africa. It belongs to the genus Phyllanthus within the family Phyllanthaceae, which includes approximately 750 species of flowering plants. This unassuming plant has gained attention for its potential medicinal properties, particularly its use in traditional medicine systems for various health conditions, including liver disorders, kidney stones, and viral infections.[2]
Botanical Description:
Habit:
Phyllanthus amarus is a diminutive herbaceous plant, typically growing between 30 to 60 centimeters in height. Its slender, delicate stems emerge from a central point and branch outwards, forming a bushy appearance.
Leaves:
The leaves of Phyllanthus amarus are small, elliptical, and arranged alternately along the stems. They have a glossy green appearance and may exhibit a slight curvature along the midrib.
Flowers:
The flowers of Phyllanthus amarus are tiny and inconspicuous, measuring only a few millimeters in diameter. They are unisexual, meaning individual plants produce either male or female flowers. The flowers are typically greenish-yellow in color and lack showy petals.
Fruit:
Following pollination, Phyllanthus amarus develops small, spherical capsules that contain numerous seeds. These capsules are initially green but may turn brown as they mature. When ripe, the capsules split open to release the seeds, aiding in dispersal.
Roots:
The root system of Phyllanthus amarus is relatively shallow, consisting of fine, fibrous roots that spread horizontally in the soil.[2,4]
Medicinal Uses: Phyllanthus amarus has a long history of use in traditional medicine systems, where it is valued for its purported medicinal properties. Some of its traditional uses include:
Hepatoprotective:
Phyllanthus amarus is believed to have hepatoprotective properties, supporting liver health and function. It is used in the management of liver disorders, including hepatitis and jaundice.
Antiviral:
This plant has been investigated for its potential antiviral activity, particularly against hepatitis B virus (HBV) and human immunodeficiency virus (HIV).
Anti-inflammatory:
Phyllanthus amarus exhibits anti-inflammatory effects, which may contribute to its use in alleviating symptoms associated with inflammatory conditions.
Diuretic:
It is also used as a diuretic, promoting urine production and helping to flush out toxins from the body.
Antioxidant:
Phyllanthus amarus contains compounds with antioxidant properties, which may help protect cells from oxidative damage caused by free radicals.[5] Phyllanthus amarus, the stonebreaker plant, is a fascinating botanical species with a rich history of traditional use and promising medicinal potential. While further research is needed to fully elucidate its mechanisms of action and therapeutic applications, this plant continues to be valued for its role in supporting various aspects of human health. As interest in natural remedies and herbal medicine grows, Phyllanthus amarus remains a subject of scientific inquiry and exploration, offering hope for the development of novel therapies and treatments. Phyllanthus amarus is a small, herbaceous plant with delicate, slender stems that can grow up to 30–60 centimeters in height. Its leaves are small, elliptical, and arranged alternately along the stems. The plant produces tiny, inconspicuous flowers that are either male or female, typically greenish-yellow in color. The fruit is a small capsule contain seeds.[3,5]


Here's a classification of the plant Phyllanthus amarus:
- Kingdom: Plantae
- Subkingdom: Viridiplantae (Green plants)
- Infrakingdom: Streptophyta (Streptophytes)
- Superdivision: Embryophyta (Land plants)
- Division: Tracheophyta (Vascular plants)
- Subdivision: Spermatophytina (Seed plants)
- Class: Magnoliopsida (Dicotyledons)
- Subclass: Rosidae
- Order: Malpighiales
- Family: Phyllanthaceae
- Genus: Phyllanthus
- Species: Phyllanthus amarus
This classification system follows the hierarchical structure used in biological taxonomy, which organizes living organisms into increasingly specific categories based on their evolutionary relationships and shared characteristics.
Classification of Phyllanthus amarus:
Kingdom:
Plantae (Plants) –
Phyllanthus amarus belongs to the kingdom Plantae, which encompasses all plants, including flowering plants, ferns, mosses, and algae.
Subkingdom:
Tracheobionta (Vascular plants) - This subkingdom includes plants with vascular tissues (xylem and phloem), allowing for the transport of water, minerals, and nutrients throughout the plant.
Superdivision:
Spermatophyta (Seed plants) - Phyllanthus amarus is a seed plant, producing seeds as part of its reproductive cycle. Seed plants include gymnosperms (such as conifers) and angiosperms (flowering plants).
Division:
Magnoliophyta (Flowering plants) - Also known as Angiosperms, this division comprises plants that produce flowers and seeds enclosed within fruits. Phyllanthus amarus is classified within this diverse group of plants.
Class:
Magnoliopsida (Dicotyledons) –
Phyllanthus amarus belongs to the class Magnoliopsida, commonly referred to as dicotyledons or dicots. Dicotyledonous plants are characterized by having two embryonic seed leaves (cotyledons), net-veined leaves, flower parts typically in multiples of four or five, and a taproot system.
Order:
Malpighiales –
This order includes a wide range of flowering plants, including tropical trees, shrubs, vines, and herbs. Phyllanthus amarus is classified within the Malpighiales order, along with other notable families such as Euphorbiaceae and Salicaceae.
Family:
Phyllanthaceae –
Phyllanthus amarus is a member of the Phyllanthaceae family, which consists of approximately 58 genera and over 2,000 species of flowering plants. This family is characterized by its diverse range of herbaceous plants, shrubs, and trees, many of which have medicinal properties.
Genus:
Phyllanthus –
Phyllanthus is a genus within the Phyllanthaceae family, comprising over 750 species distributed worldwide. Plants in this genus are commonly known as "leaf-flower" or "stonebreaker" plants. Many species within the Phyllanthus genus are used in traditional medicine for various purposes, including treating liver disorders, kidney stones, and infections. Understanding the classification of Phyllanthus amarus provides insights into its evolutionary relationships with other plant species and its place within the broader taxonomy of the plant kingdom. This knowledge can aid researchers and botanists [4,3]
Similar Species:
- Phyllanthus niruri (Chanca Piedra):
Description:
Phyllanthus niruri, also known as Chanca Piedra or "stonebreaker," is a closely related species to Phyllanthus amarus. It is a small annual herb that grows widely in tropical areas.
Medicinal Properties:
Chanca Piedra has been traditionally used for its purported ability to dissolve kidney stones, support liver health, and alleviate symptoms of urinary tract infections.
Chemical Composition:
The plant contains various bioactive compounds, including flavonoids, alkaloids, and lignans, which contribute to its medicinal properties.[6]
Clinical Studies:
Research suggests that Chanca Piedra may help prevent the formation of kidney stones and could have potential applications in liver protection and urinary tract health.
Caution:
Despite its potential benefits, individuals with certain medical conditions, such as kidney disorders or liver disease, should consult with a healthcare professional before using Chanca Piedra.[6]
- Phyllanthus emblica (Indian Gooseberry or Amla):
Description:
Phyllanthus emblica, commonly known as Indian gooseberry or Amla, is a medium-sized deciduous tree native to the Indian subcontinent. It belongs to the Phyllanthaceae family.
Medicinal Properties:
Indian gooseberry is renowned for its rich antioxidant content, particularly vitamin C. It has been used in traditional Ayurvedic medicine for its rejuvenating, immune-boosting, and anti-inflammatory properties.
Chemical Composition:
Amla contains a diverse array of bioactive compounds, including polyphenols, flavonoids, tannins, and essential oils, which contribute to its therapeutic effects.
Traditional Uses:
Indian gooseberry is used to support digestion, enhance hair and skin health, improve immune function, and promote overall well-being in traditional medicine systems.
Clinical Studies:
Research suggests that Amla may have potential anti-cancer properties due to its antioxidant and immune-modulating effects, although more studies are needed to elucidate its mechanisms of action.
Caution:
While generally considered safe when consumed in moderate amounts as a food or supplement, individuals with certain medical conditions, such as diabetes or bleeding disorders, should exercise caution and consult with a healthcare professional before using Indian gooseberry supplements.[5,6]
Extraction The term "extraction" as it is used in pharmaceuticals refers to the process of separating the chemicals in plant or animal tissues that are medicinally active from the inactive or inert components (desired and undesirable) using specific solvents during routine extraction operations. The comparatively impure liquids, semisols, or powders that are made from plants in this way are solely meant for external or oral application. In the form of tinctures and fluid extracts, some of the initially acquired extracts might be suitable for use as therapeutic agents, but others require additional processing. While decoction and hydro distillation procedures employ water as a solvent, conventional extraction methods, such as Maceration, Percolation, and Sohxlet extraction, often use organic solvents and require a significant volume of solvents and extended extraction times.[7]
Conventional Methods Used to Recover Natural Products
- Sohxlet extraction
- Thermal desorption
- Pressurized liquid extraction
- Maceration
- Steam distillation
- Phytonic desorption
- Percolation
- Membrane process
- Infusion
- Decoction
- Extraction leaching
- Surfactant mediated extraction
- Accelerated solvent extraction
- Traction technology
- Sample disruption method and
- Counter current extraction
- Enfleurage[8]
MACERATION
This method involves putting the whole or coarsely ground crude medication into a stoppered container with the solvent and letting it remain at room temperature for three days or longer, stirring often, until the soluble material dissolves. After that, the combination is strained, the marc—a moist solid substance—is pressed, and the combined liquids are clarified—either by flotation or decantation—after standing for a while.[9,10]
INFUSION
The crude medication is macerated in hot or cold water for a brief duration to create fresh infusions. These are weaker solutions of the easily soluble components of unrefined medications.[9]
DIGESTION
This type of maceration involves extracting the material using a little amount of heat. When a somewhat higher temperature is acceptable, it is employed. This results in an increase in the menstruum's solvent efficiency.[9]
MICROWAVE ASSISTED EXTRACTION
When microwaves interact with the polar molecules in the extraction media, heat is produced and the solid material's internal pressure is raised, which increases extraction efficiency when compared to conventional extraction.[10]
LEACHING
Leaching can be done in a variety of ways, including ultrasonic, microwave, and supercritical fluid extraction. Supercritical fluid extraction is a method of separation that draws soluble components out of the solid matrix by taking use of the special qualities of gases above their critical points. Because of its low critical temperature and pressure (31.1 °C, 72.8 atm), non-toxic and non-flammable characteristics, and affordable, high-purity availability, supercritical CO2 is utilized as a solvent in supercritical fluid extraction.[10]
SOHXLET EXTRACTION
The ISO 659-1988 protocol was applied to extract oil from olives using the traditional Sohxlet method. For the Sohxlet extractions, 30 g of material were weighed to the closest 10 mg. Olive sample that has been electrically milled. The quantity was moved into a 33 x 100 cellulose thimble and then put into the extraction chamber of a Sohxlet device with a 200 mL capacity. Cotton was used to plug the cellulose thimble to prevent sample particles from transferring to the distillation flask. The Sohxlet apparatus, which had a condenser, was set up on a 500 mL distillation flask with three boiling glass regulators and 300 mL of solvent. Thus, samples were extracted over the course of four hours (18–22 cycles/h) using n-hexane under reflux After cooling to ambient temperature in a desiccator, the cellulose cartridge's contents were milled and then reinserted into the thimble. Thus, the above process was repeated for two hours, for a total extraction time of eight hours (4 hours + 2 hours + 2 hours). In a vacuum rotary evaporator, the primary solvent was removed following the extraction process. After that, the material was moved into a smaller tarred flask and vacuum-concentrated until it was completely dry. Rotary evaporator for one hour at 80°C, and then let it cool once more for one hour in a desiccator. After that, the flask was weighed, and the process was repeated every 30 minutes until the difference between two successive weight readings was less than 10% (m/m). At least three extractions were carried out, and the mean data were reported.[11]
ENDOMETRIAL CANCER
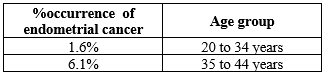
The most common cancer related to the female reproductive system is endometrial cancer(EC). Endometrial cancer lies in fourth place in most common cancer occurring in women followed by the breast, lung, and colorectal cancer. Around 142000 cases of endometrial cancer is recorded worldwide and approximately 42000 women die from this carcinoma. Recent trend in the occurrence of endometrial cancer is more in young women with fertility. It is possible to diagnose the cancer early in most women and it results in chances of increasing the survival.[15]
RISK FACTORS
50% of women with endometrial carcinoma is due the excessive fat content in body and overweight. The foremost cause is obesity and high BMI (body mass index). Other than obesity nulliparity and polycrystalline ovarian syndrome are also risk factor for the endometrial cancer. If the infertility is also accompanied then nulliparity is important risk factor that to be taken into
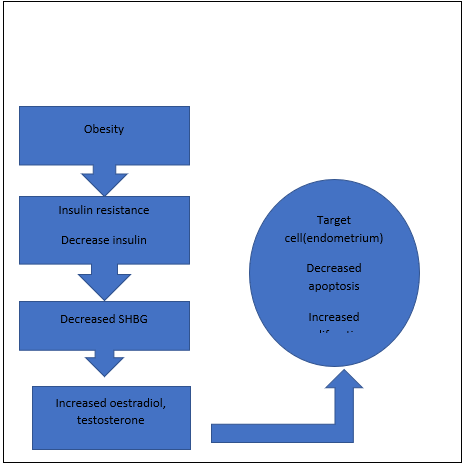
consideration. Long term exposure to excess estrogen, metabolic syndrome, history of breast cancer, long term use of tamoxifen and high serum glucose concentration are the factors that pave way for the development of the carcinoma in the tissues of endometrium. In women’s during the menopausal period, the excess body weight cause insulin resistance that leads to increased production of ovarian androgen and ultimately causing the chronic progesterone deficiency.[25,15]
SIGNS AND SYMPTOMS
Constant bleeding in the uterine in regular time interval is the most prominent symptom of endometrial cancer. The rates of endometrial cancer is 4% to 8% when the patient is having bleeding in uterine after the menstrual period. Women with endometrial cancer due to the over body weight will have different set of symptoms. The symptoms associated with endometrial carcinoma other than those related with menstrual are pain the pelvic region, pressure in pelvic, constant urge to urinate, abnormal bowel habit and fatigue. [17,19]
DIAGNOSIS
The physical examination is the basic diagnosis for endometrial cancer which includes the absolute inspection of pelvis for any bleeding, evaluation of the uterus size and position. No particular laboratory tests are needed but sometimes papanicolaou smear result can give information about presence of endometrial cancer. The fundamental methods to evaluate the endometrium are dilation and curettage. Ultrasonography is used for measurement of thickness of endometrium. The regular value of endometrium thickness is 4mm or less. Saline infusion sonohysterography is not often used but when the results of ultrasonography and biopsy is not convulsive this method is used. Hysteroscopy gives the visual form of the endometrial cavity to determine the intensity of bleeding in uterus. [16]
PATHOGENESIS
The variations in the levels of estrogen and progesterone during menstrual cycle causes the change in structure of cells in endometrium. The foremost factor that causes endometrium carcinoma is abnormal stimulation of the endometrial tissues by the estrogen. Mutations of oncogenes and tumour suppressor gene makes the proliferation of cells abnormal which leads to hyperplasia and adenocarcinoma. The carcinoma of type 1 are due to the changes in KRAS2 oncogene and PTEN tumour suppressor gene and the carcinoma of type 2 is associated with mutations in TP53 and ERBB-2 genes. Any defects in DNA repair genes causes the process of DNA repair incomplete and that lead to damage of genome eventually increases the chance of tumorigenesis.[19]

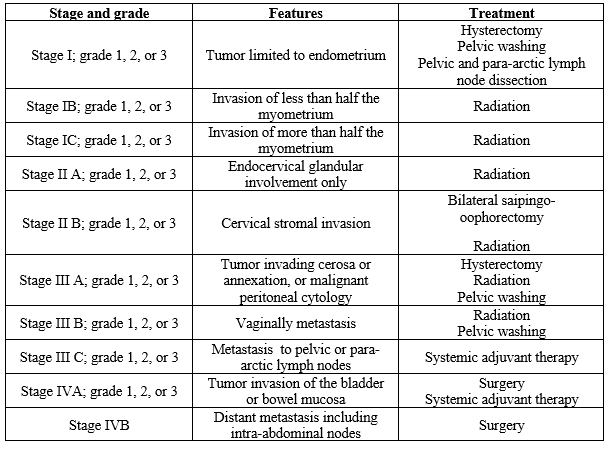
STAGING
The endometrial cancer is staged based on the surgical methods. Surgical staging has the advantages in diagnosis, prognosis, and treatment. The surgical staging includes pelvic and para aortic lymphadenectomy which also has its own risks.[21]
INVITRO TESTING METHODS FOR ANTI CANCER ACTIVITY
The research and development of new therapeutic drug for the cancer treatment asks for the need of in vitro and in vivo assays to evaluate the activity of drug molecule. Selection of appropriate method for evaluation is important since illegitimate method can result in wasting a chance of converting a potential compound into complete formulated drug.[20] The cell line is used predominantly as the screening method for anticancer drugs. New animal models were discovered to replace the cell lune method due to its imitations such as trivial in many cases and maintenance of special Clinical conditions. The Animals models used in evaluation of anti cancer activity of a drug molecules are:
- Spontaneous tumour model
- Virus induced tumour models
- Radiation induced tumour models
- Chemically induced tumour models
- Transparent tumour models
- Genetically engineered mice(GEMs)[22]
MTT ASSAY
MTT assay is one of the best methods for the measurement of viable cells without demanding the elaborate cell counting. Thus the most common use of MTT assay is to evaluate the cytotoxicity of drug molecules. The reagent used in MTT assay is 3-[4,5-dimethylthiazole -2-yl]-2,5 diphenyl tetrazolium bromide which is a mono-tetrazolium salt that comprises of quaternary tetrazole ring with positive charge. [18]
PRINCIPLE:
The main principle involved in the MTT assay is that increase or decrease in the number of viable cells is directly proportional to the mitochondrial activity. The activity of mitochondrial is evaluated due to the formation of formazan crystals from tetrazolium salt MTT. Thus, detection of formazan concentration reveals about the variations in viable cells. The formazan concentration detected by the optical density using plate reader at 540nm and 720nm.[16]
MATERIALS AND REQUIREMENTS
- Cells and controls:
class 2B bio cabinet, incubator with 5% CO at 37 degree celsius, microplates. Round bottom wells are used for suspension cells.
- Solutions:
MTT powder(sigma, St. Louis, USA) is dissolved in PBS. using the magnetic stirrer with help of magnetic stirrer and filtered using 0.22 micrometer filter. Storage condition for the solution is -20 degree Celsius.
- Solvents:
Acidified isopropanol is used as the solvent. Any fluctuations in the pH results in cloudy suspension.
- Equipment:
pipettes (0.001-1 mL), class 2B hood, benchtop centrifuge, microplate reader, O2 incubator, inverted microscope, hemocytometer.[23]
METHOD/PROCEDURE
- PLATE SETUP
The type of plate used determine the cell volume. Round bottom provides volume of 80µL and flat bottom plates gives 120µL. Features such as control wells and blank wells are necessity for every plate. Experiment type demands the number of plate required. The growth curve for the initial number of cells seeded per well is determined using a testing plate. Phosphate buffered saline(PBS) is added to fill the outer walls which maintains a minimum evaporation on the plate.
- INCUBATION
For the unstable drug, fresh plates are prepared by the addition of diluted stock solution . For the stable drugs, 30µL concentration of drug in plate is made and stored in 20oC.
- CULTURE PERIOD
For the majority of drug samples 4 days of incubation period is needed for the efficient results. For the cell lines the incubation period I’d during it’s log phase, which is 72-96 hour with 5% CO2 at a temperature of 37oC.
- MTT INCUBATION
1:10 volume of MTT solution is added for the specific incubation period. The MTT solution which is not utilized in the process can be reused. Using the plateshaker plates are shaked initially for 5 minutes followed by 900 shakes/min. Then the plates are incubated for 4-6 hour at 37o C in CO2 incubator.
- OD MEASUREMENT
The crystals are dissolved by adding the solvent acidified isopropanol. Each well is mixed properly by using a multichannel pipette. The used solvent is expelled. For an optimum results the OD is measured at 540 nm and 720 nm. By subtracting the average OD of blank control from average OD of control wells and the wells containing drugs, the percentage of living cells can be concluded.[24]


Fig. 2 A 96 well plate after the dissolution of formazan crystals using Acidified isopropanol. a) Blanks control wells, b) untreated cell control wells, c) cell line with drug C, d) cell line with drug D, e) cell line with drug E.
FACTORS AFFECTING MTT ASSAY
- Cell number and Density
- MTT Incubation time
- MTT concentration
- Cell lysate and Secretum
- Culture Media
ENDOMETRIAL CANCER ACTIVITY OF PHYLLANTHUS AMARUS
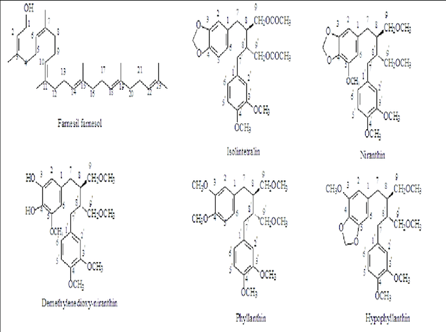
Phyllanthus species were found to include terpenoids, alkaloids, glycosides, flavonoids, tannins, and saponins in the results of an early phytochemical investigation. The main components of Phyllanthus plants are phenolic chemicals, particularly tannins. Using HPLC-MS, a detailed identification of over 100 phenolic components with a range of biological activity was achieved. It is also highlighted that distinct Phyllanthus plant portions have distinct isomers of the same chemicals. With substances including tannins, phenylpropanoids, terpenoids, phenolic compounds, flavonoids, alkaloids, saponins, and many of their glycosides, Phyllanthus species are rich in phytochemical diversity. Between 2016 and 2018, Phyllanthus spp. yielded over 81 chemicals, the bulk of which were flavonoids, triterpenoids, diterpenoids, and phenylpropanoids’. amarus is the source of flavonoid compounds known as kaempferols, which are composed of one kaempferol and three kaempferol glycosides. The glycoside rutin and quercetin were detected in the extracts of P. amarus and P. niruri L. Strong reverse transcriptase inhibition was found to be shown by the alkaloid norberbamine-2. Zephyrantine and narcyclasine-glucoside are alkaloids that have anticancer action. The chemical constituents such as alkaloids, glycosides, flavonoids and saponins are having the unique property to control the abnormal proliferation of cells. Therefore the carcinoma of endometrial tissues can be prevented by the above mentioned constituents. Phyllanthus amarus species may show an endometrial cancer activity due to the presence of constituents like quercetin, kaempferol, artemisinin and some other constituents.
CONCLUSION:
In conclusion, while Phyllanthus amarus shows promising medicinal potential with its rich phytochemical composition, including alkaloids, glycosides, flavonoids, and saponins, further research is necessary to elucidate its specific efficacy in endometrial cancer management. Integrating evidence-based traditional remedies like Phyllanthus amarus into comprehensive treatment strategies may offer valuable adjunctive support, but it should always be done under the guidance of healthcare professionals. Collaboration between traditional wisdom and modern science holds the key to unlocking novel therapeutic avenues for enhancing endometrial cancer care, empowering women with personalized treatment approaches.
REFERENCES
- Adnan, M., Patel, M., Patel, J., & Kulkarni, V. (2018). Comprehensive review on phytochemicals, pharmacological and clinical potentials of Phyllanthus amarus. Journal of Pharmacognosy and Phytochemistry, 7(1), 2962-2971.
- Gopi, S., Setty, R. S., & Rao, D. M. (2018). A review on Phyllanthus amarus. International Journal of Pharma and Chemical Research, 4(2), 59-67.
- Kaur, S., Michael, H., Arora, S., Härkönen, P., Kumar, S., & Ahonen, P. (2012). Comparative evaluation of hypolipidemic activity of Phyllanthus amarus and Phyllanthus maderaspatensis in albino rats fed with high fat diet. Iranian Journal of Pharmaceutical Research: IJPR, 11(4), 1145–1152.
- Lin, L. T., Chen, T. Y., Chung, C. Y., Noyce, R. S., Grindley, T. B., McCormick, C., & Lin, T. C. (2012). Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. Journal of Virology, 86(2), 379-395.
- Tona, L., Ngimbi, N. P., Tsakala, M., Mesia, K., Cimanga, K., Apers, S., ... & Vlietinck, A. J. (1999). Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. Journal of Ethnopharmacology, 68(1-3), 193-203.
- Yadav, A., & Patni, B. (2019). Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. Journal of Pharmacognosy and Phytochemistry, 8(1), 1550-1554.
- Rasul MG. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput. 2018;2:10-4.
- Gupta A, Naraniwal M, Kothari V. Modern extraction methods for preparation of bioactive plant extracts. International journal of applied and natural sciences. 2012 Aug;1(1):8-26.
- Singh J. Maceration, percolation and infusion techniques for the extraction of medicinal and aromatic plants. Extraction technologies for medicinal and aromatic plants. 2008;67:32-5.
- Zhang S, Dai S, Finkelman RB, Graham IT, French D, Hower JC, Li X. Leaching characteristics of alkaline coal combustion by-products: A case study from a coal-fired power plant, Hebei Province, China. Fuel. 2019 Nov 1;255:115710.
- Virot M, Tomao V, Colnagui G, Visinoni F, Chemat F. New microwave-integrated Soxhlet extraction: an advantageous tool for the extraction of lipids from food products. Journal of chromatography A. 2007 Dec 7;1174(1-2):138-44.
- Zubair MF, Atolani O, Ibrahim SO, Adebisi OO, Hamid AA, Sowunmi RA. Chemical constituents and antimicrobial properties of Phyllanthus amarus (Schum & Thonn). Bayero Journal of Pure and Applied Sciences. 2017 Sep 28;10(1):238-46.
- Park DW, Choi DS, Ryu HS, Kwon HC, Joo H, Min CK. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer letters. 2003 Jun 10;195(2):185-92.
- Arora V, Quinn MA. Endometrial cancer. Best Practice & Research Clinical Obstetrics & Gynaecology. 2012 Jun 1;26(3):311-24.
- Wilson JK, Sargent JM, Elgie AW, Hill JG, Taylor CG. A feasibility study of the MTT assay for chemosensitivity testing in ovarian malignancy. British journal of cancer. 1990 Aug;62(2):189-94.
- Ryan AJ, Susil B, Jobling TW, Oehler MK. Endometrial cancer. Cell and tissue research. 2005 Oct;322:53-61.
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. The Lancet. 2005 Aug 6;366(9484):491-505.
- Bahuguna A, Khan I, Bajpai VK, Kang SC. MTT assay to evaluate the cytotoxic potential of a drug. ||| Bangladesh Journal of Pharmacology|||. 2017 Apr 8;12(2):115-8.
- Wright JD, Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. The Lancet. 2012 Apr 7;379(9823):1352-60.
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer research. 1987 Jun 15;47(12):3239-45.
- Burke WM, Orr J, Leitao M, Salom E, Gehrig P, Olawaiye AB, Brewer M, Boruta D, Herzog TJ, Shahin FA, SGO Clinical Practice Endometrial Cancer Working Group. Endometrial cancer: a review and current management strategies: part II. Gynecologic oncology. 2014 Aug 1;134(2):393-402.
- Reinhold U, Tilgen W, editors. Chemosensitivity testing in oncology. Springer Science & Business Media; 2002 Oct 31.
- Van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Cancer cell culture: methods and protocols. 2011:237-45.
- Ghasemi M, Turnbull T, Sebastian S, Kempson I. The MTT assay: utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. International journal of molecular sciences. 2021 Nov 26;22(23):12827.
- Berchuck A, Boyd J. Molecular basis of endometrial cancer. Cancer. 1995 Nov 15;76(S10):2034-40.


 Venkata G Karthik Reddy*
Venkata G Karthik Reddy*
 Giresha Naidu M
Giresha Naidu M
 Gowtham Gowda M R
Gowtham Gowda M R
 Hari Kishor R
Hari Kishor R
 Haritha R
Haritha R
 Hruthika B
Hruthika B








 10.5281/zenodo.13337464
10.5281/zenodo.13337464