Abstract
A simple, rapid and precise and accurate method was developed for the estimation of Artemether in bulk & tablet dosage form. Analysis was performed on a Phenomenax C18, 250 x 4.6 mm column 82:21 Acetonitrile : water, the mobile phase filtered through to 0.45 µm membrane filter degassed. as mobile phase, a flow rate 1.0 mL/min, column temperature 30°C and UV detection at 216 nm. ART were well resolved on the stationary phase and the retention time for Artemether was 4.509 min. The calibration curves were linear in the concentration range of 50-500 ?g/mL for ART. Intra-and inter-day relative standard deviations for both the components were <2.0%. The percentage recoveries obtained for ART within the limit.
Keywords
Artemether, HPLC, Method Development, Validation.
Introduction
Development of simple and reproducible analytical methods for estimation of drugs is very important part of quality control and assurance. Chemically Artemether is 3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-methoxy-3,6,9-trimethyldecahydro-12H-3,12-epoxy[1,2]dioxepino[4,3-i]-2-benzopyran. Artemether is an antimalarial agent used to treat acute uncomplicated malaria. It is administered in combination with lumefantrine for improved efficacy. This combination therapy exerts its effects against the erythrocytic stages of Plasmodium spp. and may be used to treat infections caused by P. falciparum and unidentified Plasmodium species, including infections acquired in chloroquine-resistant areas.. The structure is shown in fig.1

Figure No.1: Structure of Arthemeter
MATERIAL AND METHODS
Apparatus and software
- HPLC( Agilent) computer loaded EZ Chrom Elite software was used for all the HPLC measurements. The column was a Phenomenax C18, 250 x 4.6 mm column used. The absorbance spectra of the reference and test solutions were carried out in 1cm quartz cells over the range of 200-400 nm.
Reagents and materials
Artemether (99.5% purity) was received as gift samples from Micro Orgo Chem. HPLC grade Acetonitrile & Water.
Standard Preparation (Stock Solution):
Preparation of Artemether standard solution Approximately 20 mg of artemether reference standards were accurately weighed on weighing balance. The weighed quantity was transferred to a 100 ml volumetric flask. 7 ml Chloroform was added to the flask to ensure complete solubilization, followed by the addition of 80 ml of acetonitrile. The volume was filled up to the mark with 0.05% trifluoroacetic acid. The resulting solution contained 200 µg/ml of artemether. The solution was filtered through 0.45 µm membrane filter.
Preparation of Sample Solution:
The tablets were weighed and crushed to a finely powdered state. An accurately weighed portion of the powder, equivalent to about 20 mg of artemether, was transferred to a 100ml volumetric flask followed by the addition of 7ml of chloroform. The solution was sonicated for 15 min and addition of 80 ml of acetonitrile. The volume was filled up to the mark with 0.05% trifluoroacetic acid. The solution was filtered through 0.45µm membrane filter.Different batches of test formulations and one batch of reference were analyzed using the validated method. Chloroform was added to ensure the complete solubilization of the samples. Six replicates of each batch were assayed for the analysis.
Selection of Chromatographic Condition:
Proper selection of the method depends up on the nature of the sample, its molecular weight and solubility. The drugs selected in the present study. Thus normal Phase HPLC was selected for the initial separation because of its simplicity, suitability, ruggedness and its wider usage.
Initial Separation Condition:
The mobile phase selected to elute the drug from the stationary phase was methanol &
acetonitrile and HPLC grade water, because of its favorable UV transmittance
Preparation of buffer:
0.1g of hexane sulphonic acid was weighed into a 100 ml beaker, dissolved and diluted to 100 ml with HPLC grade water, the flask was shaken until the particles get dissolved and volume was made up to the mark with HPLC grade water. The pH was adjust to 4 with orthophosphoric acid.
Preparation of Mobile Phase:
Acetonitrile HPLC (82%) & Water (21%) were mixed andfiltered through to 0.45 µm membrane filter & degrassed.
Diluent Preparation:
Acetonitrile was used as diluent.
Preparation of Standard Solution: An accurately weighed of 20 mg of artemether was transferred into 100 ml volumetric flask and add 7 ml of Chloroform, addition of 80 ml of acetonitrile. The volume was filled up to 0.05 % of trifluoroacetic acid. The resulting solution concentration of 200 µg/ml.
OPTIMIZED METHOD
Optimized method for the estimation of Artemether by HPLC was finally achieved by using the following chromatographic condition.
Table No. 1 Optimizes Method

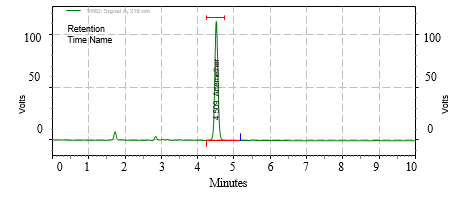
Figure 2. Chromatogram for Optimized Method
RESULT & DISCUSSIONS
VALIDATION:
1. System suitability
Sample solution of Artemether was injected three times into HPLC system as per test procedure. The system suitability parameters were evaluated from standard chromatograms obtained by calculating the %RSD of retention times, tailing factor, theoretical plates and peak areas from three replicate injections.
Table No. 2 System Suitability Parameter of Artemether
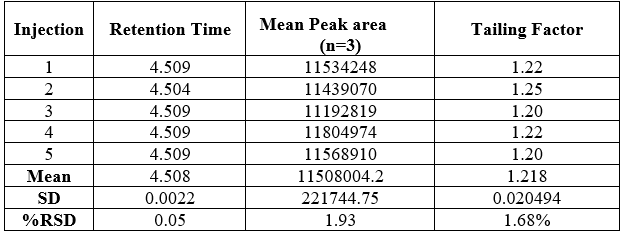

Figure 3. Chromatogram for System suitability
2. Specificity
Specificity is the ability to assess unequivocally the analyte in the presence of components which may be expected to be present. A particular analytical procedure's lack of specificity may be addressed by additional supporting analytical techniques.

Figure 4. Chromatogram For without sample (Blank)
3. Linearity & Range Studies:
Six different concentration ranging 50% to 500% of artemether labelled claim for linearity standard solution ranging 50µg/ml to 500µg/ml. The calibration curve obtained by plotting peak area against concentration and injected under optimized chromatographic condition.
Table No. 3 Concentration and mean peak area of Artemether for Linearity Study


Figure 5. Linearity Studies Artemether
4. Precision:
The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions.

Figure 6. Chromatogram for Precision
5. Accuracy (Recovery)
The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found. This is sometimes termed trueness.
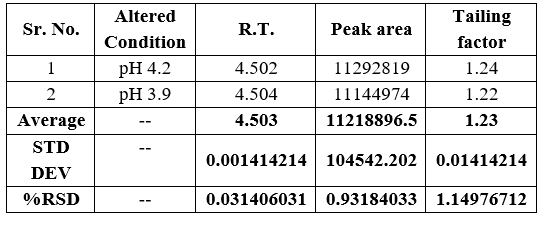
Figure 7. Chromatogram for Accuracy (Recovery)
6.Robustness
Robustness studies were carried out using slight modifications in chromatographic such as:
- Change in flow rate by ±10%
- Change in pH by ±0.2
- Change in wavelength by ±2

Table No. 4 Robustness study observation of Artemether with changing the Flow rate

Table No. 5 Robustness study observation of Artemether with changing the pH

Table No. 6 Robustness study observation of Artemether with changing the Wavelength

Figure 8. By changing the wavelength by 214nm
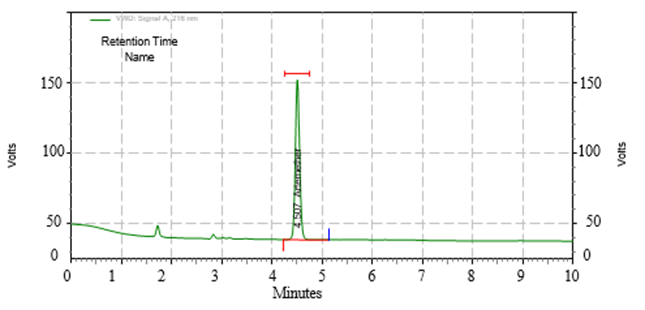
Figure 9. By changing the wavelength by 218nm
SUMMARY & CONCLUSION
The developed HPLC technique is simple, precise and accurate. As the drug is sensitive to degradation, selectivity is an important validation parameter. Statistical analysis proves that the method is reproducible and selective for the analysis of Artemether in pharmaceutical dosage forms. It can be used to determine the purity of the drug available from various sources. This study was conducted to develop a new facile HPLC based analytical method for the determination of artemether (20 mg) in a newly developed formulation. Various advantages were offered by this method which includes easily constitutable mobile phase and shorter run time with high resolution of the analytes’s peaks. This newly developed analytical method has been validated according to parameters provided in ICH guidelines. The method has been found to be very simple and convenient to perform; sensitive and specific for the objective drugs. Moreover the method is accurate, precise and robust over a wide range of analytes’s concentration. Therefore, in the light of the study, the proposed method can be used for analysis of formulation of artemether in any analytical setting of either a pharmaceutical industry or research organization or any academic institution which houses an HPLC instrument.
REFERENCES
- Chauhan A., Mitttu B., and Chauhan P., “Analytical Method Development and validation : A Concise Review”. J Anal and Bioanal Tech: 2015, 6:1, 1-5.
- Sweetman SC. Martindale: The Complete Drug Reference, Antimalarials, Artemisinin derivatives, Artemether Pharmaceutical Press: London, UK, 37th ed., Volume A, 2011, pp. 650-52.
- Green MD, Mount DL, Wirtz RA. Authentication of artemether, artesunate and dihydroartemisinin antimalarial tablets using a simple colorimetric method. Trop Med Int Health 2001; 6:980-82.
- Atemnkeng MA, De Cock K, Plaizier Vercammen J. Quality control of active ingredients in artemisinin-derivative antimalarials within Kenya and DR Congo. J Trop Med Int Health 2007; 12: 68–74.
- Arun R, Smith A. Development of analytical method for lumefantrine by UV spectrophotometry. Asian J Chem 2011; 23: 1764-66.
- Karbwang J, Na-Bangchang K, Congpuong K, Molunto P, Thanavibul A. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur J Clin Pharmacol 1997; 52: 307–10.
- Navaratnam V, Mansor SM, Chin LK, Mordi MN, Asokan M, Nair NK. Determination of artemether and dihydroartemisinin in blood plasma by high-performance liquid chromatography for application in clinical pharmacological studies. J Chromatogr B. 1995; 669: 289–94.
- Karbwang J, Na-Bangchang K, Molunto P, Banmairuroi V, Congpuong K. Determination of artemether and its major metabolite, dihydroartemisinin, in plasma using high-performance liquid chromatography with electrochemical detection. J Chromatogr B. 1997; 690: 259–65.
- Sandrenan N, Sioufi A, Godbillon J, Netter C, Donker, M, Valkenburg V. Determination of artemether and its metabolite, dihydroartemisinin, in plasma by high-performance liquid chromatography and electrochemical detection in the reductive mode. J Chromatogr B 1997; 691: 145–53.
- Souppart, C., Gauducheau, N., Sandrenan, N. and Richard, F. Development and validation of a high-performance liquid chromatography-mass spectrometry assay for the determination of artemether and its metabolite dihydroartemisinin in human plasma. J Chromatogr B 2002; 774: 195–03.
- Skoog DA, Holler FJ, Nieman TA. Principles of instrumental analysis: Douglas A. Skoog, F. James Holler, Timothy A. Nieman. 5th ed. Belmont (CA): Brooks/Cole. 1998. Page No.1-2.
- A. K. M. Ayatullah Hosne Asif, Abdur Razzaque, Md. Zayedul Hasan. A Brief Overview of Different Analytical Techniques for Material and Chemical Analysis. International Journal of Instrumentation Science 2020, 7(1): 1-12.
- Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of analytical chemistry. Cengage learning; 2013. Page No. 2-3.
- Christian GD, Dasgupta PK, Schug KA. Analytical chemistry. John Wiley & Sons; 2013 Oct 7. Page No. 6-9.
- Gurdeep R, Chatwal S, Anand K. Instrumental methods of chemical analysis. Himalaya publishing house; 2016.
- Malviya R, Bansal V, Pal OP, Sharma PK. High performance liquid chromatography: a short review. Journal of global pharma technology. 2010 Jun 9;2(5):22-6.
- LaCourse ME, LaCourse WR. General instrumentation in HPLC. InLiquid Chromatography 2017 Jan 1 (pp. 417-429). Elsevier.
- Antony R Gerardi, Jennifer L Lubbeck, Christa L Colyer. Dimethylditetradecylammonium bromide (2C14DAB) as a self-assembled surfactant coating for detection of protein-dye complexes by CE-LIF. Journal of Solid State Electrochemistry.2009; 13 (4), 633-638.https://en.wikipedia.org/wiki/Validation
- Guideline ICH. Validation of analytical procedures: text and methodology. Q2 (R1). 2005 Nov;1(20):05
- Jatto E, Okhamafe AO. An Overview of Pharmaceutical Validation and Process Controls in Drug Development. Tropical Journal of Pharmaceutical Research. 2002; 1(2):115-22.
- Epshtein NA. System suitability requirements for liquid chromatography methods: controlled parameters and their recommended values. Pharmaceutical Chemistry Journal. 2020 Aug; 54(5):518-25.
- Bose A. HPLC calibration process parameters in terms of system suitability test. Austin Chromatography. 2014; 1(2):1-4.
- Tiryaki O, Baysoyu D, Aydin G, Secer E. Setting system suitability parameters for performance optimization of GC-NPD detection for pesticide residue analysis. Gazi University Journal of Science. 2009 Jul 1; 22(3):149-55.
- Aashigari S, Goud R, Sneha S, Vykuntam U, Potnuri NR. Stability studies of pharmaceutical products. World J Pharm. Res. 2018 Oct 25; 8:479-92.
- Bajaj S, Singla D, Sakhuja N. Stability testing of pharmaceutical products. Journal of applied pharmaceutical science. 2012 Mar 24; 2(3):129-38.
- ICH Expert Working Group. ICH guideline Q1A (R2) stability testing of new drug substances and products. In International Conference on Harmonization 2003 Feb (Vol. 24).
- Guideline EM. Guideline on stability testing: stability testing of existing active substances and related finished products. CPMP/QWP/122/02, Rev. 1, 2003, 1/18; 2003 Jan.
- Iqbal Ahmad, Muhammad Ali Sheraz, Sofia Ahmed. Stability of Drug and Drug Product. Higher Education Commission – Pakistan; 201
- Shi B, Yu Y, Li Z, Zhang L, Zhong Y, Su S, Liang S. Quantitative analysis of artemether and its metabolite dihydroartemisinin in human plasma by LC with tandem mass spectrometry. Chromatographia 2006; 64: 523–30.Hodel EM, Zanolari B, Mercier T, Biollaz J, Keiser J, Olliaro P, Genton B. A single LC-tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J Chromatogr B 2009; 877: 867–86.
- César IC, de Aquino Ribeiro JA, de Souza Teixeira L, Bellorio KB, de Abreu FC, Moreira JM et al. Liquid chromatography-tandem mass spectrometry for the simultaneous quantitation of artemether and lumefantrine in human plasma: application for a pharmacokinetic study. J Pharm Biomed Anal 2011; 54: 114-20.
- César IC, Nogueira FHA, Pianetti GA. Simultaneous determination of artemether and lumefantrine in fixed dose combination tablets by HPLC with UV detection. J Pharm Biomed Anal 2008; 48: 951–54.
- Sridhar B, Hanumantha Rao K, Sai Srinivas TV, Seshu madhuri V, Madhuri K, Seshagiri Rao JV. method for the simultaneous estimation of Artemether and Lumefantrine in pharmaceutical dosage forms. J Adv Pharm Sci 2010; 1: 95-99.
- Sunil J, Sanjith Nath M, Samba Moorthy U. HPLC method development and validation for simultaneous estimation of Artemether and Lumefantrine in pharmaceutical dosage forms. Int J Pharm Pharmaceut Sci 2010; 2: 93-96.
- Rahul Singh, Ashish Pathak, Pooja Chawla, “Method Development and Validation for Simultaneous Estimation of Ketorolac and Sparfloxacin by RP-HPLC”. Indian J.Pharm.Biol.Res. 2013; 1(4):95-101.
- C. Roy validated stability- indicating RP-UPLC method for simultaneous determination of sodium methylparaben, sodium propylparaben and ketorolac tromethamine in topical dosage form, Indian journal of pharmaceutical science 2013; 75(2).
- Dhiraj A. Khairnar, Chetan S. Chaudhari and Sanjay P. Anantwar, Method Development And Validation Of Ketorolac Tromethamine In Tablet Formulation By RP-HPLC Method. IJPSR, 2014; Vol. 5(9): 3696-3703.
- Vikas P, Sharma G S and Seema R. RP-HPLC Method for Simultaneous Estimation of Artemether and Lumefrantine. Int. J. Univers. Pharm. Life Sci., 2011; 1(1): 31-43.
- Sridhar B Hanumantha R K , Saisrinivas T V,Madhuri V S, Madhuri K and Seshagiri R. A validated RP-HPLC method for the Simultaneous Estimation of Artemether and Lumefrantine in pharmaceutical dosage forms. Int. J. Adv. Pharm. Sci., 2010; 1(1): 95.
- Kakade R B and Kaylankar TM. Revearsed phase liquid chromatographic method for simultaneous determination of Artemether and Lumefrantine in pharmaceutical preparation. Int. J. ChemTech Res., 2011; 3(3): 1722-7.
- Sunil J, Sanjithnath M and Sambamoorthy U. Method development and validation for the HPLC for the simultaneous estimation of Artemether and Lumefrantine in pharmaceutical dosage forms. Int. J. Pharm. and Pharm. Sci., 2010; 2(4): 93-96.
- Nagarsenker M and Tayade N. Validated HPTLC method of analysis for Artemether and its formulations. J. Pharm. Biomed. Anal., 2007; 43: 839-44.
- Sandrenan N, Sioufi A,Godbillon J, Netterb C, Danker M and Valkenburg C V. Determination of Artemether and its metabolite, dihydroartemisinin, in plasma by HPLC and electrochemical detection in the reductive mode. J. Chromatogr. B, 1996; 691: 145-154.
- Souppart C, GauducheauN, Sandrenan N and Richard F. Development and validation of an HPLC -Mass Spectrometry Assay for determination of Artemether and its metabolite, dihydroartemisinin, in human plasma. J. Chromatogr. B, 2002; 774: 195-203.
- Fleckenstien L, Naik H and Murry D. Development and validation of an HPLC-Mass Spectrometry Assay for determination of Artemether and its metabolite, dihydroartemisinin, in human plasma. J. Chromatogr. B, 2005; 816: 233-42.
- Aweeka F Huanga L, Jayewardenea A and Li X Marzan F Lizak P. Development and validation of an HPLC -Mass Spectrometry Assay for determination of Artemether and its metabolite, dihydroartemisinin, in human plasma. J. Pharm. Biomed. Anal., 2009; 50: 959-65.


 Priyanka Chavhan*
Priyanka Chavhan*














 10.5281/zenodo.11517460
10.5281/zenodo.11517460