Abstract
Nano structured lipid carrier(NLC) are the 2nd generation lipid Nanoparticle formulated using Trigonella foenum-graecum l. loaded into NLC as Buccal patch as a dosage form to enhance the efficiency of the drug for the treatment of Hyperglycemia. Using Stearic acid and Soybean oil as lipid phase and Span 60 with water as aqueous phase mixed together completely using magnetic stirrer to form a emulsion. Formed NLC emulsion loaded into the buccal patch as a dosage form. Among the formulations N1 to N8, formulation N4 consider as the best with the drug release of 97%. Formulation N4 incorporated into 3 buccal patch N4B1, N4B2, N4B3 with different concentration of excipients. Formulation N4B2 produce maximum drug release of 89.43% and its kinetic release value R2 is 0.9967 follows first order kinetics is considered as best formulation with proper film formation. In future the dissertation work can be pursued into In-vivo studies. According to ICH guidelines the method was Formulated and Evaluated.
Keywords
Trigonella foenum-graecum l. Nano Structured lipid carrier, Buccal patch, Hyperglycemia, Release Kinetics.
Introduction
Novel drug delivery system
The novel system is the novel drug delivery system. Recent discoveries into the pharmacokinetic and Pharmacodynamic behavior of drugs have made the creation of the ideal drug delivery system easier. The carriers known as innovative drug delivery systems (NDDS) help keep medication doses within therapeutic ranges for extended periods of time. Novel medication delivery systems have a number of benefits over traditional drug delivery methods.
- For an extended length of time, the ideal therapeutic medication retention in the blood or tissue may be sustained.
- Extended periods of time at pre-determined release rates may be achieved.
- For drugs with a short half-life, the duration could be extended
- It may be possible to eliminate side effects by targeting the location of action.
- Frequently dosage and medication waste
With the goals of minimizing drug degradation or loss, preventing harmful side effects, improving drug bioavailability, and encouraging and facilitating the accumulation of the drug in the necessary bio- zone (site), different kinds of drug delivery systems have been developed, and some are currently under development. There aren't any number innovative carriers that have been proven effective in delivering drugs in a targeted and regulated manner. It is crucial to assess the terminologies used under the many main categories of innovative drug delivery systems effectively.
- Drug action is provided at a predetermined pace by sustained or controlled drug –- delivery systems, which offer a continuous (zero-order) release of the medication at levels in the blood that are therapeutically efficacious
- Drug action is achieved by localized drug delivery systems through either spatial or temporal regulation of drug release (typically at a rate that is rate-limiting) in the target area. [1]
Lipid based nanoparticles:
Lipid-based nanoparticles (LBNPs) such as liposomes, solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) have received great attention in drug discovery and cancer treatment. These nanoparticles can transport hydrophobic and hydrophilic molecules, display very low or no toxicity, and increase the time of drug action by means of a prolonged half-life and a controlled release of the drug [2] Nanostructured lipid carriers (NLCs) spring up as second generation of lipid nanoparticles to overcome the shortcomings of first generation i.e. SLNs. Biodegradable and compatible lipids (solid and liquid) and emulsifiers are used for the preparation of NLCs. Liquid lipids (oil) incorporation causes structural imperfections of solid lipids leading to a less ordered crystalline arrangement which avert drug leakage and furnish a high drug load.[3,4]
Advantages of NLC [5-8]
- Enhanced drug release characteristics and loading capacity, as well as consistent drug incorporation over storage
- Rate pre-programmed drug delivery systems work by controlling the release of drug molecules by system design, which regulates the molecules' molecular diffusion.
- Increasing drug loading capacity and drug release pattern modulation.
Components of NLC
Lipid is the main ingredient of nanostructure lipid carriers, which controls drug loading capacity, action prolongation, and formulation stability. To produce NLC, solid lipids such as fatty acids, waxes, steroids, diglycerides, and monoglycerides have been employed.[10] For the development of lipid nanoparticles, physiologically acceptable, biodegradable, non-toxic, and generally recognized as safe (GRAS) lipids are favored.
Selecting the right lipids is crucial before using them to create nanoparticulate carriers. Numerous properties of nanocarriers are influenced by the kind and structure of the lipid. It has been argued that the most practical criterion for selecting an appropriate lipid is the solubility or apparent partition coefficient of the bioactives in the lipid. Interpretation is provided by the drug molecules solubility in lipid, which influences drug loading and encapsulation effectiveness.[11] The degree of crystallization of the different lipids used also influences the size, charge, efficacy, and entrapment of the medication. [10]
Types of NLC [12]
Type I - Very imperfect matrix
Type II - Amorphous types
Type III - Multiple matrix
Type I (Very faulty matrix):
Compared to solid lipid, liquid lipid (oil) is employed in Type I NLC at a lower concentration. Blending oil and solid lipids to create an o/w nano-emulsion results in solid particles that crystallize when the mixture cools from a molten state to room temperature. This process creates an incomplete, highly disordered lipid matrix that provides space for drug molecules and an amorphous drug structure.
Type II (Amorphous type):
Particles of the amorphous type of Nano structured lipid carrier were formed by a well-regulated lipid mixture; these particles were solid rather than crystalline. It is necessary to maintain this indeterminate situation.
Type III (Many types):
A significant oil concentration is present in Type III NLC. The two lipids separate phases during the crystallization process. They have a miscibility gap that causes a small, oily nano-compartment to precipitate at a specific temperature. Higher amounts of liquid lipid added to the lipophilic phase demonstrate the benefits of the solid matrix in preventing drug leaks when lipids lack drug solubilities, whereas liquid lipid exhibits great solubility for lipophilic drug.
Solid Lipids [13-15]
A mixture of different chemical substances with melting points more than 40°C.
Solid lipids such as this are readily tolerated
- Suitable for usage by humans.
- Moreover biodegradable in vivo.
Examples include Cutina CP 8, dynasan, precifac, stearic acid, beeswax, and carnauba wax.
Liquid lipids
These liquid lipids are acceptable and well-tolerated for usage by humans.
Among them are castor oil, oleic acid, davana oil, olive oil, miglyol, cetiol V, and so forth.
Surfactants
The kind and amounts of surfactants have an impact on the effectiveness and quality of NLC. It has been discovered that the choice of surfactant significantly affects the toxicity, physical stability, and crystallinity of NLC.[16] Drug permeability and the degree of drug dissolution are also influenced by surfactant systems. Surfactants are selected according to their effect on particle size, lipid modification, hydrophilic-lipophilic balance (HLB) value, and mode of administration. Because of their amphipathic character, surface active agents, or emulsifiers, are adsorbed on the interface, where they lessen the tension between the lipid and aqueous phases.[17]
Colloid particle crystallization occurs concurrently with solidification during NLC formation; however, the significant increase in particle surface area that occurs during crystallization causes the system to become unstable. Therefore, surfactant is necessary to enhance the nanoparticles' interface quality in order to achieve stability.[18] The stability of NLCs can be controlled by altering the composition of the surfactant system, which in turn affects how miscible the chemical components are.[16]
Buccal Patches:
Buccal drug delivery is the medication delivery via the cheek mucosal membranes. bypassing the hepatic portal system and gastrointestinal tract to increase the bioavailability of oral medications that would otherwise be metabolized by the hepatic firstpass. The intermediate gastrointestinal tract's pH and digestive enzymes also prevent the medication from degrading.[19,20]
MATERIALS AND METHODS
Materials
Trigonella foenum-graecum L. Seeds (Fenugreek) was purchased from the retail store, Coimbatore. Stearic acid , Soybean oil, Span 60, HPMC, Glycerine , SLS were used which are of laboratory grade and available at college.
Collection of Trigonella foenum-graecum L. seed:
The seed of Trigonella foenum- graecum L. was from Coimbatore, Tamilnadu. The plant was Identified and Authenticated by Dr. S. S Hameed, Scientist ‘F’& Officer-in- Charge, The Tamilnadu Agricultural University, Coimbatore.
Extraction Process of seed of Trigonella foenum-graecum L.
Seed of Trigonella foenum- graecum L. (500g) were extracted by Maceration method. Initially powdered seed extracted with 800 ml of PET ether to remove fatty acid, followed by 800ml Ethanol. To get the residual extract, solvent heated at 30 °C for 8 hrs and evaporated. The residual extract was washed in water and used in the further studies.

Fig 1: Extraction of fenugreek seeds
Formulation of Trigonella foenum-graecum L. Loaded NLC based Buccal patch:
The drug loaded NLC system was prepared using an “micro emulsion method”. Initially Lipid phase was prepared by taking accurate amount of Trigonella foenum- graecum L. Was accurately weighed and added to mixture of stearic acid and Soybean oil and then melted at 30?C to form a uniform and clear oil phase. Then 10ml of aqueous phase was prepared by dispersing the surfactant (span 60) in distilled water and then heating it at the same temperature. The hot aqueous phase was added to the oil phase at 70°C under magnetic stirring at 500 rpm. A translucent emulsion was obtained by continuous agitation and stirring using the magnetic stirrer.
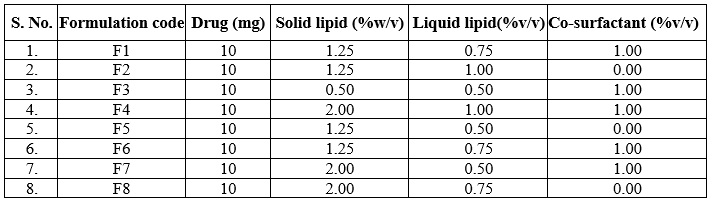
Table 1: Formulation of FSE- NLC N1 to N8
CHARACERIZATION of NLC:
Particle size distribution
The Malvern VR Zeta sizer was used to measure the particle size and polydispersity index (PDI) of the FSE-loaded NLC formulations. The dispersions were analyzed at 25ºC with a 90º angle of detection after being diluted 1:200 with double-distilled water.
Entrapment Efficiency and Drug loading capacity
By centrifuging the FSE-loaded NLC for two hours at 7000 rpm, the EE% and DL% were determined. After that, the sample supernatant was 67esicca, suitably diluted with 0.1NHcl, and examined at 270 nm with a UV spectrophotometer. The following formula was used to determine the EE% and DL%:
EE%=W(Total)–W(Free)*100
W(Total)
and
DL%=W(Total)–W(Free)*100
W(Lipid)
Where,
EE%=the percentage of encapsulation efficiency,
W(Total)=the amount of added drug during preparation of NLCs
W(Free)=the amount of free drug in the clear supernatant fluid after centrifugation. W(Lipid)= the amount of lipid added during preparation of NLCs.
Zeta potential
A dispersion system like NLC's charge stability was assessed using the zeta potential. It was employed to measure the bilayer's electrical charge magnitude. The vesicle surface charge was crucial in characterizing the behavior of NLC. Compared to an uncharged NLC, a charged NLC is more stable against aggregation and fusion. The dynamic light scattering approach can be used to determine each NLC's zeta potential. Zeta potentials between -10 and +10 mV are considered neutral, while values between -30 and +30 mV are thought to be strongly cationic and strongly anionic. In a centrifugation tube, 5 ml of NLC emulsion was added. The tube was centrifuged for 10 minutes at 14000 rpm at a temperature of approximately 4oC. The samples were then analyzed using a Malvern Zetasizer to determine their zeta potential.
Entrapment Efficiency
Centrifugation was used to remove an unentrapped medication from the produced NLC for 30 minutes at 4500–5000 rpm in a 15 ml centrifugation tube. After discarding the supernatant solution, 15 ml of phosphate buffer saline 7 were added to a centrifuge tube containing NLC dispersion. This process was then carried out three times. The resulting purified NLC was then put in a bath sonicator for ten minutes after being diluted 1:10v/v (NLC emulsion: Phosphate buffer saline pH 7). The UV spectrophotometer was used to measure the drug molecules that were entrapped and measured at 270 nm. Using the formula, the amount of drug entrapped and the drug loading capacity will be determined;
Entrapment efficiency (?) = Amount of drug entrapped /total amount of drug)×100
Invitro Drug release studies
In vitro drug release of all 5 formulations were studied by means of Dialysis diffusion method. Himedia dialysis membrane 50 with molecular weight ranges from 1200-14000 daltons were used indicated has the capacity to accommodate 1.61 ml/cm. The donor compartment i.e, dialysis bag was soaked in warm water for removal of glycerol for about 30 minutes and then the NLC emulsion was then transferred to purified dialysis bag and dipped in a 60 ml receptor compartment containing phosphate buffer saline 7 and the medium is subjected to magnetic stirring at150 rpm at 30oC for 24hours. At regular time interval 1ml of sample were collected and diluted to 10 ml with phosphate buffer saline pH 7 consequently, fresh medium of 1ml phosphate buffer were replaced to the medium. The absorbance of samples were analyzed at 270nm by using UV-spectrophotometer.
Kinetic release studies
Drug release kinetics of 5 different formulations were calculated by using a software Microsoft Office Excel Add-In. The in vitro drug release data was used in various kinetic equations to understand the mechanism of drug release by determining the correlation coefficient and “n” value.
- Zero-order, as cumulative% drug release Vs time
- First-order, as log cumulative %drug retained Vs time
- Higuchi’s model, as cumulative % drug release Vs Square root of time
- Korsmeyer- peppa’s model, as log cumulative % drug release Vs log time and determine the “n” value from the slope.
- This study was carried out to identify the kinetic drug release model of the formulation. The kinetic release of model was assessed using highest correlation coefficient (R2) value formulation.
Formulation of Trigonella foenum- graecum l. Loaded NLC based Buccal patch:
The best Trigonella foenum- graecum L. loaded Nano structured lipid carrier emulsion has to been capsulated into the Buccal patch by solvent casting method. The required amount of Fenugreek seed extract (FSE), HPMC, SLS, Glycerine were added to the 20 ml of distilled water and stirred for 20 minutes uniformity is attained. To this above mixture the accurately weighed amount of Nano structured lipid carrier emulsion was added and stirred. The mixture was then transferred to the petridish and a funnel was kept in a inverted position for 24 hours. After 24 hours the patch was removed and kept in a desicators and dried.

Fig 2: Solvent casting method

Fig 3: Buccal Patch
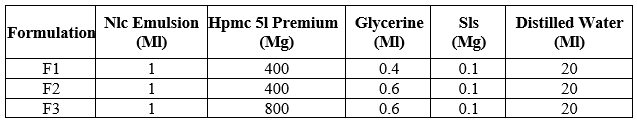
Table 2: Composition of FSE Loaded NLC based Buccal patch
Evaluation of FSE loaded NLC based Buccal Patch
Physicochemical Evaluation
Interaction Studies: The purpose of this study is the compatibility between the drug and the excipients guarantee sample interaction. Should an interaction arise, it could impact the drug product's stability and bioavailability. Therefore, FT-IR spectral measurements were used to conduct interaction experiments.
Thickness of the patch: Five films were selected from each formulation, and a Venier caliper was used to measure each film's thickness at various locations. The standard deviation and average film thickness were calculated. [21,22]
Weight uniformity: 3 patches were selected from each formulation and put through the IP procedure's weight variations test using a Shimadzu digital balance. Each buccal patch weight was deducted from the average weight of the five buccal films. We computed the mean ± SD values for each formulation. [21,22]
Folding endurance: Using a sharp blade,3 patches of each formulation measuring 2x2 cm were cut. A tiny patch strip was folded repeatedly at the same location until it burst to test folding endurance. The folding endurance value was the number of times the patch could be folded in the same direction without breaking. The average value was determined and noted. [23]
Percentage moisture content: The buccal patches were precisely weighed and stored in anhydrous calcium chloride-filled desiccators. The patches were removed and weighed after 3 days. By applying the formula to measure moisture loss (%), the moisture content (%) was ascertained.[24]
Percentage moisture content= [(Initial weight - Final weight) / Final weight] × 100
Drug content: Using a magnetic stirrer, the prepared buccal patch was dissolved in 100 ml of pH 7 phosphate buffer solution (PBS) for 12 hours. The mixture was then sonicated for 30 minutes to ensure that 200 mg of FSE and NLC emulsion were present. Phosphate buffer pH 7 was used to dilute 1ml of the filtrate to 10 ml following filtration to remove insoluble residue. The UV spectrophotometer was used to test the absorbance at 280 nm respectively. [25,26]
Content uniformity test: It is measured by taking ten films of each preparation on separate 100 ml volumetric flasks. Next, 100 ml of pH 7 phosphate buffer is added, stirring for 24 hours, and then the mixture is filtered and UV spectroscopically detected at 280 nm. The average of these 10 films is used to get the final reading. Buccal patches pass the test of content uniformity if 9 out of 10 contain drug content between 85 and 115% of the stated value and one has content not less than 75 to 125% of the specified value. However, an extra 20 patches were tested for drug content if the drug content of 3 patches fell between 75% and 125%. The buccal patches passed the test if the range of these 20 patches was between 85% and 115%.[27]
INVITRO EVALUATION
In-vitro drug release studies:
In vitro drug release all different formulations were carried out by Open Edge tube method. The cellophane membrane was mounted between donor and receptor compartment such that the mucosal surface facing the donor compartment. The Buccal patch was fixed on between donor and receptor compartments were clamped together and placed in water bath maintained at 37±0.5? C. The volume of the receptor cell was 25ml and the effective surface area available for permeation were 4.9602 cm2. The receptor compartment filled with phosphate buffer of pH 7. The Hydrodynamics of the receptor fluid was stirred at 600 rpm with star head magnet. Samples of 1 ml were withdrawn at specific interval of time. The same volume of phosphate buffer pH 7 was added to receptor compartment to maintain the sink condition and the samples were analyzed at 280 nm by using UV spectrophotometer.
Kinetic release studies
Drug release kinetics of 5 different formulations were calculated by using a software Microsoft Office Excel Add-In. The in vitro drug release data was used in various kinetic equations to understand the mechanism of drug release by determining the correlation coefficient and “n” value.
- Zero-order, as cumulative% drug release Vs time
- First-order, as log cumulative % drug retained Vs time
- Higuchi’s model, as cumulative % drug release Vs Square root of time
- Korsmeyer- peppa’s model, as log cumulative % drug release Vs log time and determine the “n” value from the slope.
This study was carried out to identify the kinetic drug release model of the formulations. The kinetic release of model was assessed using highest correlation coefficient (R2) value formulations.
RESULTS AND DISCUSSIONS
Physical Characterization:
Physical characteristics has impact on the stability, Product Performance, appearance and Processibility. Physical characteristic examination was played major role in the early development of dosage form. Hence, physical characteristics of Trigonella foenum-graecum L. was examined and the observation were reported in the table
Organoleptic Property of fenugreek seeds:
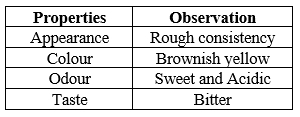
Table 3: Organoleptic properties of pure drug Trigonella foenum- graecum L
Solubility studies:
Solubility played an important role in drug effectiveness without the utilization of proper solvent the drug not be absorbed by our body leads to low bioavailability. Hence, solubility of a drug was determined using various solvents. The 1 mg of was dissolved in various solvents and the results found were shown in table
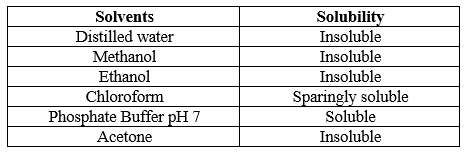
Table 4: Solubility profile of drug determination of wavelength range
Determination of Wave length maxima
10 mg of the drug Trigonella foenum – graecum L. was dissolved in phosphate buffer saline pH 7 and the maximum absorption from dilution of 100 µg/ml was found to be 270nm. Concentration of 1000 µg/ml of Trigonella foenum-graecum L. drug was dissolved in phosphate buffer saline pH 7 and scanned over a wavelength range of 200-400 nm using UV-spectrophotometer and the wavelength maxima was determined.
Construction of Calibration Curve
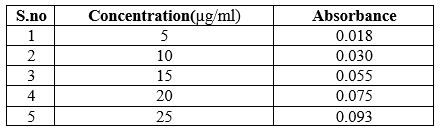
Table 5: Absorbance data of various concentration of Trigonella foenum – graecum
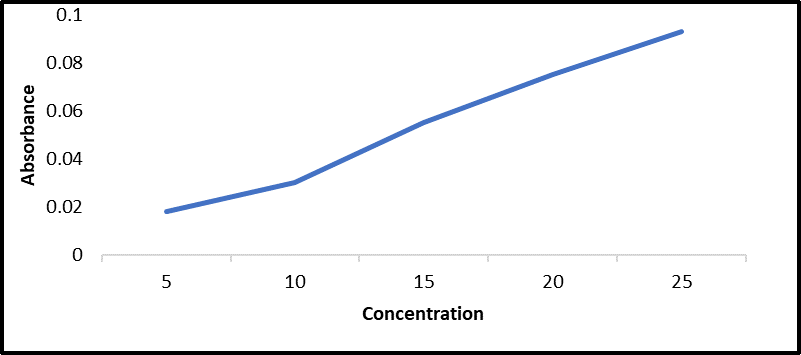
Figure 4: Standard graph of Trigonella foenum-graecum L
Compatible studies FT-IR:
FT-IR studies were carried out by using potassium bromine disc pellet method. The drug- excipient compatibility studies were carried out with an intent to identify, quantify and predict potential interactions (physical or chemical) along with the impact of these interactions on the manufacturability, quality and performance of the final drug product. The spectra of drug and other excipients was analyzed by using FT-IR matching approach,
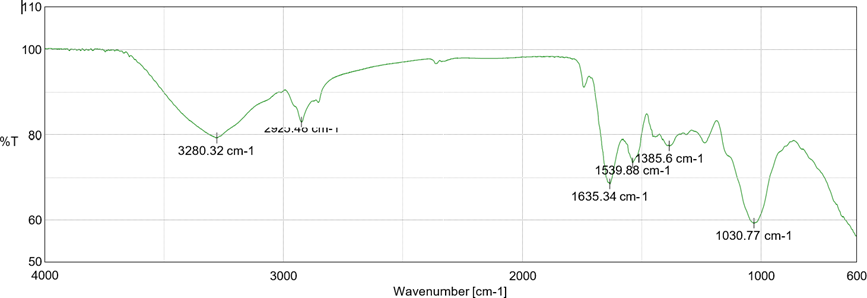
Fig 5: FT-IR spectrum of Pure drug

Table 6: FT-IR data of Pure drug
FT-IR studies revealed that there is no interaction between drug and polymer by ensuring no any new peak appearance or disappearance of already existed peaks indicated that there was not any chemical interactions between drug and excipients.
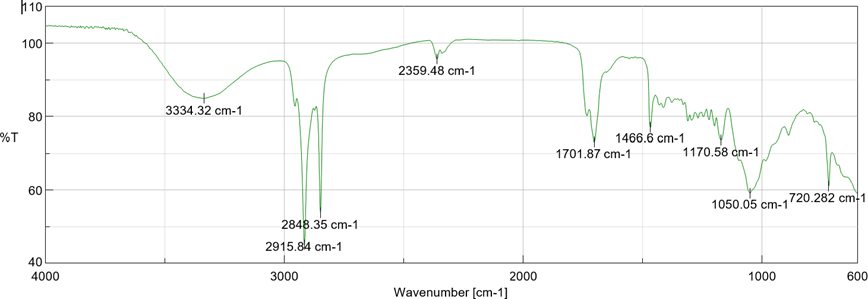
Fig 6: FT-IR spectrum of Drug + Span 60
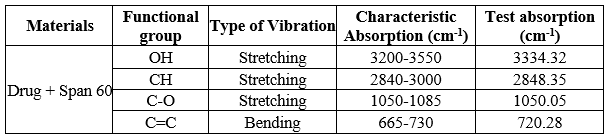
Table 7: FT-IR data of Pure drug + Span 60
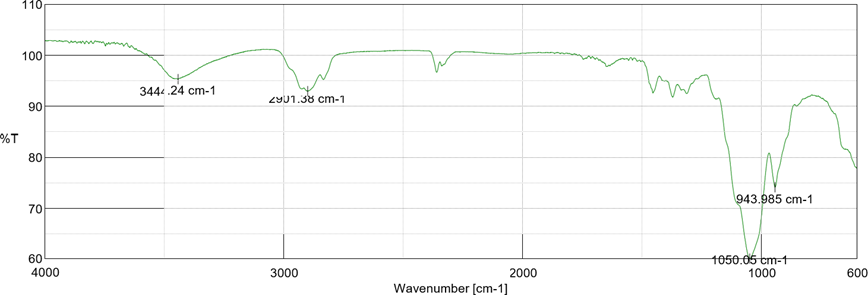
Fig 7: FT-IR spectrum of Drug + HPMC
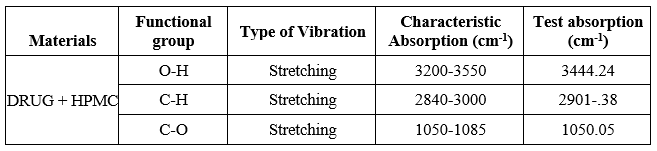
Table 8: FT-IR data of Pure drug + HPMC
pH
The pH ranges of all the formulations were studied to ensure formulations did not produced any irritation effect to the body.
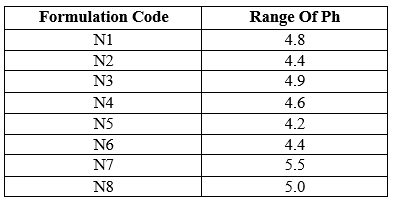
Table 9: PH of formulations
The pH ranges were found between 4.2 – 5.5 which indicated the formulations did not produce any irritation effect to the mucus membrane.
Evaluation of Trigonella foenum - graecum L. Loaded NLC:

Fig 8: Formulation of Trigonella foenum -graecum L. Loaded Nano Structured lipid carrier
Nano structured lipid carrier were prepared by Micro- Emulsion method using magnetic stirrer. These formulations were contained drug (Fenugreek seed),non-ionic surfactant (span 60 ) and soybean oil in various composition. The characteristics of formulations and release of drug were determined by its characteristics evaluation
Particle size Determination
The particle size distribution along the mean diameter of the all different composition of Nano structured lid carrier formulations were measured by using Dynamic Light Scattering Particle Size Analyzer (Malvern instruments). The particle size ranges all formulations from N1 to N8 were found to be in a nanosize range which indicated that all formulations has a smooth spherical surface. The particle size ranges of N1, N2, N3, N4, N5,N6 ,N7 and N8 were found to be , 73nm, 55.67nm, 120nm, 40.94nm, 153nm, 267nm, 210 nm, 235nm. The increase in particle size indicated, increase in surfactant concentration would increase the particle size range. The N4 formulation contained Non-ionic surfactant and soybean oil in the ratio of 1:1 shows the least particle size range of 270nm among all other NLC formulations. The particle size data reported that all NLC formulations from N1 to N8 having a highest polydispersity index of about 0.384PI respectively
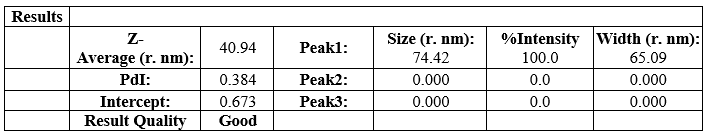
Table 10: Particle size range of N4 formulation
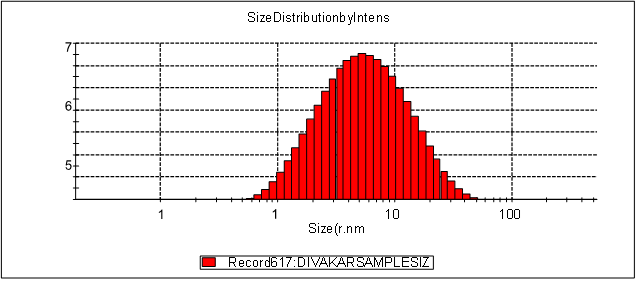
Fig 9: Particle size range of N4 formulation
Zeta Potential
All prepared NLC formulations were evaluated for its stability study. The zeta potential of all formulations were determined by using Zeta meter to measure the vesicle surface charge ( zeta potential). The stable NLC zeta potential ranges were between ± 30mV. The zeta potential of all NLC formulations were found be. The zeta potential range of N4 data showed in the figure given below;
Results
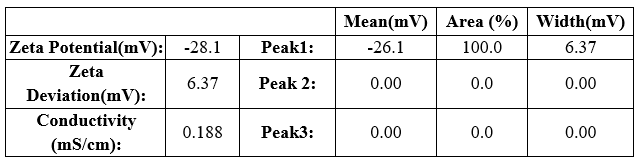
Table 11: Zeta potential of N4 formulation
Result quality: Good
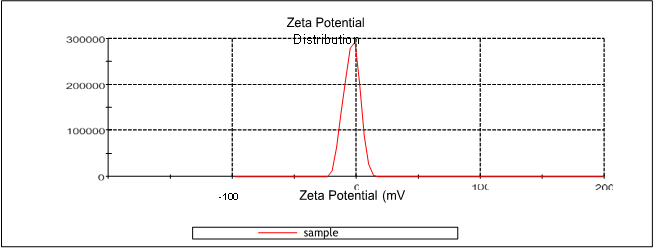
Figure 10: Zeta potential of N4 formulation
Entrapment Efficiency
This evaluation was carried out to ensure the percentage amount of drug entrapped in the NLC formulation whose vesicle was formed using Span 60. The Entrapment efficiency of all formulations were found to be within the range of about 90.5 % to 97.8 %
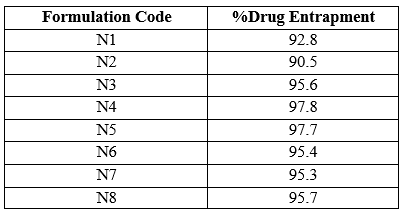
Table12: Entrapment efficiency of all formulations
Among all other formulations N4 showed maximum percentage of drug entrapment of about
97.8.
In Vitro Drug Release
In vitro drug release of Trigonella foenum-graecum L. loaded NLC formulations were determined by dialysis diffusion method (open edge tube method). In vitro drug release studies were carried out to ensure the safety, efficacy, product performance, batch to batch uniformity and bioavailability of a drug to produce the desired therapeutic activity. Hence, in- vitro drug release of all different NLC formulations were analyzed and percentage cumulative drug release were determined. The cumulative percentage drug release all 8 formulations were reported in table 16 by taking time in hours on X-axis and cumulative % drug release on Y-axis
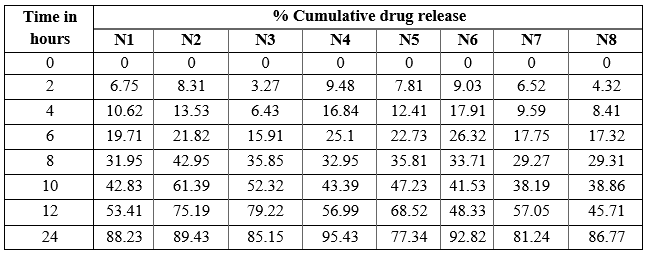
Table 13: % cumulative drug release of N1 to N8
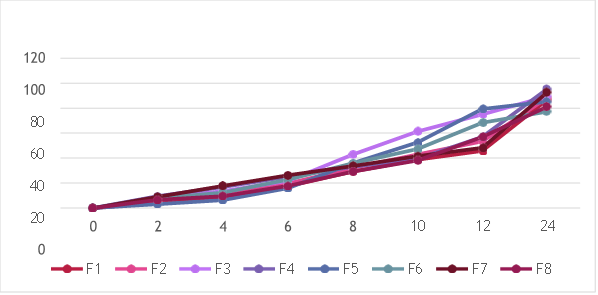
Fig 11: % cumulative drug of N1 to N8
The N4 formulation showed the highest percentage of cumulative drug release. The initial cumulative percentage of drug release was found to be 9.48% at end of 2 hours and then increased drug release of about 43.39% at 10 hours and at the end of 24 hours it showed maximum drug release was found to be 95.43%.
Selection of best formulation
The N4 NLC formulation was found to best among all because compared to other formulations it had a particle size of 40.94 nm, zeta potential range of about -28.1mV showed that formed NLC were more stable with a PDI Of about 0.384PI, entrapment efficiency were reported to be 97.8% and the in vitro release of drug was found to be 95.43%. Therefore, N4 formulation was selected as the best NLC emulsion to incorporate into buccal Patch for efficient drug delivery.
Kinetic release study
Different models such as Zero –order kinetics ( % amount of drug release versus time ), First- order kinetics (log percentage of drug remaining to release versus time),Higuchi (Percentage amount of drug unreleased versus square root of time) and Korsemeyer –Peppas (log percentage of drug released versus log time) were applied and assessed for the kinetic release of all prepared NLC emulsion. The suitable kinetic model (drug release mechanism) of drug release was selected based on regression coefficient (nearer to value of 1). The kinetic parameters of all N4 formulations were reported in the table 17
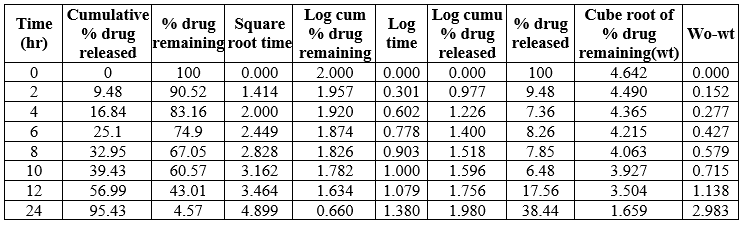
Table 14: Kinetic release of N4 formulation
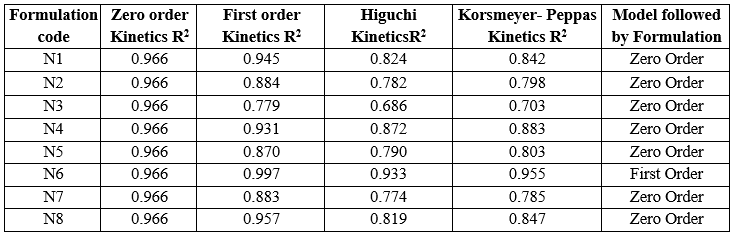
Table 15: Kinetic parameters of N1-N8 formulation
The data showed the N1-N5 and N7, N8 formulatoin was fitted into Zero –order release kinetic mechanism and N6 was fitted into a First order kinetic mechanism
Formulation of Trigonella foenum- graecum l. loaded NLC based Buccal Patch:
Physicochemical characterization: The N4 formulation was found to be optimized formulation. This optimized N4 NLC emulsion was further incorporated into buccal patch using solvent casting method.
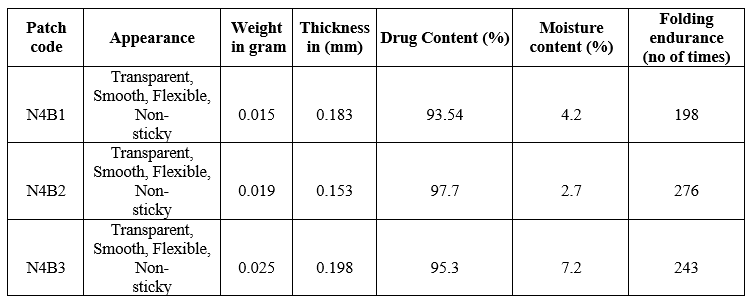
Table 16: Effect of polymer on physicochemical parameters
From the above characteristics N4B2 formulation showed the least thickness range of about0.153mm with reduced patch weight of about 0.019g, showed highest amount of drug content of 97.7 %moisture content was found to be 2.7% and folding endurance was about 276 reported that this formulation could easily penetrated through the Buccal mucosa and increased product performance than other formulations.
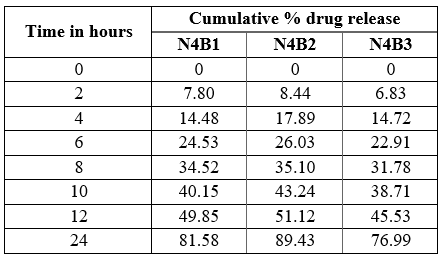
Table 17: Cumulative % drug released at `T’ of NLC Buccal patch
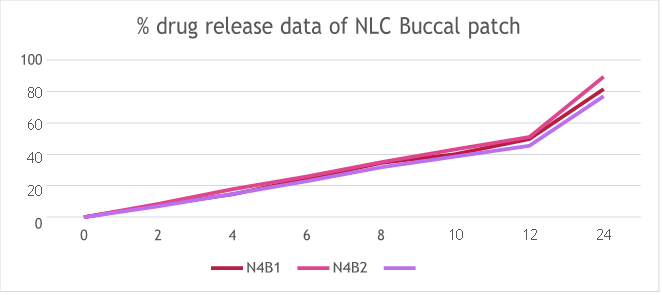
Fig 12: % drug release data of NLC Buccal patch
Kinetic release studies
Different models such as Zero –order kinetics (% amount of drug release versus time), First-order kinetics (log percentage of drug remaining to release versus time), Higuchi (Percentage amount of drug unreleased versus square root of time) and Korsemeyer –Peppas (log percentage of drug released versus log time) were applied and assessed for the kinetic release of all prepared NLC emulsions. The suitable kinetic model (drug release mechanism) of drug release was selected based on regression coefficient (nearer to value of 1). The kinetic parameter of all 3 formulations were reported in the table 21,22 and 23.
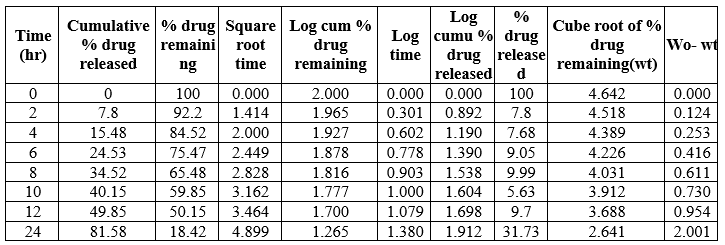
Table 18: Kinetic Release of N4B1formulation
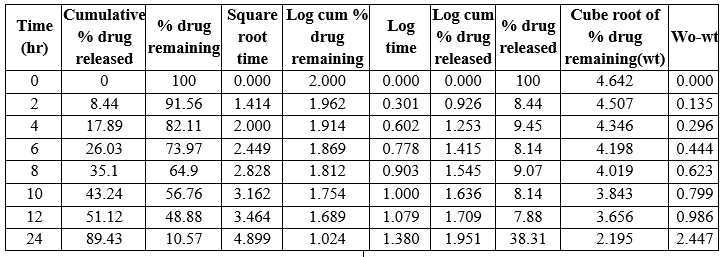
Table 19: Kinetic Release of N4 B2 formulation
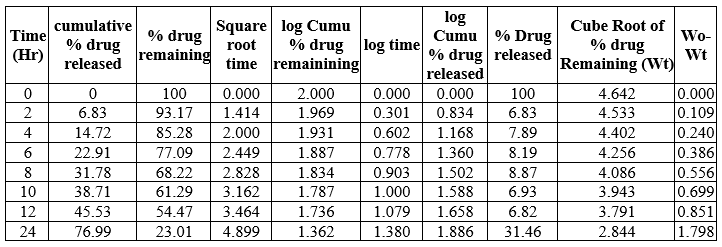
Table 20: Kinetic release of N4B3 formulation
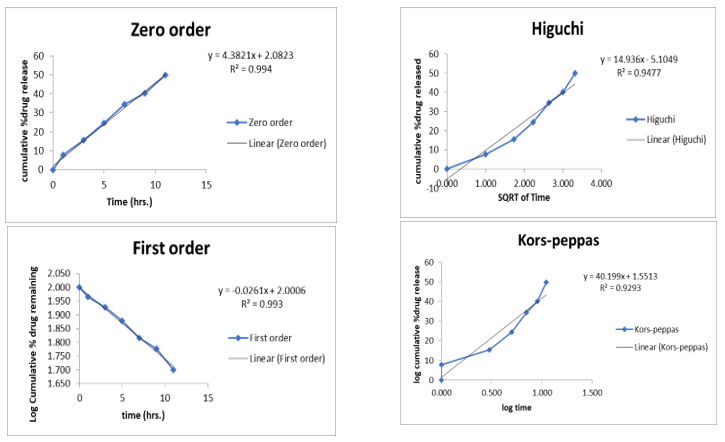
Fig 13: Kinetic Parameters curve of N4B2
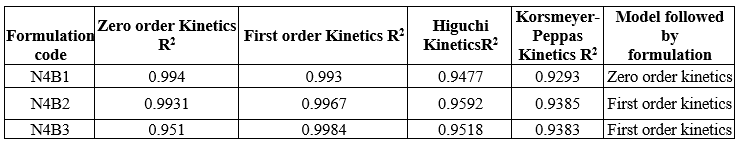
Table 21: Kinetic parameter of Trigonella foenum – graecum l. Loade NLC Buccal patch
The N4B1 follows zero order kinetics, N4B2 and N4B3 formulation were fitted into first order kinetic release model
SUMMARY AND CONCLUSION
The research was carried to present the concept of formulation of Nanostructured lipid carrier incorporated into buccal patch for the treatment of hyperglycemia. Among the various types of formulation, the NLC type of formulation was selected because of its increasing the bioavailability, drug loading and solubility of the drug in different conditions and environments. The concept NLC emulsion formulated using Microemulsion method was then incorporated into Buccal patch by Solvent casting technique delivered the drug at controlled rate and release for a long period of time. Thereby it reduced the frequency of dosing, increased the bioavailability of drug, increased patient compliance and reduced the cytotoxicity. The herbal drug Trigonella foenum- graecum l. were selected as active pharmaceutical ingredient in present research rather than the selection of synthetic drug. The utilization of herbal drug showed that it could able to avoid the side and adverse effects produced by the synthetic drug on chronic administration. Fenugreek were traditionally utilized a natural herb to cure hyperglycemia. Hence, the formulation of Trigonella foenum- graecum l was loaded NLC buccal patch. First stage of research were focused on the compatability studies to ensure safety, stability, effectiveness and product performance of drug with other excipients. Hence, compatability studies were measured by FT-IR interaction studies of drug and pure drug with polymer, non-ionic surfactant and other excipients were studied. FT-IR studies revealed that there was no interaction between drug and polymer by ensuring no any new peak appearance or disappearance of existed peaks. The pH of all formulations were found to be in excellent ranges. The particle size distribution of all formulations were in optimum ranges and N1 showed the least particle range of about 40.94 nm. The zeta potential of all formulations were between the range of - 26.76mV to 33.58 mV and N2 showed zeta potential of -28.1mV reported more stable NLC were formed. The entrapment efficiency of all formulations showed range from 90.5 to 97.8% and the highest entrapment of drug was observed in N4 . The Invitro drug release showed N4 attained maximum percentage of drug release of 95.43 % at the end of 24hours. On general N4 was considered to be the best NLC formulation among all. The N4 formulation was fitted into zero order kinetic release model with R2 value of 0.966. N4 Trigonella foenum- graecum l. loaded NLC emulsion incoporated into different polymeric composition of Buccal patch. Among all three formulation N4B2 showed the least thickness of 0.153 mm ensured easy penetration, weight of 0.019 g, highest drug content of about 89.43%,uniformity in drug content was found to be 94.16%, moisture content of 2.7% and folding endurance of about 276 times. In vitro drug release of N4B2 showed highest % cumulative drug release of 89.43% and then it fitted into first-Order kinetic model with R2 value of 0.9967. On conclusion, this formulation produced excellent drug release ensured that this was effective formulation for the treatment of hyperglycemia. In future, animal studies could be developed to ensure about the exact predictability of effectiveness of the formulation on chronic administration.
REFERENCE
-
-
-
- Mohd. Gayoor Khan* THE NOVEL DRUG DELIVERY SYSTEM, World Journal of Pharmaceutical sciences, Volume 6, Issue 7, 477-487
- Ozpolat B., Sood A.K., Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014;66:110–116
- López-García R, Ganem-Rondero A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Occlusive Effect and Penetration Enhancement Ability. J Cosmet Dermatol Sci Appl. 2015;5(2):62–72.
- Jain P, Rahi P, Pandey V, Asati S, Soni V. Nanostructure lipid carriers: a modish contrivance to overcome the ultraviolet effects. Egypt J Basic Appl Sci. 2017;4(2):89–100.
- Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242:121–8
- Karnati v chandana*, n. Vishal gupta, Sandeep kanna, Nanostructured lipid carriers: The Frontiers in Drug delivery, Asian Journal of Pharmaceutical and Clinical research, Vol 12, Issue7, 2019
- Müller RH, Petersen RD, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59:522–30
- Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415:232–43.
- Muchow M, Maincent P, Muller RH. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm. 2008;34:1394–405
- Noor NM, Sheikh K, Somavarapu S, Taylor KMG. Preparation and characterization of dutasteride-loaded nanostructured lipid carriers coated with stearic acid-chitosan oligomer for topical delivery. Eur J Pharm Biopharm. 2017;117:372–84.
- Shah R, Eldridge D, Palombo E, Harding I. Lipid Nanoparticles: Production, Characterization and Stability. UK: Springer; 2015.
- Sahoo L. Nanostructured lipid carrier (NLC)- A Promising Drug Delivery for Transdermal Application. J. Pharm. Sci.& Res. 2020; 12(4): 475-487.
- Chen PC, Huang JW, Pang J (2013) An investigation of optimum NLCsunscreen formulation using taguchi analysis. J Nanomater pp: 1-11.
- Joshi M, Patravale V (2006) Formulation and Evaluation of Nanostructured Lipid Carrier (NLC)–based Gel of Valdecoxib. Drug Dev Ind Pharm 32: 911-918.
- Laso? E, Sikora E, Ogonowski J (2013) Influence of process parameters on properties of Nanostructured Lipid Carriers (NLC) formulation. Acta Biochem Pol 60: 773-777.
- Karn-Orachai K, Smith SM, Phunpee S, Treethong A, Puttipipatkhachorn S, Pratontep S. et al. The effect of surfactant composition on the chemical and structural properties of nanostructured lipid carriers. J Microencapsul. 2014;31(6):609–18.
- Jaiswal P, Gidwani B, Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):27–40.
- Han F, Li S, Yin R, Liu H, Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Colloids Surf A Physicochem Eng Asp. 2008;315(1-3):210–6.
- Tangri P, Recent advances in oral mucoadhesive drug delivery system: A review, International Journal of Pharmaceutical Research and Development, 2011, 3(2), 151- 161.
- Murali krishna K, Nagaraju T, Gowthami R, Rajashekar M and Yamsani SK, Comprehensive Review on Buccal Delivery, International Journal of Pharmacy, 2012, 2(1), 205-217.
- Alagusundaram M, Chengaiah B, Ramkanth S, Angala PS, Chetty M, Dhachinamoorty Formulation and evaluation of mucoadhesive buccal films of ranitidine. Int J Pharmtech Res. 2009;1:557–63
-
-
-
- New Delhi: Indian pharmacopoeia published by the controller of Publication; 2007. pp. 241–2
- ALIX, D.; GHANIA, D. Synthesis and characterization of sulphated ?, ? and ? cyclodextrins : application to the complexation of Acyclovir. Carbohydr. Res., v.38, n.21, p.2185-2193, 2003
- Attama A, Akpa PA, Onugwu LE, Igwilo G. Novel buccoadhesive delivery system of hydrochlorothiazide formulated with ethyl cellulose hydroxypropyl methylcellulose interpolymer complex. Scientific Res Essay. 2008;3(6):26–33.
- Birudaraj R, Berner B, Shen S, Li X. Buccal permeation of busipirone: mechanistic studies on transport pathways. J Pharm Sci 2005;94:70-8.
- Harris D, Robinson JR. Bioadhesive polymers in peptide drug delivery. Biomaterials 1990;11:652–8.
- Singh S, Virmani T, Virmani R, Kumar P, Mahlawat G. Fast dissolving drug delivery systems: formulation, preparation techniques and evaluation. Universal Journal of Pharmaceutical Research. 2018; 3(4): 56-64


 S. Divakar*
S. Divakar*
 K. Shanthini
K. Shanthini


































 10.5281/zenodo.12730715
10.5281/zenodo.12730715