Abstract
Sugarcane bagasse is a rich source of cellulose, hemicellulose, lignin, and some other extracts. Sugarcane bagasse generated in very high amounts has been a big issue for industries and the environment globally for the past few years. Therefore, proper pretreatment is important to efficiently solubilize the lignin that shows cellulose and hemicellulose for various enzymatic actions. Pretreatment decreases the biomass i.e., the crystallinity of cellulose, structural complexity of cell wall, and lignification process for its effective use in biorefinery. The present research describes the utility of sugarcane bagasse as a sustainable and renewable cellulose substrate for the production of industrially important multifarious value-added excipients i.e. Micro Crystalline Cellulose.
Keywords
Sugarcane bagasse, Microcrystalline Cellulose, Lignin, Cellulose crystallinity, Structural complexity, Saccharification.
Introduction
The oral route is considered the most promising route to produce the instantaneous pharmacological effect of drugs. Oral delivery is considered the most favored route of drug delivery in the pharmaceutical industry. Effective drug delivery via the oral route may depend on factors such as gastric emptying, GI transit time of dosage form, amount of drug release from dosage form, and site of absorption of various drugs.[1]
Many of the dosage forms administered via oral route possess several limitations such as varying GI transit time because of various factors of gastric emptying leading to non-uniform drug absorption profiles, incomplete or partial drug release, and less residence time of the dosage form in the stomach. Due to this, the incomplete absorption of drugs happens which have an absorption site in the upper part of the small intestine, as the drug passes down the absorption site, the remaining quantity stays unabsorbed. [1] This is due to the given advantages,
- It is considered the most convenient and uncomplicated route for drug delivery.
- Its administration method is very easy compared to other routes.
- It helps to improve patient compliance and most cost-effective method.
- Oral solid dosage forms such as have been formulated and developed nowadays since they are the most effective routes of administration.
- The oral route administers at least 90% of all drugs used to produce systemic effects.
- Tablet represents the unit dosage form in which one dose of the drug has been accurately placed.[1]
Tablet - The tablet is designed to be released rapidly after oral administration or the tablet is dissolved and administered as a solution. Pharmaceutical tablets are unit dosage form, solid, biconvex, and flat dishes, prepared by compressing a drug or a mixture of a drug, with or without excipients.” [2]
IDEAL CHARACTERISTICS [2] –
- They may vary in shape, size, and weight depending on the amount of active medicinal substance and the route or mode of administration.
- The tablet may be coated or uncoated, and consist of one or more active substances with or without excipients including diluents, binders, glidants, lubricants, coloring matters which should be given by the component authority, and flavoring substances.
- It is the most popular dosage form and 72% of the total medicines are dispensed in the form of tablets and a very well-known dosage form for its patient compliance due to self and easy administration.
Advantages [2]
- They are unit dosage forms and offer the greatest capabilities of all oral dosage forms for the greatest precision and least content variability.
- Low cost among all oral dosage forms and are easiest and cheapest for packaging.
- Product identification requires no additional processing steps when employing embossed or monogrammed punch face.
- Provides the greatest ease of swallowing with the least tendency for hanging up above the stomach, especially when provided tablet disintegration is not excessively rapid.
- They lend to certain special release profile products, like, enteric coated or delayed release profiles.
- Easy large-scale production than other oral dosage forms.
Disadvantages [3]
- Some drugs oppose the compression into dense compacts due to their amorphous nature and low-density character.
- Drugs having poor wetting, less dissolution properties and optimum absorption window may be difficult to formulate, as tablets that will still provide adequate drug bioavailability.
- Bitter-tasting drugs, drugs with non-handling odor, or drugs that are sensitive to atmospheric moisture may require encapsulation before compression.
Excipients - “Pharmaceutical excipients are defined as the substances other than the active pharmaceutical ingredient, that has been appropriately evaluated for safety and are intentionally included in a drug delivery system but do not show the pharmacological effect” [4]
The excipients allow the drug substance to be applied to the patient in the right form and support the way and place of action without being active themselves. There are a variety of excipients in each class of it which firstly made compatibility with the drug or API to form stable and effective dosage form without being engaged in the pharmacological activity. [4]
Excipients have different roles in various formulations, some of the major roles assigned to the excipients can be [4],
- In the processing of the drug delivery system during its manufacture.
- Protect, support, or increase stability, bioavailability, or patient acceptability.
- In product identification, enhance any attribute of the overall safety.
- In the effectiveness and delivery of the drug in use.
- Assist in maintaining the integrity of the drug product during storage.
- Provide stable retention to the API.
Some of the widely used excipients are listed below [5],
- Diluents
- Binders
- Flavouring agents
- Disintegrants
- Colorants
- Lubricants
- Glidants
- Preservatives
microcrystalline cellulose [6]
- MCC is defined as “purified, partially depolymerized cellulose” produced by treating alpha cellulose, derived from lingo-cellulosic biomass with mineral acids.
- MCC is used as an excipient, binder, and adsorbent in the pharmaceutical industry, and as a stabilizer, anti-caking agent, fat substitute, additive, and emulsifier in the food industry.
- As gelling agents, stabilizers, and suspending agents in the beverage industry, and as fat substitutes, thickeners, stabilizers for emulsions, reinforcing agents for cement-based composite materials, and binders in cosmetics, which are derived from hardwood.
- In the pharmaceutics field, microcrystal cellulose is used as a tablet filler ingredient.
- Microcrystalline cellulose (MCC) is a pure partially depolymerized cellulose synthesized from ?-cellulose precursor (type I?). It is obtained as a pulp from fibrous plant material, with mineral acids using hydrochloric acid to reduce the degree of polymerization.
- The MCC can be synthesized by different processes such as reactive extrusion, enzyme-mediated, steam explosion, and acid hydrolysis.
- It is manufactured by spray-drying the neutralized aqueous slurry of hydrolyzed cellulose. It is a valuable additive in pharmaceutical manufacturing, food, cosmetics, and other industries.
Composition - Sugarcane bagasse, a fibrous residue consisting of nearly 31–44?llulose, 20–33% hemicellulose, 18–34% lignin, 1.0–9.1% ash, and some other components. Typically, SCB is a highly heterogeneous material consisting of 40-45% of cellulose (mainly ?-67 Cellulose) which have more crystalline domains, 30-35% of hemicellulose, a heterogeneous polysaccharide consisting of xylose, arabinose, galactose, and mannose sugars.[6]
Materials - Sugarcane bagasse is a bio-waste, procured from a sugarcane juice shop of Chandan Tiwari, at Anandwan Chowk, Warora. Aspirin and other excipients were procured from Prachi Chemicals, Nagpur
Method
Collection of Sugarcane Bagasse
- Sugarcane bagasse is a bio-waste, obtained after extracting the juice from sugarcane. We got SB from a juice shop and the shopkeeper was Chandan Tiwari.
- His shop is located at Anandwan Chowk, Warora. We received the Bagasse for our project work and First of all, we washed the bagasse with water, so the dirt was removed from it.
- Then, spread the SB, under the sunlight, so it gets dry properly.
- We repeated the process several times, so the remaining sugar particles get removed from it.
- We had brought about 1 kg of bagasse which after washing and drying, we got about 900 grams of bagasse. It took about 2 days to finish this step.
- After washing, we chopped the dried bagasse into fine pieces and converted it into small particles with the help of a mixer grinder whose RPM was 18000.
- We have repeated this process several times to get the desired size of particles. After doing the process, we weighed it and we got approximately 740 grams of sugarcane bagasse.
- This step took us up to 4 hours. Then we packed the crushed SB powder in a butter paper wrapped it in a suitable packet and stored it till the next step started.
Extraction of Sugarcane Bagasse
- For extraction of cellulose from SCB, a 3-step delignification was implemented with acid, alkaline pre-treatment, and oxidation process.
- The grounded SCB was treated with 5% HNO3, at 80°C for 2 hours with occasional stirring.
- The reaction was quenched with distilled water and filtered. Then the residue was neutralized by washing with water.
- After drying, the residue was further treated with 2N NaOH at 80°C for 2 hours with stirring.
- Lignin was removed as filtrate and residue was neutralized with distilled water to get a pH 7
- The delignified biomass was then bleached with 10% CH3COOH/NaClO at 80°C for 2 hours with occasional stirring.
- The resulting solution was diluted with distilled water and filtered. Cream white pulpy cellulose was extracted.
- The cellulose was subjected to depolymerization in the presence of 10% H2O2 with 0.5 ml of H2SO4 at 80°C for 5 hours.
- A white turbid solution was resulted. After filtration, the depolymerized cellulose was dried in an oven overnight at 60°C and ground to produce a white, crystalline powder.

Figure No. 01 – Extraction of MCC from Sugarcane Bagasse
Evaluation of MCC isolated from Sugarcane Bagasse
1. Organoleptic Characters
2. Identification Tests
3. Fourier Transform Infra-Red Spectroscopy –
-
-
- Preparation of KBr Plate – Weigh accurately 5 mg of dry IR grade KBr in a mortar. Grind the KBr until there is no evidence of crystallinity and uniform distribution of the KBr.
- Mixing the sample with KBr - Mix a small amount of MCC with KBr powder on the plate. The ratio of sample to KBr should be optimized to obtain a good signal-to-noise ratio.
- Pressing the sample - Carefully press the mixture of microcrystalline cellulose and KBr using a hydraulic press or manual press to form a thin, uniform pellet. Apply sufficient pressure to ensure good contact between the sample and KBr.
- Collect baseline spectrum - Before analyzing the sample, collect a background spectrum by scanning an empty KBr plate. This will be used for baseline correction during data analysis.
- Place the sample on the FTIR spectrometer - Once the sample pellet is prepared, carefully place it on the sample holder of the FTIR spectrometer.
- Collect FTIR spectrum - Set up the FTIR spectrometer to collect spectra in the mid-infrared range (4000 to 400 cm-1). Scan the sample over the desired wavelength range.
- Interpretation - Interpret the results based on peaks observed in the spectrum. Assign peaks to specific vibrational modes of cellulose and other functional groups present in the sample.

Figure No. 02 – FTIR of Isolated MCC
Formula Preparation
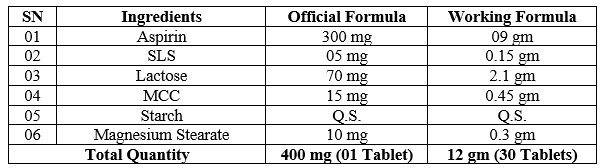
Table No. 01 – Formula for Aspirin tablet using isolated MCC from Sugarcane Bagasse
Tablet Punching
Punching of Tablet by Single Punch Tablet Machine [9] -
- The first step is to develop a formulation that is suitable for direct compression.
- This involves selecting appropriate APIs, excipients, and any other necessary additives.
- The formulation should have good flow properties, compressibility, and uniformity.
- Weight the API and all the excipients and mix all of them.
- Then this mixture is triturate in mortar and pestle and passes through the sieve no.100.
- After passing through the sieve the premix for the tablet is prepared and then this premix is placed into to hopper.
- The upper punch moves up and the lower punch moves down to create a cavity in the die and the feed from the hopper falls into the die cavity.
- The upper punch moves downwards to compress the powder into tablets and the lower punch moves upward to eject the compressed tablet.
- The whole process repeats until the feed material is exhausted.
Evaluation Parameter for Aspirin Tablet[9]
Pre-Compression Parameters - Various flow properties like bulk density, tapped density, angle of repose, carr's compressibility index, and Hausner’s ratio were performed to match the pre-compression as per IP.
Post-Compression Parameters
1. General Appearance – The general appearance of the tablets from each formulation batch was observed. The general parameters are shape, color, presence, or absence of odor were evaluated visually by randomly observing any tablet from formulated batches.
2. Tablet Dimensions – The physical dimensions of the tablets such as thickness and diameter are essential for acceptance and tablet uniformity. The measurement of the thickness and diameter of the tablets is carried out by using a digital thickness tester.
3. Weight variation test – Twenty tablets were selected at random, individually weighed in electronic balance and the average weight was calculated. The uniformity of weight was determined according to the I.P. specification
4. Hardness Test – The tablet was held between a fixed and moving jaw of hardness tester. The scale was adjusted to zero and the load was gradually increased until the tablets fractured. The value of the load at the point gives a measure of the hardness of the tablet.
5. Friability test – It is done in Roche friability apparatus where the tablets are subjected to the combined effect of abrasion and shock by utilizing a plastic chamber that rotates at 25 rpm for dropping the tablets at a distance of 6 inches with each rotation. Pre-weighed 10 tablets are placed in the friability, which is then operated for 100 revolutions (4 minutes). The tablets are then dusted and reweighed.
6. Dissolution test –
- The dissolution test apparatus consists of a cylindrical vessel with a hemispherical bottom and made of glass or transparent plastic having a 1000 ml volume capacity.
- The vessel is immersed in a water bath whose temperature is maintained at 370 0.5°C.
- The vessel is fitted with a cover having 4 holes. One hole for the shaft, a second for putting the thermometer, and the rest two for sampling.
- The shaft is attached to a variable speed-driven motor that rotates at 25-150 rpm. Place 1000 ml of dissolution medium (for example distilled water, hydrochloric acid (pH 1.2), and Phosphate buffer (pH 7.40) into the vessel.
- The vessel is placed in a water bath. The temperature is maintained at 30°C. In the case of paddle-type apparatus the tablet is placed in a vessel and stirred using a paddle.
- But in the case of basket-type apparatus, the tablet is placed in the basket Start the motor, and the speed is adjusted to 100 rpm or as mentioned in the monograph.
- At the specified interval as mentioned, the sample is withdrawn and filtered.
- Replace the volume withdrawn for sampling with a fresh dissolution median to maintain a constant volume.
- The samples are tested by analytical methods such as UV, chromatography, etc. to measure the proportion of drug dissolved.
7. Disintegration test –
- Disintegration testing determines the time required for the breaking of tablets when placed in a liquid medium.
- The apparatus used to perform the test is known as the disintegration test apparatus. The apparatus consists of a water bath which is filled with water up to the mark mentioned.
- Place 1000 ml beakers into the water bath. A basket holding 6 tubes open at the top. The bottom is covered with a 10-mesh screen.
- The basket rack assembly is dipped in the medium in 1000 ml beakers.
- The temperature of the liquid is maintained at 37°C. One tablet is placed into each tube.
- The assembly moves up and down at a specified rate (30 times per minute). A cylindrical disk made of transparent plastic is also placed over the tablet.
- The disc should impart little pressure on the tablet. The time to disintegrate of tablet and fall through the screen is noted.
RESULTS AND DISCUSSIONS
Evaluation of Microcrystalline Cellulose
Organoleptic Test - The results of the preliminary study are given below,

Table No. 02 – Organoleptic evaluation of Isolated MCC
Identification Tests – The results of identification tests are given below,
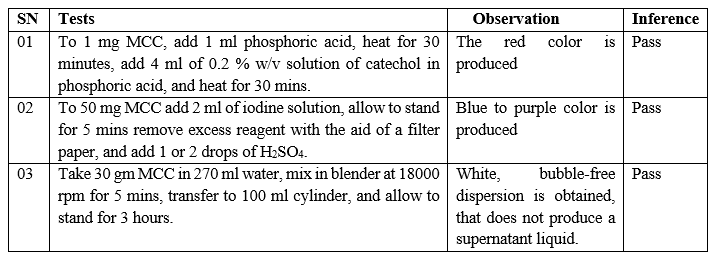
Table No. 03 – Identification tests of isolated MCC
FTIR Spectroscopy
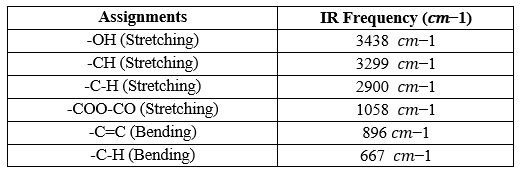
Table No. 04 – IR Functional groups and their frequencies with MCC
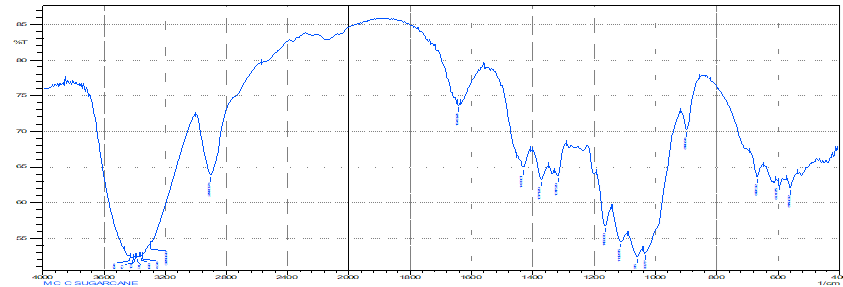
Graph No. 01 - FTIR Spectrum of Isolated Microcrystalline Cellulose
Evaluation of Aspirin prepared using MCC extracted from Sugarcane Bagasse
Organoleptic Test - The results of the preliminary study are given below,

Table No. 05 – Organoleptic Evaluation of Aspirin
Evaluation Parameter For Aspirin Tablet
Pre - Compression Parameters
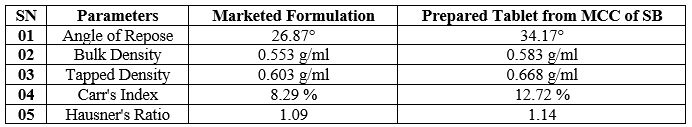
Table No. 06 – Pre – compression of aspirin tablets
Post - Compression Parameters

Table No. 07 – Post – compression of aspirin tablets
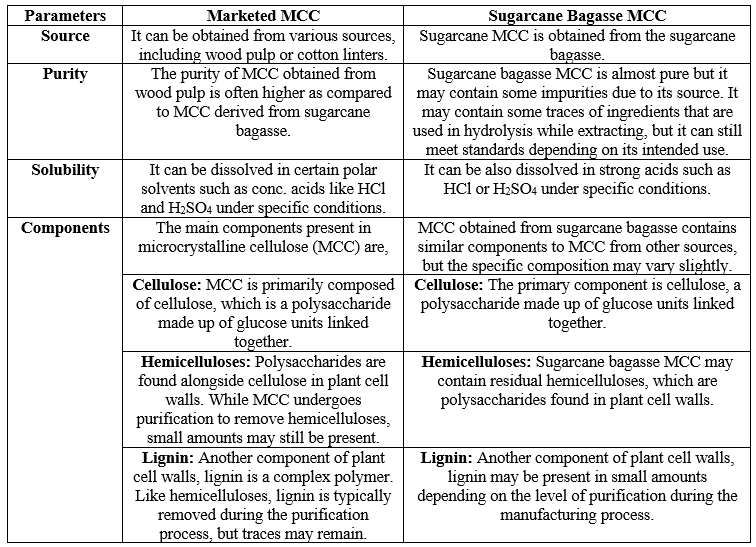
Table No. 08 – Comparison between Marketed MCC and MCC isolated from Sugarcane Bagasse
CONCLUSION
From the performed data, it can be concluded that the formulation prepared using MCC extracted from sugarcane bagasse shows nearly the same pre and post-formulation criteria as the Marketed formulation but a little variation in flow properties can be seen. The variation may be due to the solvents, apparatus, and process handling, and can get improved results after utilizing good sources and utilities, etc. The MCC extracted from the sugarcane bagasse meets all essential standards as per Indian Pharmacopoeia and with marketed Aspirin Tablets and can be used for future perspectives.
REFERENCE
- Leon Lachman, Herbert A. Lieberman and Joseph L. Kanig. The Theory and Practice of Industrial Pharmacy, Varghese publishing house, Mumbai; 3rd edn, 1987: 293-5.
- Indian Pharmacopoeia, Ministry of Health and Family Welfare. Ghaziabad, India, 3: 2186-88; 2010.
- Lachman, L.; Lieberman, H. A.; Kanig, J. L. Theory and Practice of Industrial Pharmacy, Varghese Publishing House: Bombay, pp. 293-345, 430-456, 1991.
- Raymond C., Paul J. Sheskey, Scan C. Owen. “Handbook of Pharmaceutical Excipients”, Pharmaceutical Press, London, 5th edition, 334-335, 278-282, 2006.
- Reymond.C.Rowe. Hand Book of Pharmaceutical Excipients”, Fourth Edition 354- 357.
- Shlieout G, Arnold K, Muller G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS PharmSciTech. 2002;3:E11
- Suzuki T, Nakagami H. Effect of crystallinity of microcrystalline cellulose on the compatibility and dissolution of tablets. European Journal of Pharmaceutics and Biopharmaceutics. 47:225-230
- Landín M, Martínez-Pacheco R, Gómez-Amoza JL, Souto C, Concheiro A, Rowe RC. Effect of batch variation and source of pulp on the properties of microcrystalline cellulose. International Journal of Pharmaceutics. 1993;91:133-141
- Indian Pharmacopoeia Commission. "Indian Pharmacopoeia 2007."
- Shlieout G, Arnold K, Muller G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS PharmSciTech. 2002;3:E11
- Suzuki T, Nakagami H. Effect of crystallinity of microcrystalline cellulose on the compatibility and dissolution of tablets. European Journal of Pharmaceutics and Biopharmaceutics. 47:225-230
- Landín M, Martínez-Pacheco R, Gómez-Amoza JL, Souto C, Concheiro A, Rowe RC. Effect of batch variation and source of pulp on the properties of microcrystalline cellulose. International Journal of Pharmaceutics. 1993;91:133-141
- Chauhan YP, Sapkal RS, Sapkal VS, Zamre GS. Microcrystalline cellulose from cotton rags (waste from garment and hosiery industries). International Journal of Chemical Sciences.:681-688
- El-Sakhawy M, Hassan ML. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydrate Polymers. 2007;67:1-10
- Chuayjuljit S, Su-uthai S, Charuchinda S. Poly(vinyl chloride) film filled with microcrystalline cellulose prepared from cotton fabric waste: Properties and biodegradability study. Waste Management & Research. 2010;28(2):109-117
- Rashid M, Gafur MA, Sharafat MK, Minami H, Miah MAJ, Ahmad H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohydrate Polymers. 2017;170:72-79
- Uesu NY, Pineda EA, Hechenleitner AA. Microcrystalline cellulose from soybean husk: Effects of solvent treatments on its properties as acetylsalicylic acid carrier. International Journal of Pharmaceutics. 2000;206:85-96
- Suvachittanont S, Ratanapan P. Optimization of micro crystalline cellulose production from corn cob for pharmaceutical industry investment. Journal of Chemistry and Chemical Engineering. 2013;7:1136-1141
- Gaonkar SM, Kulkarni PR. Improved method for the preparation of microcrystalline cellulose from water hyacinth. Textile Dyer and Printer. 1987;20(26):19-22
- Gaonkar SM, Kulkarni PR. Microcrystalline cellulose from coconut shells. Acta Polymer. 1989;40:292-293


 Chitralekha Gunaji Therkar*
Chitralekha Gunaji Therkar*
 Vishal K. Biswas
Vishal K. Biswas
 Dharti S. Borkar
Dharti S. Borkar
 Ritik N. Chende
Ritik N. Chende
 Priyanka R. Boratwar
Priyanka R. Boratwar











 10.5281/zenodo.13201616
10.5281/zenodo.13201616