Abstract
Momordica charantia, commonly known as bitter gourd, is a widely recognized medicinal plant used traditionally to treat various ailments, including inflammatory conditions. The leaves of M. charantia are particularly rich in bioactive compounds such as saponins, flavonoids, and alkaloids, which exhibit potent anti-inflammatory properties. This review aims to provide a comprehensive overview of the formulation and evaluation of an anti-inflammatory gel derived from M. charantia leaves, examining its potential therapeutic applications.The review begins with an exploration of the phytochemical profile of M. charantia leaves, emphasizing the significance of the identified compounds in mitigating inflammation. Saponins and flavonoids are highlighted for their ability to modulate inflammatory pathways, while alkaloids contribute to analgesic effects. Understanding these compounds is crucial for formulating an effective gel. In discussing the formulation process, the review details the selection of appropriate gel bases, including carbomers, xanthan gum, and sodium alginate, focusing on their rheological properties and compatibility with skin. Extraction methods, such as cold extraction and Soxhlet extraction, are analyzed for their efficiency in preserving the bioactivity of the leaf extracts. The incorporation of extracts into gel bases is outlined, ensuring uniform distribution for optimal therapeutic outcomes.The evaluation of the anti-inflammatory gel encompasses several key aspects. Physicochemical properties, such as pH, viscosity, and stability, are assessed to ensure formulation integrity. In vitro studies, including albumin denaturation and membrane stabilization assays, are employed to evaluate anti-inflammatory efficacy, while cytotoxicity assays determine the safety profile of the gel. Furthermore, in vivo studies utilizing animal models provide insights into the gel’s effectiveness in reducing inflammation and assessing potential side effects. Clinical trials are essential for confirming the gel’s efficacy and safety in human subjects, paving the way for its integration into modern therapeutic practices. This review underscores the potential of M. charantia leaves as a source of natural anti-inflammatory agents and highlights the importance of rigorous scientific evaluation in developing effective topical treatments. In conclusion, the formulation of an anti-inflammatory gel from M. charantia leaves represents a promising area of research, bridging traditional knowledge with contemporary pharmaceutical practices. This review serves as a foundation for future studies aimed at optimizing the gel formulation and exploring the therapeutic applications of M. charantia in treating inflammatory conditions, ultimately contributing to the development of safe, effective, and natural healthcare solutions.
Keywords
Antiinflammatory, gel, Phytochemicals, Flavonoids, Saponins, Gel formulation, Chronic inflammation, Extraction methods, Momordicacharantia.
Introduction
Inflammation is a complex biological response to harmful stimuli, including pathogens, damaged cells, and irritants. While it plays a crucial role in the body’s healing process, chronic inflammation is implicated in various diseases, such as arthritis, diabetes, cardiovascular diseases, and cancer. The management of inflammation has become a significant focus in modern medicine, leading to an increased interest in natural anti-inflammatory agents derived from plants. Momordica charantia, commonly known as bitter gourd or bitter melon, has been utilized in traditional medicine for centuries. Predominantly found in Asia, Africa, and the Caribbean, this plant is celebrated not only for its culinary uses but also for its wide array of medicinal properties. In particular, the leaves of M. charantia have gained attention for their pharmacological potential, which includes anti-inflammatory, antioxidant, and antimicrobial effects. The therapeutic applications of M. charantia are deeply rooted in various traditional practices, where it is used to treat conditions such as fever, infections, and gastrointestinal disorders.

Fig: Momordica charantia leaves
Recent scientific investigations have begun to validate these traditional uses, identifying a range of bioactive compounds within the leaves that contribute to their therapeutic properties. Among these, flavonoids, saponins, and alkaloids stand out for their demonstrated ability to modulate inflammatory pathways and reduce oxidative stress. For example, flavonoids are known for their capacity to Inhibit pro-inflammatory cytokines, while saponins may enhance immune response and exhibit analgesic properties. These findings support the potential of M. charantia leaves as a valuable source for developing effective anti-inflammatory agents. In the quest for novel therapeutic formulations, topical applications, such as gels, offer a promising route for delivering anti-inflammatory agents directly to the site of inflammation. Gels provide several advantages, including ease of application, localized treatment, and enhanced patient compliance. The formulation of an anti-inflammatory gel from M. charantia leaves not only harnesses the plant’s medicinal properties but also offers a modern pharmaceutical approach to treating inflammatory conditions. This review aims to synthesize current knowledge regarding the formulation and evaluation of an anti-inflammatory gel from M. charantia leaves. It will explore the extraction methods of bioactive compounds, the selection of suitable gel bases, and the evaluation techniques used to assess the gel’s physicochemical properties, efficacy, and safety. By integrating traditional knowledge with contemporary scientific methodologies, this review seeks to pave the way for further research into the therapeutic applications of M.charantia, ultimately contributing to the development of effective, natural remedies for inflammatory diseases. Inflammation is a vital biological response that serves as a protective mechanism against infection, injury, and harmful stimuli. It involves a complex interplay of immune cells, signaling molecules, and vascular changes aimed at restoring tissue homeostasis. While acute inflammation is beneficial and essential for healing, chronic inflammation can lead to detrimental effects and is associated with a wide range of health issues, including autoimmune disorders, metabolic syndrome, and neurodegenerative diseases. The management of inflammation, therefore, is a critical aspect of modern healthcare, prompting the search for effective and safe anti-inflammatory agents.
In recent years, there has been a resurgence of interest in natural products, particularly those derived from medicinal plants, due to their potential therapeutic benefits and lower side-effect profiles compared to synthetic drugs. One such plant is Momordica charantia, commonly referred to as bitter gourd or bitter melon. This plant is native to tropical and subtropical regions and is widely cultivated for its edible fruits and leaves. Beyond its culinary uses, M. charantia has a long history in traditional medicine systems, particularly in Ayurveda and Traditional Chinese Medicine, where it is used to treat a variety of ailments, including diabetes, infections, and inflammatory conditions. The pharmacological properties of M. charantia are attributed to Its rich phytochemical composition. The leaves contain numerous bioactive compounds, including flavonoids, saponins, terpenes, alkaloids, and phenolic acids, which contribute to their antioxidant, anti-inflammatory, and antimicrobial activities. Research has demonstrated that these compounds can modulate key inflammatory pathways, such as the nuclear factor kappa B (NF-?B) signaling pathway, which plays a central role in the inflammatory response. For instance, studies have shown that extracts from M. charantia leaves can inhibit the production of pro-inflammatory cytokines, thereby reducing inflammation at the cellular level. The formulation of an anti-inflammatory gel from M. charantia leaves presents an innovative approach to harnessing these therapeutic properties. Gels are versatile pharmaceutical forms that offer several advantages for topical application, including enhanced penetration of active ingredients, ease of application, and the potential for sustained release of bioactive compounds. The development of a gel formulation allows for direct application to inflamed tissues, providing localized relief while minimizing systemic side effects. In developing this anti-inflammatory gel, several critical factors must be considered, including the selection of appropriate gel bases, the extraction methods for obtaining bioactive compounds, and the evaluation of the gel’s physicochemical properties and therapeutic efficacy. The choice of gel base influences the viscosity, stability, and skin compatibility of the formulation. Various agents, such as carbomers, xanthan gum, and hydroxypropyl methylcellulose (HPMC), can be employed to create a gel with desirable characteristics. Moreover, extraction methods play a pivotal role in determining the potency the gel. Techniques such as cold extraction, maceration, and Soxhlet extraction can be employed to maximize the yield of bioactive compounds while preserving their integrity. Following formulation, rigorous evaluation through in vitro and in vivo studies is essential to assess the anti-inflammatory efficacy and safety profile of the gel.This review seeks to comprehensively analyze the formulation and evaluation processes of an anti-inflammatory gel derived from M. charantia leaves. By synthesizing existing research and outlining the methodologies involved, this review aims to contribute to the understanding of M. charantia as a source of natural anti-inflammatory agents and highlight its potential for future therapeutic applications. Ultimately, the integration of traditional knowledge with modern pharmaceutical practices can lead to the development of effective, safe, and accessible treatments for inflammatory conditions, addressing a significant need in contemporary healthcare.
OBJECTIVE:
- To extract bioactive compounds from Momordica charantia leaves using suitable solvents to maximize anti-inflammatory properties.
- To develop a stable gel formulation that incorporates the leaf extract, optimizing for viscosity, texture, and spreadability to ensure ease of application.
- To characterize the physicochemical properties of the gel, including pH, viscosity, consistency, and microbial stability
- To assess the in vitro anti-inflammatory activity of the gel through standardized assays, such as measuring inhibition of inflammatory mediators and cytokines.
- To conduct stability studies to evaluate the gel’s shelf life and performance under different storage conditions.
- To evaluate the safety profile of the gel through irritation tests and potential side effects in suitable animal models.
- To compare the efficacy of the formulated gel against established anti-inflammatory treatments, providing evidence for its potential as an alternative therapeutic option.
Active Compounds :
The leaves of Momordica charantia contain several bioactive compounds, including:
- Saponins
- Flavonoids
- Alkaloids
- Phenolic acids
These compounds contribute to the anti-inflammatory effects observed in various studies.
Method and Process
- Preparation of Plant Extract:
- Collection: Fresh Momordica charantia leaves are collected, washed thoroughly, and air-dried.
- Extraction: Grind the dried leaves into a fine powder. Use a suitable solvent (e.g., ethanol or water) for extraction. Perform maceration or Soxhlet extraction for 24-48 hours.Filter the extract using Whatman filter paper and concentrate it using a rotary evaporator.
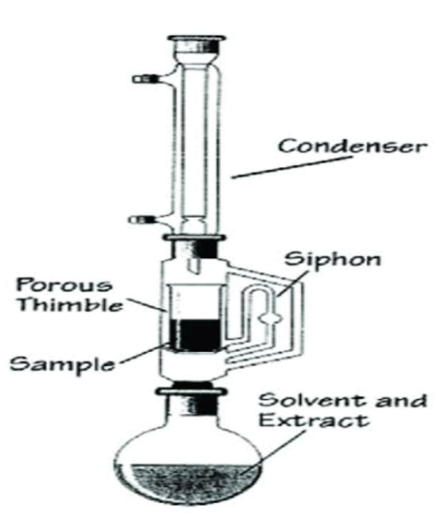
Fig : Extraction of plant
- Formulation of Gel
- Ingredients:Extract of Momordica charantia (calculated based on desired concentration). Gel base (e.g., carbomer, hydroxypropyl methylcellulose).Preservatives (e.g., phenoxyethanol). Distilled water.
- Procedure:Disperse the gel base in distilled water and mix until a uniform gel is formed.Slowly add the concentrated plant extract to the gel base while stirring continuously to ensure even distribution.Adjust the pH of the gel (ideally between 6-7) using triethanolamine or citric acid.Homogenize the mixture to achieve a smooth consistency.
- Characterization of Gel:
- Physicochemical Properties:
- pH Measurement: Using a pH meter.
- Viscosity: Measured using a viscometer.
- Spreadability: Assess by applying a specific weight to the gel and measuring the diameter of spread.
- Stability Testing: Conduct accelerated stability studies under varying temperature and light conditions.
- In Vitro Anti-Inflammatory Activity :
- Assays:COX Inhibition Assay: Measure the inhibitory effect on cyclooxygenase enzymes.
- Cytokine Release: Use cell lines to assess the release of pro-inflammatory cytokines (e.g., TNF-?, IL-6) after treatment with the gel.
- Safety and Irritation Tests :
- Irritation Test: Apply the gel to a small area of healthy skin in an animal model or human volunteers to observe for any adverse reactions.Acute Toxicity Studies: Assess systemic toxicity if required.
- Comparative Efficacy :
- Standard Control: Use a known anti-inflammatory drug (e.g., diclofenac) for comparative analysis in the in vitro assays.
- Data Analysis :
- Analyze the data statistically to determine the significance of anti-inflammatory effects compared to controls. Use software like SPSS or GraphPad Prism. This comprehensive approach ensures the formulation is effective, safe, and ready for potential therapeutic applications.
Evaluation of the Gel:
- Physicochemical Properties:
- pH: Should be neutral to avoid skin irritation.
- Viscosity: Important for application ease.
- Stability: Subject to temperature and time assessments.
- In Vitro Studies:
- Anti-inflammatory Activity:Assessed using assays like the albumin denaturation and membrane stabilization tests.
- Cytotoxicity Tests: Ensure the gel is safe for use.
- In Vivo Studies:
- Animal models can be used to assess the anti-inflammatory efficacy and observe any side effects.
- Clinical Trials:
- Further evaluation in human subjects is necessary to confirm efficacy and safety.
CONCLUSION:
The formulation of an anti-inflammatory gel from Momordica charantia leaves shows promise due to its rich phytochemical profile and demonstrated therapeutic potential. Future studies focusing on optimizing the formulation and conducting comprehensive clinical trials will be essential for validating its effectiveness and safety for public use.
REFERENCES
- Shekhawat MS, Shekhawat NS, Harish K, Phulwaria M, Gupta AK. High frequency plantlet regeneration from nodal
- Segment culture of female Momordica dioica (Roxb). J Crop Sci Biotechnol. 2011; 14(2): 133-137.
- Lohiya, N. K., & Singh, S. (2017). Anti-inflammatory and analgesic activity of Momordica charantia in experimental models. Indian Journal of Pharmacology, 49(5), 673-677.
- Das, D., & Gupta, M. (2020). Role of Momordica charantia in the management of inflammation and metabolic disorders. Phytotherapy Research, 34(3), 563-577.
- Chaudhary, S., & Kumar, A. (2017). Therapeutic potential of Momordica charantia: A review. International Journal of Herbal Medicine, 5(2), 57-63.
- Maji, S., & Mandal, A. (2018). Herbal gel formulation: A review. Journal of Pharmaceutical Sciences and Research, 10(6), 1470-1474.
- Jain A, Soni M, Deb L, et al. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. Leaves. J Ethnopharmacol. 2008; 115: 61-66.Doi: 10.1016/j.jep.2007.09.009.
- Schaefer H, Renner SS. Phylogenetic relationships in the order Cucurbitales and a new classification of the gourd Family (Cucurbitaceae). Taxon. 2011; 60(1): 122-138. Doi: 10.1002/tax.601011.
- Bharathi, K., & Vasantharaj, S. (2016). Phytochemical and pharmacological potential of Momordica charantia. International Journal of Pharmacy and Pharmaceutical Sciences, 8(4), 8-15.
- Bhadra, S., et al. (2019). Anti-inflammatory activity of Momordica charantia leaf extracts in a rat model. Journal of Ethnopharmacology, 234, 170-178
- Ghosh, D., & Gupta, S. (2016). Natural products as anti-inflammatory agents: A review of Momordica charantia. Asian Journal of Pharmaceutical and Clinical Research, 9(4), 10-15 11) Kumar, S., & Kumar, V. (2019). Momordica charantia: An overview
- of its bioactive compounds and health benefits. Journal of Food Science and Technology, 56(10), 4332-4341.
- Niranjan, R., & Prasad, S. (2020). Potential of Momordica charantia leaves in the management of inflammation. International Journal of Research in Pharmacy and Science, 10(2), 45-53.
- Sharma, S., & Sharma, R. (2019). Formulation and evaluation of herbal anti-inflammatory gel. Journal of Drug Delivery and Therapeutics, 9(5), 116-121.
- Singh, B., et al. (2019). Pharmacological activities of Momordica charantia: A review. Pharmacognosy Reviews, 13(25), 125-133.
- Srinivasan, K. (2019). Anti-inflammatory properties of Momordica charantia extracts: A comprehensive review. Food Science and Human Wellness, 8(1), 30-36.
- Subramaniam, S., et al. (2020). Formulation strategies for herbal gels: Current trends and future prospects. Pharmaceutical Biology, 58(1), 1-15.
- Tiwari, P., & Tiwari, A. (2020). Role of herbal drugs in the treatment of inflammation. Journal of Drug Research, 1(1), 22-29.
- Vennila, S., et al. (2020). Phytochemical screening and anti-inflammatory potential of Momordica charantia extracts. International Journal of Pharmacognosy and Phytochemical Research, 12(4), 145-151
- Vijayakumar, M., & Sharmila, G. (2018). Evaluation of Momordica charantia leaf extract for its anti-inflammatory activity. Asian Pacific Journal of Tropical Biomedicine, 8(5), 245-251.
- Wahab, A., et al. (2019). Formulation and characterization of herbal gel from Momordica charantia leaf extracts. Journal of Medicinal Plants Studies, 7(4), 01-06.


 Ankita Borade *
Ankita Borade *
 Rajashri Nimbalkar
Rajashri Nimbalkar
 Mayur Paithankar
Mayur Paithankar
 Anjali Dahake
Anjali Dahake
 Rohit Badre
Rohit Badre


 10.5281/zenodo.14268228
10.5281/zenodo.14268228