Abstract
The predominant method of medicine delivery is still oral. Novel technologies with greater performance, patient compliance, and enhanced quality have evolved in the recent past. Fast-melt tablets are specifically made to dissolve in saliva very quickly within a few seconds when placed on the tongue without the need for water. Super disintegrants are added to FMT formulations to speed up a tablet's breakdown in the mouth. The technology utilized in the production of fast-melt tablets (FMTs) can be classified as either proprietary or conventional. Conventional methods such as freeze drying, tablet moulding, sublimation, spray drying, and others, as well as proprietary methods like Zydis, Orasolv, Durasolv, Wowtab, and Flashdose, are crucial. Important components utilized in the formulation of FMTs should enable the medicine to release quickly, leading to a faster rate of dissolution. The purpose of this review is to offer insightful information to scientists and industry experts working on the creation of fast-melt tablets (FMTs).
Keywords
Fast-melting tablet, Dysphagia, Patented Technology
Introduction
Verbal Solid dosage forms are widely used due to their affordability, simplicity of usage, precise dosage self-medication, ability to prevent pain, and above all patient compliance. The tablet is the most often used dose form because it is easy to manufacture, compact, and convenient for self-administration.1,2 Many people of all ages, particularly the elderly and those with dysphagia and hand tremor, find it difficult to swallow tablets and capsules and are thus unable to take medication as directed. They are also afraid of choking while taking solid dosage forms. Because it hinders patient compliance, it is also common in younger people due to undeveloped brain systems and muscles, as well as in patients with schizophrenia. Swallowing issue affect roughly one-third of the population, particularly the young and aged. This issue affects over 50% of the population, which contributes to the high rate of non-compliance and infectious therapy.2,3,4 Pharmaceutical technologists have created a novel oral dosage form called fast- melt tablet (FMT), also known as fast disintegrating tablets, rapid melt tablets, Oro-dispersible tablets, and immediate release tablets, among other names. FMTs dissolve quickly in saliva and don't require water, typically dissolving in a matter of seconds. Fast-melt tablets provide superior biological characteristics, bioavailability, and efficacy as compared to traditional tablets.5,6 The Centre for Drug Evaluation and Research (CDER) of the United States Pharmacopoeia (USP) has just approved the technology for fast-melt (FMTs) tablets. "A solid dosage form containing medicinal substances, which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue," is how the USFDA defines a fast-melt tablet.7
Disintegration conceptual Of Fast-Melt Tablet:8
Super disintegrants are far more careful when formulating fast-melt tablets. Due to the tablet's expansion and water absorption, they enable quick breakdown. Figure-1, illustrates the concept of a fast-melt tablet, in which the super disintegrants' swelling process wets the carrier's surface, enhancing tablet disintegration and resulting in increased dissolving. The swelling capacity of the dissolving liquid and the density of the resulting matrix determine how well super disintegrants work. A higher degree of disintegration is caused by a matrix with a higher swelling capacity and density.

Fig-1: Disintegration conceptual of fast-melt tablets
2. Ideal properties of FMTs 9,10
- It should feel excellent in the mouth and not require water to dissolve or disintegrate in a matter of seconds.
- After oral administration, there should be little to no residue left in the mouth.
- They ought to work well with other excipients and taste-masking agents.
- They ought to be less sensitive to temperature and humidity in the surroundings.
- They have to possess enough mechanical strength to endure the demanding production procedure and handling afterward.
- They ought to be flexible and compatible with the processing and packaging equipment currently in use.
Table-1. Comparison of conventional tablets with fast-melt Tablets 11

ADVANTAGES 5, 12, 13
The pharmaceutical and academic sectors are both steadily and more clearly recognizing the advantages of various dosage formulations. FMTs offer the combined benefits of liquid and solid dosage forms, in addition to unique qualities listed below:
- Reduces the risk of choking or suffocation during oral administration of conventional formulations due to physical obstruction, improving safety.
- Helpful in cases such as motion sickness, sudden episodes of allergic attack, coughing, where an ultra-rapid on set of action is required.
- Easy to administer to patients who cannot swallow, such as the elderly, bedridden patients, pediatric, geriatric, psychiatric, and patients affected by renal failure.
- Ability to deliver advantages of liquid medication in the form of solid formulation.
- Using water is not necessary.
- Quick medication absorption and dissolution
- The bioavailability rises.
- Permits heavy drug loading.
- No need to chew
DISADVANTAGES 14
- Fast dissolving tablet is hygroscopic in nature so must be keep in dry place.
- They are more susceptible to degradation by humidity and temperature.
MECHANISM OF SUPER DISINTEGRANTS:5,15,16,17
In this concern, a number of mechanisms are postulated, including deformation recovery, swelling, wicking, and particle repulsive forces. It appears unlikely that a single mechanism can account for all of the disintegrants' complicated behaviour. All these suggested processes, however, offer some insight into various facets of disintegrant action.15
Swelling:
The super disintegrant experiences swelling and tablet breaking when it comes into contact with water or saliva because the aqueous phase applies additional adhesive force to it in comparison to other excipients and the medicine.16
Wicking (Porosity and capillary action):
The tablet then begins to break apart as a result of water penetrating into its center and weakening the interparticle link. since of this, it is known as capillary action since the tablet breaks eventually due to a steady rise in wetness. Larger wetting rates and shorter disintegration times are associated with increased material porosity.16
Particle Repulsive Forces:
The swelling of tablets prepared with "non-swellable" disintegrants is attempted to be explained by another disintegrant mechanism. Based on the finding that non-swelling particles also contribute to tablet disintegration, Guyot-Hermann developed a particle repulsion theory. Water is necessary for the disintegration mechanism, which is based on the electric repulsive forces between particles. Repulsion is secondary to wicking, according to research findings.5
Due to deformation:
When tablet compression occurs, dissolved particles deform; these distorted particles return to their original shape when they come into touch with water or aqueous solutions. The starch's propensity to expand was occasionally increased when granules experienced considerable distortion during compression. The increasing size of the deformed particles causes the tablet to shatter. Investigations on this possible starch mechanism have only recently begun.17
EXCIPIENTS 18,19,20
Several excipients are used in the manufacture of quick melting tablets, including super disintegrants, lubricants, binders, flavour and sweeteners, fillers, and surface-active agents. Among these excipients' most important qualities are their rapid breakdown and rapid release of medicine.
Super disintegrants:
The fast-melt tablet formulation relies heavily on the super disintegrants. It facilitates the pills' rapid disintegration in the mouth, which causes them to dissolve swiftly. Super disintegrants include sodium starch glycollate, sodium carboxy methyl cellulose, modified corn starch, and cross povidone. The flowability of sodium starch glycollate is better than that of sodium croscarmellose. Crosspovidone has a very compactable and fibrous character.
Flavour and sweeteners:
By adding flavour and taste-masking agents, the items are made more appealing and palatable for patients. These ingredients have been added to assist counteract bitterness, and some of the active ingredients taste bad. Flavour oils, aromatic flavouring oil, and peppermint flavour. Aspartame, fruit essences, citrus oils, vanilla, and sugar derivatives are examples of flavouring agents.
Fillers:
Fast-melting tablet formulation also heavily relies on fillers. It improves the majority of the dose form. Sorbitol, calcium carbonate, mannitol, and magnesium carbonate are a few examples of fillers that are used.
Binders:
In order to make the powder more cohesive and help it form granules, binders are added to the formulation. After compaction, such grains create a cohesive mass or compact mass of tablets. These binding agents include, among others, HPMC (Hydroxypropyl methylcellulose), polyvinyl alcohol, and polyvinyl pyrrolidone.
Surface active agents:
Surface-active ingredients are crucial to the creation of FMTs. By lowering interfacial tension, it improves quick melt tablet solubilization. Sodium lauryl sulfate, sodium dodecyl sulfate, and polyoxymethylene sorbitan fatty acid esters are a few examples of surface-active compounds.
Lubricants:
Lubricants are not as essential as any other excipients. It is used to improve the mechanism of the transportation of drugs through the gastrointestinal tract and remove granular properties. Some lubricants are talc, Stearic acid, Magnesium stearate, polyethylene glycol, liquid paraffin, magnesium lauryl sulphate, colloidal silicon dioxide.
VARIOUS TECHNIQUES USED IN FAST-MELT TABLET (FMTS) FORMULATION:
There are two basic categories that may be utilized to classify the technology used to prepare Fast-melt tablets. Conventional technologies and patented technologies fall under this category.21
CONVENTIONAL TECHNOLOGY FOR FMTs FORMATION.
Fast-melt tablet formulation has been accomplished through a range of techniques. This article has addressed the eight primary techniques that are commonly used to make these tablets.21
- Lyophilization
- Direct Compression
- Tablet Moulding
- Mass Extrusion
- Spray Drying
- Nanonisation
Lyophilization or Freez-drying 22
Freeze drying is the process in which water is sublimed from the product after it is frozen. With this method, an amorphous porous structure that dissolves quickly is produced. The process of freeze-drying has shown to boost bioavailability and improve absorption. The key disadvantages of lyophilization technology are that it is expensive and time consuming; fragility makes traditional packaging inappropriate for these products and low stability under pressure situations.

Fig – 2: Lyophilization or Freeze drying method
Direct Compression Method 23
The disintegrant addition technology (direct compression) is the most preferred technique to manufacture the tablets due to certain advantages:
- Higher dosages can be used, and the tablet's final weight can be greater than it would be using other techniques.
- The simplest method of producing the tablets; standard tools and readily accessible excipients are employed.
- There aren't many processing stages required.
- Economic viability.
The disintegrant effectiveness is highly influenced by the size and hardness of tablets. Tablets that are huge and hard take longer to dissolve than usual. Small, extremely soft pills have little mechanical strength. In order to get rapid disintegration and high dissolution rates, the best sort and concentration of disintegrant should be selected. Disintegration time, however, stays relatively constant or even rises over the threshold concentration level.

Fig – 3: Direct compression method
Tablet moulding method:22
There are two types of moulding processes: solvent method and heat method. In order to create a wetted mass (compression moulding), the powder blend is moistened with a hydro-alcoholic solvent and then compressed at low pressure in molded plates. After that, the solvent is eliminated by air-drying. These tablets have a porous structure that speeds up dissolving and are less compact than compacted tablets. The moulding approach yields tablets that are easier to scale up for industrial manufacture than the lyophilization method.

Fig – 4: Tablet moulding method
Mass extrusion method:24
This approach uses a solvent mixture of water-soluble polyethylene glycol, commonly methanol, to soften a combination of the active medication and other components. The liquefied material is forced through a syringe or extruder to create a cylinder, which is then divided into uniform pieces using hot blades to create tablets. Drugs with bitter tastes can have their granules coated with the dried cylinder to cover up their bitter flavour.
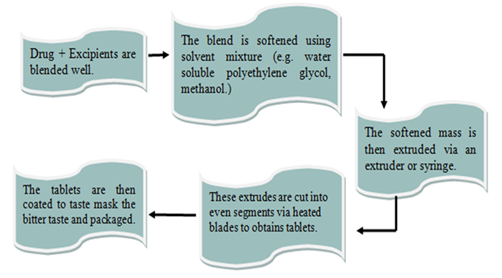
Fig – 5: Mass extrusion method
Spray-Drying method:24
Spray-drying in order to create fast-melt Tablets. The formulations included mannitol as a bulking agent, sodium starch glycolate or croscarmellose as a disintegrant, and both hydrolyzed and non-hydrolyzed gelatin as a supporting ingredient for the matrix. Additional enhancement of disintegration and dissolution was achieved by adding an acid (like citric acid) or an alkali (like sodium bicarbonate). The aforementioned suspension was compacted into tablets, and the porous powder was produced by spray drying it. In an aqueous media, tablets made using this technology demonstrate a disintegration time of less than 20 seconds.
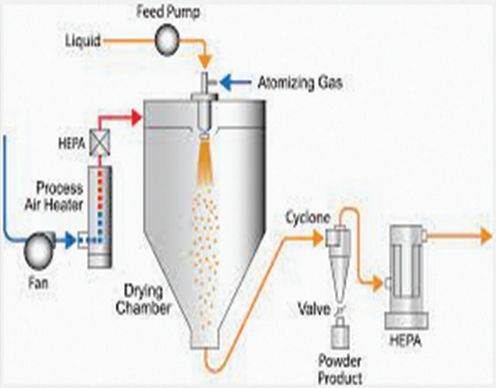
Fig – 6: Spray drying method
Sublimation method:24
In this procedure, inert volatile chemicals such as urea, urethane, naphthalene, camphor, etc. are added to other excipients and the blend is compressed into a tablet. When a tablet comes into touch with saliva, the pores in its structure caused by the sublimation of volatile substance dissolve the tablet. Furthermore, a variety of solvents, including as benzene and cyclohexane, can be employed as pore-forming agents. Using this technique, mouth-dispersing tablets with a highly porous structure and good mechanical strength have been created.
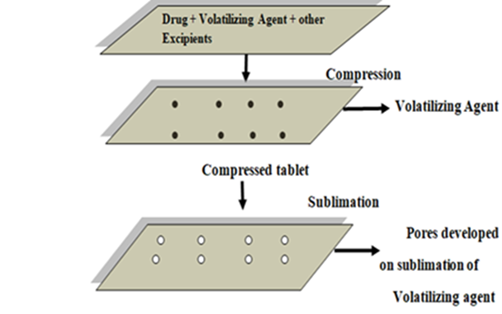
Fig – 7:sublimation method
Nanonization:24
Nanonization comprises lowering drug particle size to nano size by wet-milling, stabilizing nanocrystals with chosen stabilizers, and integrating them into FMTs. For medications that are not very water soluble, this approach has the benefit of improved absorption, bioavailability, and economical production.
PATENTED TECHNOLOGIES FOR FMTs FORMATION: 2,26,27,28
The rapid disintegration of fast-melt tablet is commonly ascribed to the rapid penetration of water into the tablet matrix, which causes the tablet to disintegrate quickly. Pharmacies have patented a number of technologies that have been developed based on various parts of formulation and processes. Here is a description of patented technology:
- Zydis Technology.
- Durasolve Technology.
- Orasolve Technology.
- Flash Dose Technology.
- Wow Tab Technology.
- Flash Tab Technology.
- Oraquick Technology.
- Quick –Dis Technology.
- Nanocrystal Technology.
Zydis Technology
R.P. Scherer, Inc. created Zydis, the first technology for modern tablets to be commercialized. In order to make Zydi's tablets, the medication is either lyophilized or freeze-dried within a gelatin matrix. It comes packaged in a unique blister pack and is extremely lightweight and delicate. Since very little water remains in the medication for microbial attack as a result of freeze drying, the preparation is also self-preserving. Zydi's technique produces tablets that break down in a matter of seconds.
Durasolv technology
This method, created by CIMA Labs, produces tablets that include lubricants, fillers, and the medicine. The tablets exhibit good stiffness and are manufactured using standard tableting equipment. They have good stiffness and can be packed in standard tabletting equipment. This method works well with tablets that include few active components.
Orasolv Technology
This technology was created by CIMA Labs, wherein tablets are produced through direct compression at a lower pressure than in standard DC. In addition to the effervescent disintegrators, the active component is taste-masked. Soft and friable, the generated tablets are packaged in a specially made pick-and-place system.
Flash Dose Technology
Using a special spinning mechanism, the Flash Dose technology creates a crystalline structure that resembles floss—think cotton candy. The active medication can then be added to this crystalline sugar and compacted into a tablet. Fuisz has patented this process, which goes by the name "Shear form." The finished product's surface area for dissolution is very high. Once on the tongue, it immediately dissolves and disperses. The "floss" in "flash dose" tablets is a self-binding shear form matrix. There are two types of shear form matrices that are produced using flash heat processing.
Wow tab Technology
For some years, the mouth-dissolving/disintegrating tablet formulation known as Wow tab has been available in the Japanese market. The WOW on the Wow tab indicates that the tablet should be administered "With Out Water." The Wow tab technique makes use of excipients that resemble sugar, such as mannitol. Low moldability saccharides (fast dissolving) and high moldability saccharides (excellent binding property) are combined in this technique. The two distinct saccharide types are mixed together to create a tablet formulation that has the right amount of hardness and rate of mouth dissolve. The Wow tab formulation is slightly more stable in the environment than Zaydis or OraSolv because of its notable hardness. It works well with blister packaging as well as traditional bottle packaging. In less than 15 seconds, the Wow tab product dissolves swiftly.
Flash tab Technology
The Flash tab technology is patented by Prographarm Laboratories. With this technology, an active substance in the form of microcrystals is prepared into a tablet that dissolves quickly. Conventional processes such as coacervation, extrusion-spherization, basic pan coating techniques, and microencapsulation can be used to manufacture drug micro-granules. The granulated combination of excipients made by wet or dry granulation is combined with the microcrystals of micro-granules of the active ingredient, and then compacted into tablets. The traditional tableting technology was used for all processing, and the resulting tablets are said to have a disintegration period of less than a minute and good mechanical strength.
OraQuick technology
The oral dissolving tablet OraQuick is made using a unique taste-masking process. Production is accelerated and enhanced since flavor masking eliminates the need for solvents. OraQuick produces less heat during manufacturing than other fast dissolving processes, making it appropriate for drugs that are sensitive to heat. OraQuick promises to cover up the taste and disappear in a matter of seconds.
Quick-Dis technology
Rapid-Dis Technology Lavipharm has created the perfect intraoral mouth dissolving medicine delivery technology to meet the market's unmet demands. The innovative intraoral medication delivery device, known by its trademark Quick-DisTM, is a flexible, thin, and rapidly dissolving film that is a Lavipharm-exclusive patented technology. The tongue is positioned with the film either on top or below. It is held in place at the application location and quickly releases the active ingredient for systemic or local absorption. The Quick-DisTM film with a 2 mm thickness typically disintegrates in 5 to 10 seconds. Quick DisTM film with a 2 mm thickness dissolves in around 30 seconds.
Nanocrystal Technology
Elan's unique Nanocrystal technology can facilitate formulation and enhance drug activity and end product attributes for tablets that dissolve orally. Particle size reduction raises surface area, which speeds up the rate of dissolution. The application of nanocrystal technology can achieve this with predictability and efficiency. Small drug particles, usually smaller than 1000nm in diameter, known as nanocrystals are created by milling the drug material using a special wet milling method. Water-soluble components are mixed with drug nanocrystal colloidal dispersion, blisters are filled, and the mixture is lyophilized. The resulting wafers are extremely durable yet quickly dissolve with very little water.
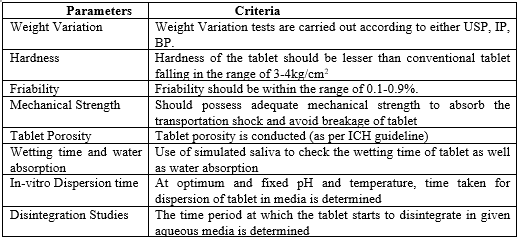
Evaluation Parameters:11
To ascertain the tablet's quality, it is critical to assess the medications that are produced. The essential evaluation parameters are listed below-
Table 2: Evaluation parameters of Fast-Melt Tablets
CONCLUSION:
For more over ten years, manufacturers have been interested in FMTs due to their potential benefits over conventional dosage forms. These benefits include enhanced patient compliance, convenience, bioavailability, and rapid beginning of action. Some of these technologies yield formulations of FMTs that are sufficiently strong mechanically and dissolve quickly in the mouth without the need for water. These dosage forms are always being improved by formulation technologies, which results in even more advantages with less disadvantages. A firm can prolong market exclusivity and provide a more convenient dosage form or dosing regimen for its patient base by developing a new dosage form.
REFERENCES:
- Yadav G, Kapoor A, and Bhargava S. Fast Dissolving Tablets Recent Advantages: A Review. 2012; 3(3): 728 -36.
- Divate S, Kavitha K, Ganesh N. S. Fast Disintegrating Tablets – An Emerging Trend. Int. J. of Pharm. Sci. Rev. and Res. Feb. 2011; 2(6): 18-22
- Nilesh S. M, Prakash W.P. Fast Dissolving Tablets: A Review of Formulation and Evaluation Strategies. Int. J. of Creat. Res. Thoughts. May 2024; 12(5):440-53
- Kumar, R. S., & Devi, M. G. A Review Article on Fast Dissolving Tablets. Int. J. of Health Sci. May 2022; 6(S2): 13–98.
- Neeta, Dureja H, Dahiya J. Fast Dissolving Tablets: An Overview Novel Science. Int. J. of Pharm. Sci. 2012; 1(5): 228-32.
- Gupta S, Mali. R. R, Goel. V. Novel study in fast dissolving drug delivery system: a review. Indian J. Pharm. Biol. Res. 2015; 3(1):93-107.
- Chandan S, Varun D, Ashish G, Dabeer A. Orally disintegrating tablets: A Review. Int J. of Pharm & LIFE Sci. 2010;1(5):250-56.
- Jyothi, K. Akhil Kumar, M. Mamatha, N. Mamatha. Formulation And Evaluation of Fast Dissolving Tablets of Zidovudine Using Sublimation Technique. World J. of Pharm. Res. June 2018; 7 (13): 912-24.
- Mangal M, Thakral S, Goswami M. Comparison Study Between Various Reported Disintegrating Methods for Fast Dissolving Tablets. Afr. J. Basic. Appl. Sci. 2012;4(4): 106-09.
- Aarti J, Sonali J, Ganesh D. Oro dispersible tablets: A comprehensive review. Res J Pharm Technol. Jan. 2014; 7(3):368-75
- Kushagra K, Gauravi X, Suresh K. J. Fast-Melt Tablets (FMTs): Revolutionizing rapid relief-an in-depth review of swift dissolve technology. Int. J. Pharm. Res. Allied Sci. 2016; 5(2):311-22.
- Siddiqui N, Garg G, Sharma PK. Fast dissolving tablets: preparation, characterization and evaluation: an overview. Int J. Pharm Sci Rev Res 2010; 2:87-96.
- Samita G, Gaurav K. Fast Dissolving Drug Delivery and its Technologies. J. Pharm. Innov. 2012; 2 (1): 34-39.
- Gnanaprakash M, Gomathi M, Jothimanivannan C. Fast dissolving tablet. Int. J. Pharm. Sci. Rev. Res., 2023 April 15; 7(1): 7-14.
- Pahwa R, Piplani M, Sharma PC, Kaushik D and Nanda S. Orally disintegrating tablets-friendly to paediatrics and geriatrics. Arch. Apll. Sci. Res. 2010; 2: 35-38.
- Nannjkar D, Shinde J. Recent Trends of Mouth Dissolving Tablet: An Overview. Int. J. Pharm. Sci. Rev. Res. June 2020; 62(2): 157-65.
- Bhusnure O.G. Gholve S.B, Giram P.S, Thonte S.S. Role of Super disintegrating in Fast Dissolving Tablets. Sept 2015; 4 (2):263-81.
- Roy D, Anjan De, Biswas. S, Das. A. An Overview on Mouth Dissolving Tablets. J. of Huazhong. Univ of Sci. and Technol. May 2022; 50(4):1-30.
- Arya A, Chandra A. Fast drug delivery system: A review. Scholars Research Library. 2010; 02(2):350-36.
- K. Abdul Razak, A. Seetha Devi, A. Anka Rao, V. Vasu Naik. Orally Disintegrating Tablets: A Review. Int. J. of Pharm. And Chem. Res. March 2015;1(1): 25-30.
- Bano S, Kumar S, Sharma P. Fast-Melt Tablets (FMTs): Revolutionizing rapid relief-an in-depth review of swift dissolve technology. Int. J. of Bio. and Pharm Sci. Arch, 2023, 06(02), 8–36.
- Momin MM, Dev A. Fast dissolving tablets: a novel approach. Indian Journal of Pharmaceutical and Biological Research. 2015 Jan 1;3(1):18
- Babu A, Akhtar Md. S. Overview of formulation & evaluation of fast dissolving tablet: A promising tablet dosage form. J of Appll. Pharm. Res. July 2020; 8 (3): 01 – 09.
- Karodade A.G, Aniket P. Wable, kharbal G. Fast dissolving tablet. Biological and Pharmaceutical Sciences, 2024, 27(01), 001–007.
- Masih A, Kumar A, Singh S, Tiwari A.K. Fast dissolving tablets: A review. Int J Curr Pharm Res 2017; 9:8-18.
- P. Nand, N Vashist, Anand A, Drabu S. Mouth Dissolving Tablets- A Novel Drug Delivery System. Dec 2010; 3(1):01-7.
- Chandan S, Varun D, Ashish G, Dabeer A. Orally disintegrating tablets: A Review. Int J. of Pharm & LIFE Sci. 2010;1(5):250-56.Organic Commun. 2022 Jul 1; 15:288-96.


 Baby Lynthong*
Baby Lynthong*
 Fathima Thafheema
Fathima Thafheema
 Dr. M. Mallikarjuna Gowda
Dr. M. Mallikarjuna Gowda









 10.5281/zenodo.13955749
10.5281/zenodo.13955749