Abstract
Depression is a prevalent mental health disorder with significant societal and economic burdens. Recent research has highlighted the intricate relationship between the gut microbiota and depression, emphasizing the bidirectional communication along the microbiota-gut-brain system modulation, HPA axis regulation, and brain-gut axis communication. Probiotics, particularly strains of Lactobacillus, Bifidobacterium, Faecalibacterium, and Clostridium have emerged as potential therapeutic agents for depression due to their ability to modulate the gut microbiota and influence neurochemical pathways. Studies in both animal models and clinical trials have demonstrated the antidepressant effects of probiotics, offering promising avenues for novel interventions in the management of depression and related disorders. Further research is needed to elucidate the precise mechanisms of action and optimize probiotic-based therapies for depression.
Keywords
Depression, Gut microbiota, Microbiota-gut-brain axis, HPA axis, Brain-gut axis, Probiotics
Introduction
Depression is a mental health condition marked by slowed thinking, persistent feelings of sadness, and a lack of motivation to engage in activities[1]. It often comes with thoughts of self-harm and physical symptoms like headaches or stomachaches. These aspects not only affect individual's well-being but also strain their relationships and impact their ability to work, leading to significant economic costs for families and society. Unfortunately, studies suggest that depression is becoming more common among the general population, highlighting the urgent need for effective interventions and support systems[2].
Depression's impact on global health is profound.It ranks as the third leading cause of global disability in healthy life years. Additionally, it is now recognized as the fourth most common illness worldwide, affecting nearly 4.4% of populations in both developed and developing countries[3]. These statistics underscore the widespread nature of depression and highlight the urgent need for comprehensive strategies to address its prevalence and impact on individuals and societies globally. The projected rise in depression by 2030 is indeed concerning, and the COVID-19 pandemic has certainly exacerbated mental health challenges worldwide. The increase in depression cases due to the pandemic underscores the importance of prioritizing mental health support and resources, both during and after public health crises. It highlights the need for accessible and effective mental health services to address the growing burden of depression and other mental health disorders[4]. Depression is a complex disorder with multifaceted origins, involving a combination of genetic, environmental, and psychological factors. The various hypotheses provides valuable frameworks for understanding some of the mechanisms involved in depression:
Monoamine Reduction Hypothesis:
This hypothesis suggests that depression is associated with a deficiency in certain neurotransmitters, particularly serotonin, norepinephrine, and dopamine. Medications like SSRIs (Selective Serotonin Reuptake Inhibitors) target this imbalance by increasing the availability of serotonin in the brain [5].
Hypothalamus-Pituitary-Adrenal (HPA) Axis Over activation:
Chronic stress can lead to dysregulation of the HPA axis, resulting in excessive cortisol production. This prolonged stress response is implicated in the development and persistence of depressive symptoms [6].
Brain-Derived Neurotrophic Factor (BDNF) Reduction:
BDNF is a protein that supports the survival and growth of neurons. Reduced levels of BDNF have been observed in individuals with depression, and this may contribute to structural changes in the brain associated with the disorder[7].
While these hypotheses provide valuable insights, it's important to recognize that depression is a heterogeneous condition with diverse underlying causes. Research continues to uncover new factors involved in its pathogenesis, including inflammation, neuroplasticity, and genetic predispositions. A comprehensive understanding of depression's etiology is crucial for developing more effective prevention and treatment strategies. Research exploring the microbiota-gut-brain axis has uncovered a fascinating new etiology towards understanding depression[8].
GUT MICROBIOME AND DEPRESSION
The gut microbiota plays a crucial role in maintaining human health and the immune system, with numerous neuroscientific studies indicating its significance in the development of brain systems[9]. The relationship between the gut microbiota and the brain is bidirectional, as evidenced by research on the microbiome-gut-brain axis. There is substantial evidence linking anxiety and depression disorders to the community of microbes residing in the gastrointestinal system[10] . Various approaches such as modified diet, increased intake of fish and omega-3 fatty acids, as well as macro- and micro-nutrients, prebiotics, probiotics, synbiotics, postbiotics, fecal microbiota transplantation, and regulation of 5-HTP, offer potential avenues for altering the gut microbiota as a treatment strategy[11]. However, there is a limited number of preclinical and clinical research studies assessing the effectiveness and reliability of these therapeutic approaches for depression and anxiety. This article examines relevant research on the correlation between gut microbiota and depression/anxiety, along with the diverse therapeutic possibilities for modifying the gut microbiota.
Mechanisms of gut microbiota in depression[13]
The connection between gut microbiota and depression is a burgeoning area of research within the field of neuroscience and psychiatry. Here's an overview of the mechanisms involved (Figure 1).
Neurotransmitter Production:
The gut microbiota plays a crucial role in the production and regulation of neurotransmitters such as serotonin, dopamine, and gamma-aminobutyric acid (GABA). Serotonin, in particular, is heavily involved in mood regulation, and its production in the gut is influenced by the composition of gut bacteria.
Immune System Modulation:
The gut microbiota interacts with the immune system, influencing its activity and response. Dysbiosis, an imbalance in gut bacteria composition, can lead to chronic low-grade inflammation, which has been linked to the development of depression. Inflammation can disrupt neurotransmitter metabolism and neuronal function, contributing to depressive symptoms.
Hypothalamic-Pituitary-Adrenal (HPA) Axis:
The HPA axis is a complex system involved in the body's response to stress. Dysregulation of the HPA axis is commonly observed in depression. The gut microbiota can communicate with the HPA axis through various pathways, including the release of signaling molecules and metabolites. Dysbiosis may disrupt HPA axis function, leading to an exaggerated stress response and increased susceptibility to depression.
Brain-Gut Axis Communication:
There is bidirectional communication between the gut and the brain, known as the brain-gut axis. The gut microbiota communicates with the central nervous system through neural, endocrine, and immune pathways. Changes in gut microbiota composition can alter this communication, influencing mood and behavior.
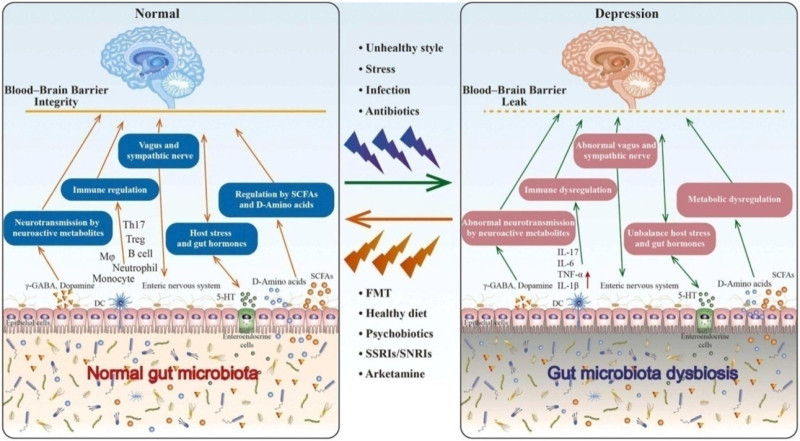
Figure.1 : The connection between gut microbiota and depression is mediated by the brain-gut-microbiota axis. Unhealthy lifestyles, stress, and infections can disrupt gut microbiota balance, contributing to depression. Various interventions like fecal microbiota transplantation, diet changes, psychobiotics, and antidepressants can restore balance, alleviating depressive symptoms. These approaches promote beneficial bacteria growth, produce neurotransmitters benefiting brain function, and modify neurotransmitter levels, offering promising avenues for improving mental health[12].
AMELIORATIVE EFFECT OF PROBIOTICS IN DEPRESSION
The term "probiotic" emerges from a linguistic fusion, drawing from Latin ("pro") and Greek ("bios"), signifying "for life." The historical utilization of fermented dairy items underscores the enduring presence and application of probiotics throughout human history. Subsequently, there has been a notable proliferation of probiotic-infused food items and probiotic supplements, available in various forms such as capsules, tablets, liquids, and powders[14].The exploration of probiotic's potential in addressing mental disorders has delved into various hypothetical mechanisms, primarily drawing from research conducted in vitro and in vivo using animal models[15]. Subsequent sections will delve deeper into the potential mechanistic role of probiotics in depression. This includes their anti-inflammatory properties, ability to restore gut permeability, modulation of neurotransmitters, attenuation of the HPA axis, and involvement in epigenetic mechanisms[16](Fig 2).
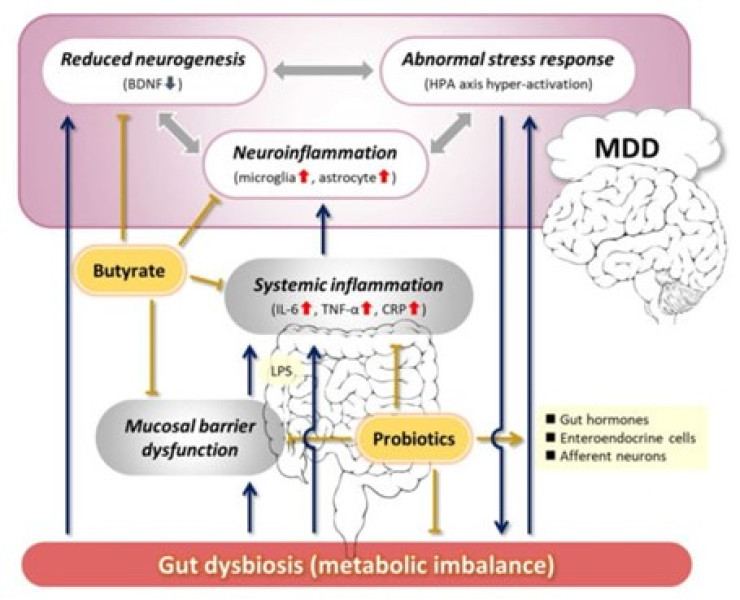
Figure 2 : The modulating role of probiotics in the underlying mechanisms of Major Depressive Disorder (MDD) via the gut-brain axis[17].
Lactobacillus
Lactobacillus is among the most extensively utilized and researched probiotic bacteria within the gut microbiota. These bacteria, identified as Lactobacillus spp., are anaerobic, gram-positive, peroxidase-negative, non-spore-forming rods that thrive under microaerobic conditions. Being a natural component of the healthy microbiota in the human gut, vagina, and oral cavity, Lactobacilli are generally regarded as safe microorganisms, posing low pathogenic risks and lacking the capability to transfer antibiotic resistance traits to pathogens[18].Therefore, Lactobacilli strains derived from natural sources have emerged as promising candidates for probiotic use. Numerous strains of Lactobacilli, such as L. plantarum, L. fermentum, L. rhamnosus, and L. casei, isolated from the gut, have been employed as probiotics. These strains offer various advantages to the host, including the alleviation of anxiety and cognitive of these Lactobacilli strains on mood, anxiety, and cognition, positioning them as potential psychobiotics[19]. One of the notable Lactobacilli strains is L. rhamnosus, known for its resilience to acidity and bile, as well as its significant adherence to human intestinal mucosal cells. Numerous animal studies have revealed the psychoactive and neuroactive attributes of a specific strain, L. rhamnosus JB-1 (JB-1), following oral administration. JB-1 has demonstrated the ability to modulate neurotransmitter levels in the brains of mice, thereby mitigating anxiety and depression-related behaviors induced by stress[20]. JB-1 consistently modulates the expression of GABAA and GABAB receptors in mice in a manner dependent on the region, thereby restoring levels of metabolites like GABA and glutamate to their baseline and diminishing corticosterone levels[21]. Researcher documented that treatment with the JB-1 strain for four weeks in BALB/c mice resulted in a 25% increase in central GABA levels[22].The antidepressant effects of JB-1 rely on the presence of an intact vagus nerve connection linking the gut and the brain[23]. Several Lactobacilli strains have been used as probiotics, including L. plantarum, L.fermentum, L.rhamnosus and L. casei, which are isolated from the gut and exertvarious benefits to the host, including attenuation of anxiety and cognitive improvement. People experiencing low mood reported increased happiness following the consumption of milk containing L. casei, whereas there was no such effect observed with the placebo[24]. Consumption of probiotics containing a mix of bacterial species, including L. casei, also led to a reduction in clinical depression and symptoms resembling depression in patients diagnosed with Major Depressive Disorder (MDD)[25]. The ingestion of Lactobacillus helveticus facilitated the recovery of rodents subjected to chronic and sub chronic stress from their depressive state[26]. Probiotic sticks containing L. helveticus, in addition to Bifidobacterium longum, reduced clinical depression and depressive-like symptoms in MDD patients[27].Research indicates that L. helveticus could potentially regulate the central NE system and HPA axis to enhance cognition, and the central 5-HT system and BDNF expression to alleviate depression[28]. The introduction of heat-killed L. paracasei through dietary intervention prevented mood decline during periods of stress in healthy individuals[29]. In mice with depression induced by corticosterone, administering either live or heat-killed L. paracasei via oral gavage demonstrated antidepressant effects comparable to or surpassing fluoxetine. Furthermore, the study revealed that live and heat-killed L. paracasei exerted their effects through distinct mechanisms: live L. paracasei elevated 5-HT levels, while heat-killed L. paracaseie levated DA levels in the brain[30].
Bifidobacterium
Bifidobacterium is a common bacterium found in the intestines, belonging to the Actinobacteria phylum and the Bifidobacteriaceae family. It is characterized as a Gram-positive bacterium that is typically non-motile, forms spores, and produces gas[31]. Mounting evidence suggests that probiotics containing Bifidobacteria have the potential to prevent and treat a range of mental and psychological disorders, including depression and anxiety[32]. In a three-blind randomized placebo-controlled trial, healthy subjects were administered a daily dose of lyophilized probiotics powder containing Bifidobacteria (2.5?×?10^9 CFU). Before and after the intervention, participants were assessed using the revised Leiden Depression Sensitivity Scale (LEIDS-r), Becker Depression Scale II (BDI-II), and Becker Anxiety Scale (BAI). The results revealed a significant reduction in overall cognitive response to depression, particularly in aggressive and reflective thinking, following the 4-week probiotics intervention. This study provided the first confirmation that a 4-week intake of multi-species probiotics positively impacted the cognitive response to natural fluctuations in mood in healthy individuals. Additionally, other research demonstrated that after treatment with Bifidobacterium infantis, maternally isolated rats exhibited reversed immune function, normalized norepinephrine concentration in the brain, and ultimately, reduced depressive behavior[33].Administering 1.0?×?10^10 CFU of Bifidobacterium longum NC3001 to adult patients with irritable bowel syndrome (IBS) and mild to moderate depression for 6 weeks resulted in significantly lower depression scores compared to the control group. Functional magnetic resonance imaging (fMRI) results revealed that Bifidobacterium longum NC3001 reduced the response of multiple brain regions, particularly the amygdala and frontal limbic regions, to fear stimuli. The decreased activation of the amygdala frontal limbic complex was associated with the reduced depression scores. These findings indicate a positive role of Bifidobacterium in the treatment of depression[34]. Numerous studies have affirmed the potential of Bifidobacteria in alleviating depressive symptoms. However, the precise mechanisms remain incompletely understood. These mechanisms might involve reducing the abundance of pathogenic bacteria, exerting anti-inflammatory effects, enhancing the integrity of the intestinal barrier, regulating tryptophan levels, influencing serotonin (5-HT) synthesis, and modulating the hypothalamus-pituitary–adrenal (HPA) axis[35].Exposure to chronic social defeat stress (CSDS) for 10 consecutive days altered the composition of intestinal microbial communities and increased the expression of interleukin-1 beta (IL-1?) in the brain in mice. This stress paradigm also led to heightened depressive behavior. However, administration of heat-sterilized Bifidobacterium breve M-16V could effectively lower the abundance levels of specific bacteria, potentially mitigating CSDS-induced depression and reducing IL-1? expression in the brain[36].
Faecalibacterium
Faecalibacterium prausnitzii, standing as the solitary species within the Faecalibacterium genus[37]. In a recent extensive cohort study, there was an inverse correlation observed between fecal levels of F. prausnitzii and depressed mood, while a positive correlation was found with quality of life[38].Faecalibacterium prausnitzii is known to produce significant amounts of butyrate through the fermentation of glucose and dietary fiber[39].F. prausnitzii also releases microbial anti-inflammatory molecules, which have the ability to suppress the proinflammatory nuclear factor (NF)-?B pathway in intestinal epithelial cells (IECs)[40]. The immunomodulatory effects align with neurochemical alterations observed in depressed mice treated with F. prausnitzii. Specifically, there was an increase in cecum short-chain fatty acids (SCFAs) and plasma interleukin-10 (IL-10) levels, alongside a decrease in corticosterone and IL-6 levels[41]. Additionally, intragastric administration of F. prausnitzii resulted in reduced colonic cytokine levels and improved intestinal permeability in mice suffering from colitis[42]. The capacity of F. prausnitzii to alleviate gut inflammation is significant enough to mitigate depressive- and anxiety-like behaviors in mice [43].
Clostridium
Treating chronic-stressed mice with C. butyricum resulted in improved depressive-like behaviors. Additionally, these treated mice exhibited increased expression of central serotonin (5-HT), brain-derived neurotrophic factor (BDNF), and glucagon-like peptide-1 (GLP-1) receptors in the brain[44]. Remarkably, when combining C. butyricum with antidepressants, approximately 70% of treatment-resistant MDD patients experienced a reduction in depression symptoms, with 30?hieving full remission[45]. These studies provide evidence supporting the antidepressant effectiveness of non-pathogenic strains of C. butyricum. However, it's important to note that certain strains of C. butyricum can be pathogenic, potentially leading to conditions such as botulism and necrotizing enterocolitis[46]. Another study found that the intake of C. butyricum increased the activity of neurogenesis-related pathways, such as BDNF, through the production of butyrate in mice[47]. While GLP-1 is recognized for its role in appetite and glucose regulation, activating central GLP-1 receptors has been demonstrated to modulate the central serotonin system and alleviate anxiety- and depressive-like behaviors in rats[48].Thus, the antidepressant mechanism of C. butyricum likely encompasses an increase in central BDNF-5-HT system and GLP-1 receptor expression facilitated by butyrate, akin to the effects observed with L. paracasei and B. infantis[49].
FUTURE PERSPECTIVE
The emerging research on the gut microbiota and its connection to depression opens up promising avenues for novel interventions in mental health. Probiotics, particularly strains like Lactobacillus, Bifidobacterium, Faecalibacterium, and Clostridium, show potential in modulating the gut microbiota and influencing neurochemical pathways associated with depression. Lactobacillus strains, such as L. rhamnosus and L. casei, have demonstrated antidepressant effects in both animal models and clinical trials. These effects are attributed to their ability to modulate neurotransmitter levels, particularly GABA and glutamate, and regulate the HPA axis, leading to reduced corticosterone levels and alleviation of depressive symptoms. Similarly, Bifidobacterium strains, like B. infantis and B. longum, have shown promise in improving depressive symptoms by influencing neurotransmitter synthesis, reducing inflammation, and modulating the HPA axis. Faecalibacterium prausnitzii, through its production of butyrate and anti-inflammatory molecules, has been linked to improvements in gut inflammation and depressive-like behaviors. Clostridium butyricum has also shown antidepressant effectiveness by increasing central serotonin and BDNF levels in the brain, potentially through the production of butyrate. However, further research is needed to fully understand the mechanisms of action of these probiotic strains and optimize their use in the treatment of depression. Future studies should focus on elucidating the precise pathways through which probiotics exert their effects, as well as exploring combination therapies and personalized approaches to treatment. Additionally, clinical trials with larger sample sizes and longer follow-up periods are necessary to establish the safety and efficacy of probiotic-based interventions for depression. Overall, the gut microbiota represents a promising target for the development of novel therapeutic strategies in mental health.
CONCLUSION
In conclusion, the emerging research on the gut microbiota and its connection to depression offers promising prospects for innovative interventions in mental health. Probiotics, including strains like Lactobacillus, Bifidobacterium, Faecalibacterium, and Clostridium, have demonstrated potential in modulating the gut microbiota and influencing neurochemical pathways associated with depression. Lactobacillus and Bifidobacterium strains have shown antidepressant effects by modulating neurotransmitter levels, reducing inflammation, and regulating the HPA axis. Faecalibacterium prausnitzii and Clostridium butyricum have also exhibited antidepressant effectiveness through their production of butyrate and modulation of central serotonin and BDNF levels. However, further research is necessary to fully understand the mechanisms of action of these probiotic strains and optimize their use in depression treatment. Future studies should explore combination therapies, personalized approaches, and larger clinical trials to establish the safety and efficacy of probiotic-based interventions for depression. Overall, targeting the gut microbiota represents a promising avenue for developing novel therapeutic strategies in mental health.
REFERENCES
- Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016 Aug 6;8(8):483.
- Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annual review of public health. 2013 Mar 18;34:119-38.
- Patel V. Why adolescent depression is a global health priority and what we should do about it. Journal of Adolescent Health. 2013 May 1;52(5):511-2.
- Renaud-Charest O, Lui LM, Eskander S, Ceban F, Ho R, Di Vincenzo JD, Rosenblat JD, Lee Y, Subramaniapillai M, McIntyre RS. Onset and frequency of depression in post-COVID-19 syndrome: A systematic review. Journal of psychiatric research. 2021 Dec 1;144:129-37.
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. Journal of clinical psychiatry. 2000 Mar 31;61(6):4-6.
- Mello AD, Mello MF, Carpenter LL, Price LH. Update on stress and depression: the role of the hypothalamic-pituitary-adrenal (HPA) axis. Brazilian Journal of Psychiatry. 2003;25:231-8.
- Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. Journal of affective disorders. 2007 Aug 1;101(1-3):239-44.
- Foster JA, Neufeld KA. Gut–brain axis: how the microbiome influences anxiety and depression. Trends in neurosciences. 2013 May 1;36(5):305-12.
- Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cellular and Molecular Life Sciences. 2019 Feb 15;76:473-93.
- Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, Rasoulpoor S, Khaledi-Paveh B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Globalization and health. 2020 Dec;16:1-1.
- Kumar A, Pramanik J, Goyal N, Chauhan D, Sivamaruthi BS, Prajapati BG, Chaiyasut C. Gut microbiota in anxiety and depression: unveiling the relationships and management options. Pharmaceuticals. 2023 Apr 9;16(4):565.
- Chang L, Wei Y, Hashimoto K. Brain–gut–microbiota axis in depression: A historical overview and future directions. Brain Research Bulletin. 2022 May 1;182:44-56.
- Limbana T, Khan F, Eskander N. Gut microbiome and depression: how microbes affect the way we think. Cureus. 2020 Aug 23;12(8).
- Zhang Q, Chen B, Zhang J, Dong J, Ma J, Zhang Y, Jin K, Lu J. Effect of prebiotics, probiotics, synbiotics on depression: results from a meta-analysis. BMC psychiatry. 2023 Jun 29;23(1):477.
- Zucko J, Starcevic A, Diminic J, Oros D, Mortazavian AM, Putnik P. Probiotic–friend or foe?. Current Opinion in Food Science. 2020 Apr 1;32:45-9.
- Rudzki L, Ostrowska L, Pawlak D, Ma?us A, Pawlak K, Waszkiewicz N, Szulc A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019 Feb 1;100:213-22.
- Suda K, Matsuda K. How microbes affect depression: underlying mechanisms via the Gut–brain axis and the modulating role of probiotics. International journal of molecular sciences. 2022 Jan 21;23(3):1172.
- Zhang Z, Lv J, Pan L, Zhang Y. Roles and applications of probiotic Lactobacillus strains. Applied microbiology and biotechnology. 2018 Oct;102:8135-43.
- Saarela M, Mogensen G, Fonden R, Mättö J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. Journal of biotechnology. 2000 Dec 28;84(3):197-215.
- Goldstein EJ, Tyrrell KL, Citron DM. Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clinical Infectious Diseases. 2015 May 15;60(suppl_2):S98-107.
- Xu M, Tian P, Zhu H, Zou R, Zhao J, Zhang H, Wang G, Chen W. Lactobacillus paracasei CCFM1229 and Lactobacillus rhamnosus CCFM1228 alleviated depression-and anxiety-related symptoms of chronic stress-induced depression in mice by regulating xanthine oxidase activity in the brain. Nutrients. 2022 Mar 18;14(6):1294.
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences. 2011 Sep 20;108(38):16050-5.
- Tette FM, Kwofie SK, Wilson MD. Therapeutic anti-depressant potential of microbial GABA produced by Lactobacillus rhamnosus strains for GABAergic signaling restoration and inhibition of addiction-induced HPA axis hyperactivity. Current Issues in Molecular Biology. 2022 Mar 22;44(4):1434-51.
- Janik R, Thomason LA, Stanisz AM, Forsythe P, Bienenstock J, Stanisz GJ. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. Neuroimage. 2016 Jan 15;125:988-95.
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences. 2011 Sep 20;108(38):16050-5.
- Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. European journal of clinical nutrition. 2007 Mar;61(3):355-61.
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016 Mar 1;32(3):315-20.
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015 Dec 3;310:561-77.
- Kazemi A, Noorbala AA, Azam K, Djafarian K. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: A double-blind, placebo-controlled randomized clinical trial. Journal of functional foods. 2019 Jan 1;52:596-602.
- Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015 Dec 3;310:561-77.
- Murata M, Kondo J, Iwabuchi N, Takahashi S, Yamauchi K, Abe F, Miura K. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Beneficial microbes. 2018 Dec 7;9(6):855-64.
- Wei CL, Wang S, Yen JT, Cheng YF, Liao CL, Hsu CC, Wu CC, Tsai YC. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain research. 2019 May 15;1711:202-13.
- Bottacini F, Ventura M, Van Sinderen D, O'Connell Motherway M. Diversity, ecology and intestinal function of bifidobacteria. Microbial cell factories. 2014 Aug;13:1-5.
- Cheng LH, Liu YW, Wu CC, Wang S, Tsai YC. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. Journal of food and drug analysis. 2019 Jul 1;27(3):632-48.
- Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan T. Effects of the probiotic Bifidobacteriuminfantis in the maternal separation model of depression. Neuroscience. 2010 Nov 10;170(4):1179-88.
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin FP, Cominetti O, Welsh C, Rieder A, Traynor J. Probiotic Bifidobacteriumlongum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017 Aug 1;153(2):448-59.
- Tian P, Chen Y, Zhu H, Wang L, Qian X, Zou R, Zhao J, Zhang H, Qian L, Wang Q, Wang G. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain, behavior, and immunity. 2022 Feb 1;100:233-41.
- Kosuge A, Kunisawa K, Arai S, Sugawara Y, Shinohara K, Iida T, Wulaer B, Kawai T, Fujigaki H, Yamamoto Y, Saito K. Heat-sterilized Bifidobacteriumbreve prevents depression-like behavior and interleukin-1? expression in mice exposed to chronic social defeat stress. Brain, Behavior, and Immunity. 2021 Aug 1;96:200-11.
- Duncan SH, Hold GL, Harmsen HJ, Stewart CS, Flint HJ. Growth requirements and fermentation products of Fusobacteriumprausnitzii, and a proposal to reclassify it as Faecalibacteriumprausnitzii gen. nov., comb. nov. International journal of systematic and evolutionary microbiology. 2002 Nov;52(6):2141-6.
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S. The neuroactive potential of the human gut microbiota in quality of life and depression. Nature microbiology. 2019 Apr;4(4):623-32.
- Duncan SH, Hold GL, Harmsen HJ, Stewart CS, Flint HJ. Growth requirements and fermentation products of Fusobacteriumprausnitzii, and a proposal to reclassify it as Faecalibacteriumprausnitzii gen. nov., comb. nov. International journal of systematic and evolutionary microbiology. 2002 Nov;52(6):2141-6.
- Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C. Faecalibacteriumprausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proceedings of the National Academy of Sciences. 2008 Oct 28;105(43):16731-6.
- Hao Z, Wang W, Guo R, Liu H. Faecalibacteriumprausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology. 2019 Jun 1;104:132-42.
- Laval L, Martin R, Natividad JN, Chain F, Miquel S, De Maredsous CD, Capronnier S, Sokol H, Verdu EF, van HylckamaVlieg JE, Bermúdez-Humarán LG. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacteriumprausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut microbes. 2015 Jan 2;6(1):1-9.
- Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology. 2019 Jun 1;104:132-42.
- Sun J, Wang F, Hu X, Yang C, Xu H, Yao Y, Liu J. Clostridium butyricum attenuates chronic unpredictable mild stress-induced depressive-like behavior in mice via the gut-brain axis. Journal of agricultural and food chemistry. 2018 Jul 24;66(31):8415-21.
- Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, Okazaki S, Yamashita S, Miura S, Miki H, Matsuda H. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clinical neuropharmacology. 2018 Sep 1;41(5):151-5.
- Cassir N, Benamar S, La Scola B. Clostridium butyricum: from beneficial to a new emerging pathogen. Clinical Microbiology and Infection. 2016 Jan 1;22(1):37-45.
- Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, Zhang H, Jin J, Chen W, Pang M, Yu J. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. BioMed research international. 2015 Oct 7;2015.


 Mumthas Beegum P. C.* 1
Mumthas Beegum P. C.* 1


 10.5281/zenodo.10868751
10.5281/zenodo.10868751