Abstract
This study aimed to develop and assess an orodispersible tablet formulation of Eletriptan hydrobromide, a selective agonist for serotonin receptors 5-HT1B and 5-HT1D, primarily used for alleviating migraine headaches. Eletriptan hydrobromide, categorized as BCS Class I, is known for its relatively short elimination half-life of approximately 4 hours. Traditional tablet forms of this drug are insufficiently rapid in onset, which impedes their effectiveness in providing immediate relief from migraine pain. The tablet formulation incorporates Eletriptan hydrobromide as the active ingredient, combined with crosscarmellose sodium as a superdisintegrant, a wax mixture as a binder, encapsulated lemon flavor for taste, aerosil as a glidant, and saccharin and mannitol as natural sweeteners and bulking agents. The study aimed to develop a formulation that facilitates rapid absorption and onset of action, enhances mouthfeel, requires no water for ingestion, and improves patient compliance. The development process was carried out in two phases: (a) taste masking and (b) tablet formulation. The drug-resin complex (DRC) was evaluated for ion exchange complex formation. The tablets were tested for various parameters including content uniformity, hardness, friability, dissolution rate, disintegration time, and weight variation.
Keywords
Eletriptan Hydrobromide, Crosscarmellose Sodium, Orodispersible Tablet, Mouth Dissolving Tablet
Introduction
Oral controlled drug delivery systems are designed to deliver medication at a predetermined rate, either locally or systemically, for a specified duration. The oral route is the most commonly adopted and convenient method for drug delivery due to its ease of administration, patient compliance, and cost-effectiveness. These systems are engineered using various approaches and materials to ensure a predictable, precise, and reproducible pattern of controlled release, or even site-specific drug delivery. In recent years, there has been an increased demand for more patient-friendly and compliant dosage forms. As a result, the development of new technologies has surged. Given the high development costs of new drug molecules, efforts are increasingly focused on creating new dosage forms for existing drugs that enhance safety, efficacy, and bioavailability while also reducing dosing frequency and production costs.[1] To meet these medical needs, pharmaceutical technologists have developed innovative oral dosage forms such as oral dispersible tablets (ODTs). ODTs are designed to be placed in the mouth, where they disperse or dissolve in saliva without the need for water, providing a quick onset of action. This method of administration is particularly beneficial as some drugs are absorbed directly through the mouth, pharynx, and esophagus as the saliva passes into the stomach. This absorption route can result in significantly greater bioavailability compared to conventional tablet forms.[2] ODTs are especially appreciated by children and the elderly, who often have difficulty swallowing conventional tablets or capsules. The simplicity and cost-effectiveness of the direct compression process used to manufacture ODTs have made this technique an attractive alternative to traditional granulation technologies. This process not only streamlines production but also ensures that the final product meets the demands for patient convenience and compliance. Superdisintegrants are often added to ODT formulations to facilitate rapid disintegration into smaller particles, enhancing dissolution. These agents are used in small amounts (1-8%, with 2-4?ing optimal) and may be combined with common disintegrants like starch. An ideal disintegrant should have poor solubility and good hydration capacity and should not form complexes with the drug. Tablets are the most popular dosage form due to their convenience, compactness, and ease of manufacturing. However, many patients, especially pediatric and geriatric populations, find it difficult to swallow tablets, leading to non-compliance and ineffective therapy. Mouth-dissolving tablets (MDTs) are designed to disintegrate or dissolve rapidly in saliva, eliminating the need for chewing, swallowing, or taking them with water. This makes MDTs particularly beneficial for pediatric, geriatric, and psychiatric patients, as well as those with swallowing difficulties. MDTs dissolve or disintegrate within a minute in the oral cavity without water and have a pleasant taste. They are also known as oral disintegrating tablets, fast-dissolving tablets, fast-melting tablets, or mouth-melting tablets. The European Pharmacopoeia has adopted the term "orodispersible tablets" for these forms, highlighting their rapid dispersion in the mouth before swallowing. Overall, MDTs address potential issues of patient compliance and provide a convenient, efficient, and effective solution for drug administration across various patient populations.[3] Drug resin complex (DRC) formation is a sophisticated and highly effective technique for taste masking, particularly useful for drugs with unpleasant or bitter flavors. This method utilizes ion exchange resins, such as Indion 234, to mask the bitter taste of drugs like Eletriptan hydrobromide. The technique is based on the principle of electrostatic interactions between the resin and the drug. In this approach, Eletriptan hydrobromide, which carries a specific ionic charge, interacts with the resin, which carries an opposite charge. This electrostatic attraction facilitates the binding of the drug molecules to the resin, forming a stable complex. The electrostatic forces create an equilibrium between the drug solution and the resin-bound drug, effectively trapping the drug within the resin matrix. This binding process significantly reduces the drug's taste, as the drug is no longer free in the oral cavity where it could be perceived.
Beyond electrostatic interactions, other forces such as Van der Waals forces and chemisorption also play a role in the complexation process. Van der Waals forces, which are weak attractions between molecules, and chemisorption, involving the formation of chemical bonds between the drug and the resin, contribute to the stability and effectiveness of the drug-resin complex. These additional interactions help ensure that the drug remains securely bound to the resin, enhancing the taste-masking effect and improving the overall stability of the dosage form.[4] Eletriptan hydrobromide is used primarily for the treatment of migraines. It alleviates migraine symptoms by targeting the underlying physiological processes. Specifically, Eletriptan hydrobromide reduces the swelling of blood vessels surrounding the brain, which is a key factor in migraine pain. Additionally, it works by inhibiting the release of certain neurochemicals from nerve endings that contribute to pain and other associated symptoms, such as nausea, sensitivity to light, and sound. By addressing these mechanisms, Eletriptan hydrobromide provides effective relief from the symptoms of migraine attacks, improving patient comfort and quality of life.
Need to Formulate Mouth Dissolving Tablets:
The demand for non-invasive drug delivery systems persists due to patients' poor acceptance and compliance with existing delivery methods, limited market size for drug companies, and the high cost of disease management. Mouth dissolving tablets (MDTs) address these issues effectively. MDTs are particularly useful for geriatric patients, especially those suffering from conditions like hand tremors and dysphagia, as well as pediatric patients who have difficulty swallowing due to underdeveloped central nervous systems and internal muscles. They are also beneficial for traveling patients experiencing motion sickness or diarrhea without easy access to water, and for patients with persistent nausea, including cancer patients undergoing chemotherapy who are too nauseous to swallow H2 blockers prescribed to prevent gastric ulceration. Additionally, MDTs are advantageous for mentally challenged, bedridden, and psychiatric patients who may have difficulty swallowing traditional tablets or capsules.[5]
Advantages of Mouth Dissolving Tablets:
Mouth dissolving tablets (MDTs) offer the combined benefits of solid and liquid dosage forms with unique advantages such as improved compliance and convenience, increased bioavailability, rapid drug therapy intervention, enhanced patient perception, safety, no need for water or chewing, improved stability, simple production, lower doses, new business opportunities, accurate dosing, and compact packaging.[6]
Challenges in Formulating Fast Dissolving Tablets:
Formulating fast dissolving tablets (MDTs) presents several challenges. Achieving the right balance between mechanical strength and disintegration time is crucial, as MDTs must disintegrate in less than a minute while remaining strong enough to withstand packaging, transport, and handling. Many MDTs are fragile, and increasing their mechanical strength often delays disintegration time. Taste masking is another significant challenge, as many drugs have a bitter taste that can affect patient compliance if the bitterness is noticeable during disintegration in the mouth. Effective taste masking ensures the drug is palatable. Additionally, MDTs should disintegrate into small particles that leave minimal or no residue in the mouth, and adding flavors and cooling agents like menthol can enhance mouth feel. MDTs also need to be resistant to environmental factors such as humidity and temperature, as they are designed to dissolve in minimal water. Finally, the technology used to produce MDTs, such as Zydis and Orasolv, can be costly due to the need for specialized equipment and packaging, which increases the final product cost.
Limitations of Mouth Dissolving Tablets:
Mouth dissolving tablets (MDTs) face limitations in certain scenarios. Drugs with large doses, such as ciprofloxacin (500 mg), are challenging to formulate into MDTs due to size constraints. Additionally, MDTs may not be suitable for patients taking anticholinergic medications or those with conditions like Sjögren's syndrome, which reduces saliva production, as these factors can hinder the effective disintegration and absorption of the tablets.[7]
MATERIAL AND METHOD
Materials:
Eletriptan hydrobromide was kindly provided as a gift sample by Ajanta Pharma, India. The formulation of the drug involved several additional excipients, each sourced from reputable suppliers to ensure quality and efficacy. Mannitol, a sugar alcohol used as a filler and sweetener, was purchased from Loba Chemicals Ltd., Mumbai. Gelucire 39/1, an emulsifying agent that helps improve the drug's solubility and bioavailability, was obtained from Gattefosse, Mumbai. To enhance the flavor profile, lemon flavor was sourced from Keva Chemicals Ltd., Mumbai. Sodium saccharin, an artificial sweetener used to mask bitterness, was acquired from Cosmo Chem, Pune. PEG 6000, a polymer used as a binder and to enhance drug solubility, was also provided by Gattefosse, Mumbai. Aerosil, a fine silica used as a flow agent and anti-caking agent, was supplied by Iatros Pharma, Pune. Indion 234, the ion exchange resin essential for taste masking, was obtained from Ion Exchange India Ltd. Finally, crosscarmellose sodium, a superdisintegrant that facilitates tablet disintegration, was purchased from Dipa Pharma, Ch. Sambhajinagar.
Each of these excipients plays a crucial role in the formulation of the drug, contributing to the overall effectiveness, stability, and patient acceptability of the final product.
Methods:
Preparation of Drug-Resin Complex (DRC) for Bitter Taste Masking:
The preparation of the drug-resin complex (DRC) for effective bitter taste masking was conducted using a batch method. Initially, 200 mg of Indion 234, an ion exchange resin, was placed in a beaker containing 100 ml of deionized water, with a conductivity of less than 2 µS/cm. The resin was allowed to swell in the water for 120 minutes to ensure adequate hydration and expansion.
Following the swelling period, 100 mg of Eletriptan hydrobromide, the drug intended for taste masking, was introduced to the beaker. The mixture was then stirred continuously for 180 minutes to facilitate the interaction between the drug and the resin. This stirring period allowed for effective electrostatic interactions and binding of the drug to the resin, forming the drug-resin complex. After the complexation process, the mixture was filtered through Whatman filter paper No. 41 to remove any undissolved particles and excess resin. The resulting drug-resin complex was then analyzed using a UV-visible double-beam spectrophotometer (Jasco V-530). This analytical step was crucial for evaluating the drug loading efficiency and verifying the formation of the complex, ensuring that the bitter taste of Eletriptan hydrobromide was adequately masked.[8]
Preparation of Orodispersible Tablets: Powder Blend Preparation:
To prepare the powder blend for orodispersible tablets, precise quantities of all ingredients were first weighed accurately. The process began with combining the drug, Eletriptan hydrobromide, with half of the crosscarmellose sodium, saccharin, and a wax mixture consisting of Gelucire 39/1 and PEG 6000 in a 2:1 ratio. This mixture was gently heated to below 50°C to melt the wax components and ensure uniform mixing.
Once the wax mixture was adequately heated, the granules were prepared using a dry granulation method. The hot mixture was passed through sieve No. 18 to obtain granules of the desired size. After granulation, the mixture was cooled, and the granules were combined with lemon flavor, the remaining half of the crosscarmellose sodium, and Aerosil. This combination was mixed thoroughly using simple spatulation to ensure even distribution of all components. [9-10]
The final powder blend was then subjected to compression to form orodispersible tablets. The tablets were prepared using a direct compression technique, which involves compressing the blend directly into tablet form without the need for additional granulation. This method ensures the tablets are easy to disperse in the mouth, providing the desired taste masking and quick onset of action. The prepared tablets were then evaluated to ensure they met the required specifications for quality and performance.
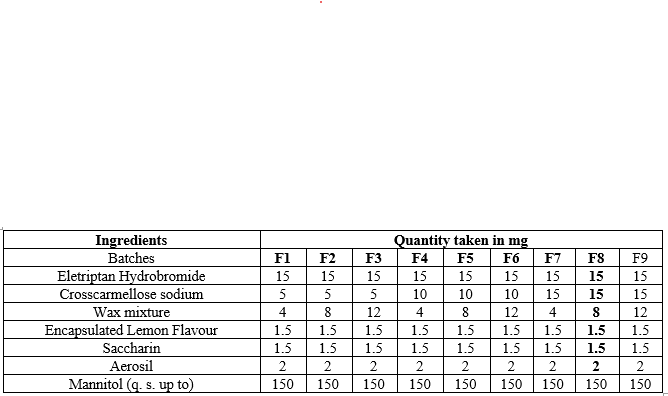
Table 1: Formulae for preparation of orodispersible tablets
Compression of Granules:
The prepared homogeneous powder blend was subjected to compression to form tablets, each weighing 150 mg. This process utilized an Rimek Mini Press II compression machine, equipped with 8 mm diameter deep concave punches specifically designed for this purpose.
During compression, the machine applied pressure to the granules, transforming the powder blend into solid tablet forms. The compression force was meticulously adjusted to achieve tablets with a hardness of approximately 2-3 kg/cm?2;, as measured using a Monsanto tablet hardness tester. This hardness level ensures that the tablets are sufficiently robust to withstand handling and packaging, while still maintaining the desired disintegration and dissolution properties necessary for effective orodispersible tablet performance. The precise control of compression parameters is crucial to ensure uniformity in tablet quality and consistency in the final product.[11-15]
FTIR Studies:
Fourier-transform infrared (FTIR) spectroscopy was employed to confirm the complexation between Eletriptan Hydrobromide and Indion 234. The samples, including Eletriptan Hydrobromide, Indion 234, their physical mixture, and the drug-resin complex (DRC), were prepared using the KBr disc method. The FTIR spectra were recorded over a range of 400 cm??1; to 4000 cm??1;. This analysis aimed to detect interactions between the drug and resin by identifying shifts or changes in the functional group peaks, which indicate complexation and the involvement of specific functional groups in the formation of the DRC.
Powder X-ray Diffraction (XRD) Studies:
X-ray diffraction (XRD) analysis was performed on Eletriptan Hydrobromide, Indion 234, and the resulting DRC to investigate their crystallographic properties. By comparing the diffractograms, the study aimed to identify any changes in the crystalline structure of the drug and resin upon complexation. This approach helps confirm whether the drug and resin interact and form a complex by examining shifts or alterations in peak positions and intensities in the XRD patterns.
Thermal Analysis:
Differential scanning calorimetry (DSC) was conducted using a Mettler Toledo 823e instrument, which was calibrated with indium and zinc standards to ensure accurate temperature and enthalpy measurements. The samples were hermetically sealed in aluminum pans and heated from 30°C to 300°C at a rate of 10°C/min. An inert atmosphere was maintained by purging nitrogen gas at a flow rate of 40 ml/min. DSC analysis provided information on the thermal behavior of the DRC, including changes in melting points or thermal events that could indicate interactions between the drug and resin, thereby confirming the formation of the complex.
Evaluation of Powder Blend:
Determination of content uniformity: Sample of powder blend was withdrawn after thorough manual mixing and then sample was analysed for drug content uniformity by procedure as mentioned earlier for assay.
Angle of repose: The angle of repose for the powder blend was determined by fixed funnel method. Angle of repose was calculated using equation:

Where,
h = height of powder heap in cm
r = radius of powder heap in cm
Tapped bulk density (TBD) and loose bulk density (LBD): Tapped density was determined by following method. A sufficient quantity of granules from each formula was taken and then was introduced in to a 10 ml measuring cylinder. It was allowed to fall under its own weight onto a hard surface from a height of 2.5 cm at 2 seconds intervals. The tapping was continued until no further change in volume was noted. LBD and TBD were calculated using following formulae;


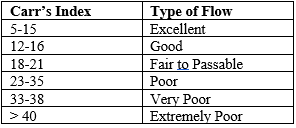
Carr’s Compressibility Index (CI): Carr’s compressibility index was calculated to assess the powder blend's flowability and compressibility. The index is derived from the difference between the tapped bulk density and loose bulk density, divided by the tapped bulk density, and expressed as a percentage:

A higher compressibility index suggests poorer flowability and greater compressibility of the powder blend.
Hausner ratio: Hausner ratio was determined for characterization of flow of powder blend. A Hausner ratio greater than 1.25 is considered to be an indication of poor flowability. Formula used was as follows:

Table 2: Relationship between % compressibility and flowability
Evaluation of Tablets:
The evaluation of tablets involves a comprehensive assessment of both pre-compression properties and post-compression characteristics to ensure quality and performance.[16-19]
Pre-compression Properties: Before tablet compression, key properties such as angle of repose, bulk density, and tapped density are measured. These properties help determine the flowability and packing characteristics of the powder blend. The angle of repose is assessed using the fixed funnel method to gauge the flowability of the powder, while bulk density and tapped density provide insights into the volume and density changes during packing.
Tablet Quality Tests:
After compression, various tests are conducted to ensure tablet quality:
Disintegration Time: Six tablets are selected randomly and subjected to a disintegration test. Each tablet is placed in the disintegration test apparatus, and a disk is added to each tube. The apparatus is then operated in a beaker containing a specified liquid for a predetermined time. The tablets are observed for their ability to disintegrate into smaller particles or dissolve completely.
Dimensions: The diameter and thickness of three tablets from each batch are measured using a digital vernier caliper. This ensures uniformity in tablet size, which is crucial for consistent dosage and appearance.
Weight Variation: Ten tablets are weighed individually, and the average weight is calculated. The individual weights are then compared with the average weight to ensure uniformity in tablet mass, which is important for accurate dosing.
Hardness: The hardness of three tablets selected at random from each batch is measured using the Monsanto Hardness Tester. This test determines the tablet’s ability to withstand mechanical stress during handling and transport. Hardness is expressed in kg/cm?2;, and the thickness is measured using a vernier caliper.
Friability: It is expressed in kg/cm. Friability of tablets were checked by Roche Friabilator, which was then operated for 4 minutes at 25 rpm. Tablets were dedusted and reweighed and % friability was calculated.

Where,
Wo = initial weight of tablets
W = weight of tablets after friability testing.
These evaluations are crucial for confirming the quality and consistency of the tablets, ensuring they meet the required specifications and perform effectively when administered.
Dissolution Testing:
Dissolution testing was conducted using a USP Type II dissolution apparatus, maintained at 37 ± 2°C with a rotation speed of 50 rpm. Nine hundred millilitres of 0.1 N hydrochloric acid (HCl) served as the dissolution medium. To monitor the dissolution process, 10 mL aliquots were withdrawn at 10-minute intervals and analyzed up to 1 hour. The withdrawn samples were assessed using a Jasco UV/VIS spectrophotometer at 221 nm, with 0.1 N HCl used as the reference medium.
Optimization Data Analysis:
The results from the experimental design were analyzed using Design Expert software (version 9). This software was utilized to create statistical models that predict the response variables based on selected process variables within the design space. The models were evaluated for statistical fit using Analysis of Variance (ANOVA) to ensure their accuracy. The optimized formulation was chosen based on the desirability function values. The polynomial regression results were visualized using 3D graphs and contour plots to illustrate the relationship between the variables and their effects on the formulation.
Preparation of Optimized Formulation:
The optimized formulation was prepared according to the recommendations provided by the software. Each batch was formulated based on the experimental design, and the formulation was evaluated for response parameters to ensure that the observed values closely matched the predicted values. This validation process was crucial to confirm the effectiveness of the optimization and the accuracy of the formulation.
RESULTS AND DISCUSSION
Confirmation of complexation:
Formation of an ion exchange complex between Eletriptan Hydrobromide and Indion 234 was confirmed by FTIR, X-ray diffraction and DSC studies. Objective was to study the interaction between drug and resin.
FTIR studies:
The infrared (IR) spectrum data for Eletriptan Hydrobromide, highlighting the characteristic bond frequencies detected in the compound. The spectrum reveals a C=C (aromatic) bond at 3064 cm??1;, indicative of aromatic ring stretching. The R-H2 (aromatic) bending vibration appears at 1468 cm??1;, and the N-H bending is observed at 1453 cm??1;, reflecting the presence of amine groups in the structure. The C-N stretching vibration is detected at 1444 cm??1;, and the C-H? stretching is noted at 2950 cm??1;. The H-Br stretching vibration, characteristic of the hydrobromide salt form, is identified at 2570 cm??1;. These frequencies collectively provide a fingerprint for Eletriptan Hydrobromide, useful for confirming its identity and purity.
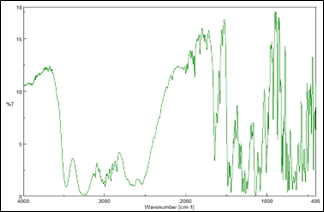
Figure 1: FTIR spectrum of Eletriptan Hydrobromide
Differential Scanning Calorimetry:
The DSC thermogram of Venlafaxine Hydrochloride was recorded using Differential scanning calorimeter (DSC 823 Mettler Toledo, Japan). The DSC thermogram (figure 2) showed the glass transition at 35.97?C, whereas it also showed a melting endotherm at 217-220?C. The appearance of glass transition clearly indicated the crystalline state of the drug, which was also confirmed by XRD which showed a characteristic crystalline state. Enthalpy of the Eletriptan Hydrobromide is 1167 cal.
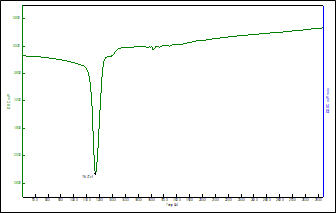
Figure 2: DSC Thermogram of Eletriptan Hydrobromide
X-ray Diffraction (XRD):
The XRD spectrum (figure 3) shows presence of any sharp distinct peak, which indicates crystalline form of drug. Crystallization affects optical, mechanical, thermal and chemical properties of the drug. X-ray diffraction is one of the analytical methods to determine the crystalline nature of Drug. Regular arrangement of atoms and molecules produce sharp diffraction peaks whereas amorphous regions result in broad peaks which are absent. The XRD study identified the crystalline nature of the venlafaxine hydrochloride distinctive peaks were evident at 2? angles of 10, 13, 15, 20, 22, 27, 35°C.
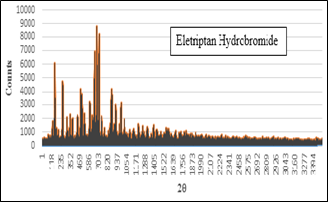
Figure 3: XRD spectrum of Eletriptan Hydrobromide
Compatibility studies:
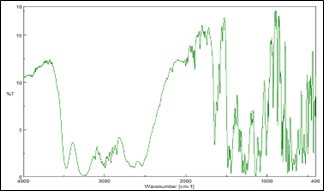
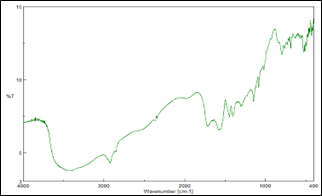
Compatibility study for Eletriptan Hydrobromide was carried out with potential formulation excipients to determine possibility of any drug-excipient interaction/incompatibility. UV Spectrophotometric and FTIR analysis showed no evidence of interaction between drug and studied excipients. A minor shifting of peak or decrease in peak intensity could be attributed to dilution effect in the mixture.
Eletriptan Hydrobromide:
Resinate:
Eletriptan Hydrobromide Tablet blend:
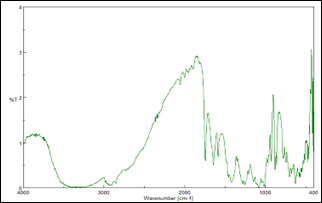
Figure 4: IR spectrum of Eletriptan Hydrobromide and excipients
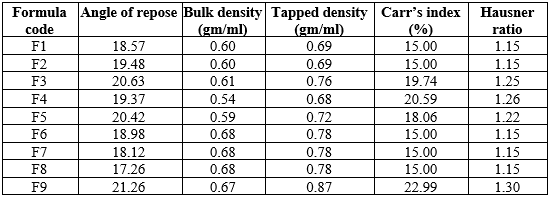
Evaluation of powder blend:
The evaluation of various powder blends, as presented in Table 3, assesses the flow properties and compressibility essential for consistent and reliable tablet production. The parameters measured include the Angle of Repose, Bulk Density, Tapped Density, Carr's Index, and Hausner Ratio. The Angle of Repose, which ranges from 18.98° to 21.26°, indicates generally good flowability for all formulations, with values below 25° suggesting excellent flow properties. Bulk Density values, spanning from 0.54 g/mL to 0.68 g/mL, represent the mass of the powder per unit volume without tapping, while Tapped Density values, ranging from 0.68 g/mL to 0.87 g/mL, reflect the powder's packing ability after tapping. Carr's Index, calculated from the difference between Tapped and Bulk Densities, ranges from 15.00% to 22.99%, with values between 5-15% indicating excellent compressibility and 12-16% signifying good compressibility. Hausner Ratio, which compares Tapped Density to Bulk Density, varies from 1.15 to 1.30, where a ratio of 1.00-1.11 denotes excellent flowability, and 1.12-1.18 indicates good flowability. Formulations F1, F2, F6, F7, and F8 exhibit good to excellent flow properties with Carr's Index and Hausner Ratio values in acceptable ranges, making them well-suited for tablet formulation. In contrast, formulations F3, F4, F5, and F9 demonstrate fair to passable flow properties, suggesting the need for further optimization to enhance their compressibility and flowability for tablet production. Overall, the data provides critical insights into the suitability of the powder blends for pharmaceutical applications, ensuring quality and consistency in the manufacturing process.
Table 3: Evaluation of powder blend
Evaluation of tablets:
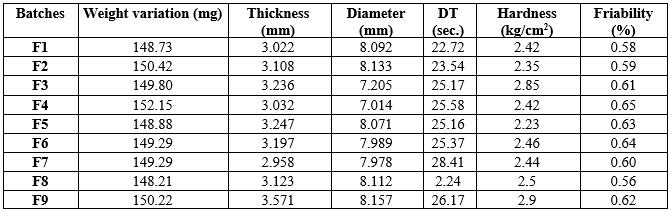
Table 4 evaluates tablet batches on key parameters: weight variation, thickness, diameter, disintegration time (DT), hardness, and friability. These factors are crucial for ensuring tablet quality and consistency in pharmaceutical production.
Batch F8 excels with optimal parameters: weight variation (148.21 mg) close to the target, acceptable thickness (3.123 mm) and diameter (8.112 mm), exceptionally fast DT (2.24 seconds), balanced hardness (2.5 kg/cm?2;), and the lowest friability (0.56%). These qualities make F8 the preferred choice for further development and production.
Table 4: Evaluation of tablets
In vitro dissolution study:
The tablets were evaluated for in-vitro dissolution profile. This graph illustrates (figure 5) the in-vitro dissolution profiles of various tablet formulations (F1 to F9) over 60 hours, with the y-axis showing the percentage of cumulative drug release and the x-axis indicating time. Notably, F8 exhibits the highest drug dissolution rate, reaching nearly 90% by the end of the period, while other formulations show slightly lower release percentages. This suggests that F8 has the most efficient drug release profile, likely due to factors such as tablet composition, excipients, preparation method, or physical properties. Understanding these profiles is vital for determining drug bioavailability and ensuring therapeutic effectiveness.
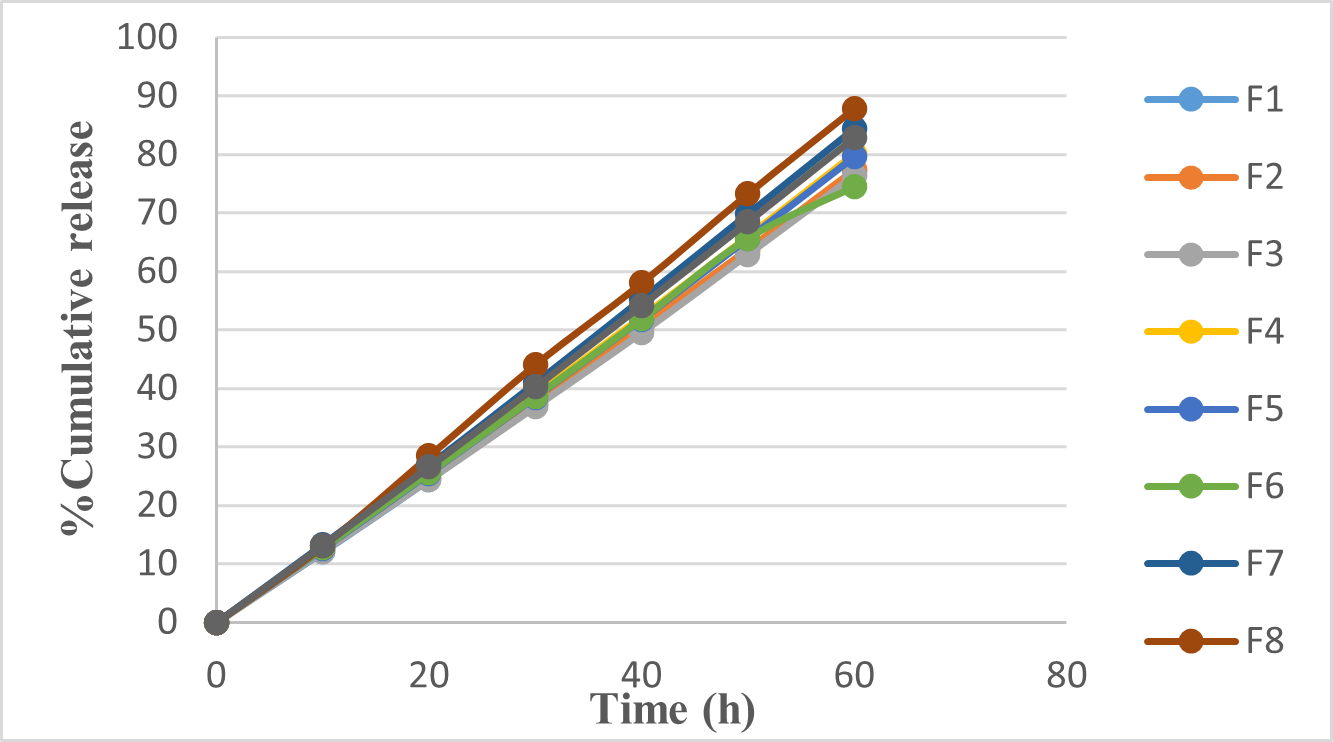
Figure 5: Dissolution of orodispersible tablet of Eletriptan Hydrobromide Optimization
Optimization of formulation:
The optimized formulation was selected using the desirability function approach with Design Expert software. The selection criteria focused on minimizing hardness, friability, and disintegration time within specified ranges. This systematic approach ensured that the final formulation not only met the desired quality standards but also exhibited improved performance characteristics, making it suitable for further development and production.
Table 5: Evaluation of orodispersible table

CONCLUSION
The study successfully demonstrated that the bitter taste of Eletriptan Hydrobromide can be effectively masked by forming an ion exchange complex with Indion 234. Indion 234, a resin specifically designed for taste masking, binds to the drug and neutralizes its bitterness. This method significantly improves the palatability of the medication, making it more acceptable for patients. The confirmation of the ion exchange complex was achieved through various analytical techniques. FTIR analysis identified the characteristic bond frequencies indicative of Eletriptan Hydrobromide, while DSC and XRD studies confirmed its crystalline nature. Compatibility studies showed no significant interaction between Eletriptan Hydrobromide and potential excipients, ensuring formulation stability. The evaluation of powder blends revealed that formulations F1, F2, F6, F7, and F8 exhibited good to excellent flow properties, making them suitable for tablet production. Conversely, formulations F3, F4, F5, and F9 require optimization to enhance compressibility and flowability.
Tablet batch F8 was identified as the most promising, demonstrating optimal parameters including minimal weight variation, appropriate thickness and diameter, rapid disintegration time, balanced hardness, and low friability. The in-vitro dissolution study further confirmed F8’s superior drug release profile, achieving nearly 90% dissolution over 60 hours. Additionally, the use of a three-by-two factorial design combined with statistical modeling proved to be a robust method for optimizing the formulations. This experimental design allowed for the systematic evaluation and adjustment of formulation parameters to achieve the desired taste masking and formulation characteristics. By utilizing the desirability function approach with Design Expert software, the study was able to select the optimal formulation based on minimizing hardness, friability, and disintegration time. Overall, the study confirms that ion exchange complexation with Indion 234 is a viable strategy for enhancing the sensory attributes of bitter drugs like Eletriptan Hydrobromide. Moreover, the application of factorial design and statistical analysis is effective in optimizing such formulations, ensuring high quality and consistency in the manufacturing process. Formulation F8, in particular, was selected for further development due to its optimal characteristics and efficient drug release profile, making it suitable for pharmaceutical applications.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally.
CONFLICTS OF INTERESTS
All authors have declared no conflict of interest.
REFERENCE
- Penta Jyothi. Mouth Dissolving Tablets- Review. International Journal of Advances in Pharmacy, Biology and Chemistry; 2012; 1(4): 412-421.
- Rewar S, Singh C J, Bansal B K, Pareek R, Sharma A K. Oral Dispersible Tablets: An Overview; Development, Technologies and Evaluation. International Journal of Research and Development in Pharmacy and Life Sciences; 2014; 3(6): 1223-1235.
- Pagar R, Ahirrao S, Yallatikar T, Wagh M. Review on Orodispersible Tablets. International Journal for Pharmaceutical Research Scholars (IJPRS), 2015; 4(1): 302-312.
- Puttewar TY, Kshirsagar MD, Chandewar AV, Chikhale RV. Formulation and evaluation of orodispersible tablet of taste masked doxylamine succinate using ion exchange resin. Journal of King Saud University (Science), 2010; 22: 229-240.
- Warad S, Warad P, Solunke, Suthar A. Taste Masking Of Highly Bitter Drug Zopiclone Using Ion Exchange Resin. Universal Journal of Pharmacy, 2013; 2(2): 77-83.
- Medicines complete. Eletriptan hydrobromide. https://www.medicinescomplete.com/mc/ martindale /current/ login.htm (Accessed 31 December 2015).
- Drugs.com. know more. Be sure. Eletriptan Hydrobromide. https://www.drugs.com/ monograph/ eletriptan-hydrobromide.htm (Accessed 4 August 2016).
- 1 mg. Eletriptan Hydrobromide. https://www.1mg.com/search/name=eletriptan + hydrobromide (Accessed 4 August 2016).
- Government of India. Ministry of health and family welfare. Indian pharmacopoeia commission Ghaziabad, 2014; I and II: 1651-1652.
- Deshpande K.B. Orodispersible Tablets: An Overview of Formulation and Technology, International Journal of Pharma and Bio Sciences, 2011; 2(1): 726-734.
- Shaikh S, Khirsagar RV, Quazi A, Fast Disintegrating Tablets: An Overview of Formulation and Technology, International Journal of Pharmacy and Pharmaceutical Sciences, 2010; 2(3): 9-15.
- Gupta AK, Mittal A and Jha KK, Fast Dissolving Tablet- A Review, the Pharma Innovation, 2012; 1-8.
- Siddiqui MN, Garg G, Sharma PK. Fast Dissolving Tablets: Preparation, Characterization and Evaluation: An Overview, International Journal of Pharmaceutical Sciences Review and Research, 2010; 4(2): 87-96.
- Ashish P, Harsoliya MS, Pathan JK, Shruti S. A Review- Formulation of Mouth Dissolving tablet. International Journal of Pharmaceutical and Clinical Science, 2011; 1(1): 1-8.
- Kumar, Kaur P, Kaur P, Piplani M. In-Vitro and In-Vivo Characterization of Mouth Dissolving Tablet: An Updated Review. Journal of Drug Delivery & Therapeutics, 2013; 3(3): 153-157.
- Deshmukh VN. Mouth Dissolving Drug Delivery System: A Review. International Journal of Pharm Tech Research; 2012; 4(1): 412-421.
- Chawla G and Jain N. Mouth Dissolving Tablets: An Overview. International Journal of Pharmaceutical Sciences and Researh, 2012; 3(9): 2919-2925.
- Madgulkar AR, Bhalekar MR and Padalkar RR. Formulation Design and Optimization of Novel Taste Masked Mouth-Dissolving Tablets of Tramadol Having Adequate Mechanical Strength, AAPS Pharm Sci Tech. 2009; 10(2): 574–581.
- Manasa KL, Ramana G and Digpati R. Formulation and Evaluation of Oral disintegrated tablets of Alfuzosin Hydrochloride using superdisintegrants. Journal of Applied Pharmaceutical Science, 2011; 01(09): 161-165.


 Nikita M. Wakchaware*
Nikita M. Wakchaware*












 10.5281/zenodo.12825405
10.5281/zenodo.12825405