Abstract
Cinnarizine is an antihistaminic drug and it is mainstay therapy for vestibular disorders, approved for nausea, vomiting, motion sickness, vertigo and tinnitus associated with Meniere’s diseases. In this work the immediate release oral jellies of Cinnarizine were formulated as delivery systems for paediatric and geriatric patients who find swallowing of tablets difficult. The oral jellies were prepared using polymers like sodium alginate, gelatin, and xanthan gum at different drug to polymer ratios by heating and congealing method. The drug and excipients compatibility study was performed using FT-IR and no interactions were found between drug and the polymers. The prepared oral jellies were evaluated for various parameters like physical appearance, pH, weight variation, spreadability, drug content, in-vitro drug release study and stability study. Physical parameters of all the formulations were within the acceptable limit. The drug content of the prepared formulations was found to be between 95.66±2.4 and 97.86±1.2 % and in-vitro drug release study was found to be between 26.76±0.22 % and 97.23±0.98 % in 45 min. The formulation F4 showed the 92.59±0.23% of drug release within 15 minutes, the MDT was lowest at 5.33 min and DE15minwas 64.43% hence it was selected as best formulation. The selected formulation F4 was found to be stable within the study period. It can be concluded that oral jelly of Cinnarizine can be successfully formulated.
Keywords
Oral jellies, Cinnarizine, ginger powder, sodium alginate, gelatin, and Xanthan gum.
Introduction
An ideal dosage regimen in the drug therapy of any disease is the one, which immediately attains the desired therapeutic concentration of drug in plasma (or at the site of action) and maintains it constant for the entire duration of treatment. Drugs are more frequently taken by oral administration. (1) It is considered most natural, uncomplicated, convenient, safe means of administering drugs which offers greater flexibility in dosage form design with low cost, convenience of self-administration and ease of manufacturing. The most evident drawback of the commonly used oral dosage forms like tablets is difficulty in swallowing, leading to patient noncompliance particularly in case of paediatric and geriatric patients. Also in patients suffering from dysphasia, stroke, thyroid disorder, Parkinson’s diseases, multiple sclerosis, nausea, vomiting, motion sickness and psychiatric patients. These are also inconvenient for bedridden patients and active working patients who are busy or traveling with no access to water. To fulfil these medical needs, pharmaceutical technologists have developed a novel oral dosage form known as Oral medicated jellies (OMJs) which dissolves rapidly in saliva, usually in a matter of seconds, without the need of water. Drug dissolution and absorption as well as onset of clinical effect and drug bioavailability may be significantly greater than those observed from conventional dosage forms. The medicated jelly has through years gained increasing acceptance as a drug delivery system. They may be prepared from natural gums, such as tragacanth, pectin, sodium alginates or from synthetic derivatives of natural substance such as methyl cellulose and sodium carboxymethyl cellulose. OMJs with good taste and flavour can increase the acceptability of bitter drugs by various groups of population. OMJs release drug in the mouth and for absorption passed through local oromucosal tissues and through pregastric (e.g., oral cavity, pharynx, and esophagus), gastric (i.e., stomach) and postgastric (e.g., small and large intestines) segments of the gastrointestinal tract (GIT). (2,3) The treatment can be terminated easily at any time, if required. (4) It provides rapid drug delivery from dosage forms with rapid onset of action. Researchers have formulated OMJ for various categories of drugs used for therapy in which rapid peak plasma concentration is required to achieve the desired pharmacological response. These include neuroleptics, cardiovascular agents, analgesics, anti-allergic, anti-epileptics, anxiolytics, sedatives, hypnotics, diuretics, anti-parkinsonism agents, anti-bacterial agents and drugs used for erectile dysfunction. (4) Cinnarizine inhibits smooth muscle cell contraction in the vasculature by blocking L-and T-type voltage-gated calcium channel. It is also known to bind to histamine H1 receptors, muscarinic (acetyl choline) receptors and dopamine D2 receptors. It is mainstay therapy for vestibular disorders, approved for nausea, vomiting, motion sickness, vertigo and tinnitus associated with Meniere’s diseases. It is also used to treat migraine. Pharmaceutically, it belongs to the BCS class ?, and drug dissolution is the rate limiting step for its absorption. It is having narrow absorption window in stomach. It has half- life about 3-4 hours. Dose of the Cinnarizine is 15 mg. (5-8) Ginger (Zingiber officinale) herbaceous perennial plant of the family Zingiberaceae and Zinger officinale possess an aromatic odour and taste, and it is a valuable source of phytochemicals. The aromatic odour is from its constituents like the Zingerone, gingerol and Shagaol. (9) Its pungent aromatic rhizome (underground stem) used as a spice, flavour, food, and medicine. It is traditionally used for its anti –nausea, anti-emetic effect. (10) In this study, we have prepared Oral jellies for drug Cinnarizine with ginger powder (flavour) and by using various gelling polymers like sodium alginate, gelatin and, xanthan gum.
MATERIALS AND METHODS
Cinnarizine was obtained as gift sample from Fleming Labs Ltd, Ahmedabad. Sodium alginate, gelatin and citric acid were procured from S.D. fine Chem Ltd. Mumbai. Xanthan gum was purchased from Himedia Laboratories Pvt. Ltd. Mumbai. All other chemicals were of analytical grade.
METHODOLOGY (11-22)
Determination of pH
A drug solution of concentration 5% w/v in distilled water was prepared and pH of the solution was determined using a pH meter. Average of three readings was computed and the values are reported as mean ±SD (n=3).
Calibration curve of Cinnarizine
Accurately weighed 100 mg of Cinnarizine was dissolved in 100 ml 0.1N HCl to get stock solution A. From the stock solution A 10ml was taken and made up to 100 ml using 0.1N HCl to get stock solution B. Different concentrations 2µg/ml to 18 µg/ml were prepared by pipetting out stock solution B as per requirement. The absorbance was recorded at 254 nm using U.V spectrophotometer (Shimadzu UV-1601, Japan) against suitable blank.
Preparation of Cinnarizine oral jellies
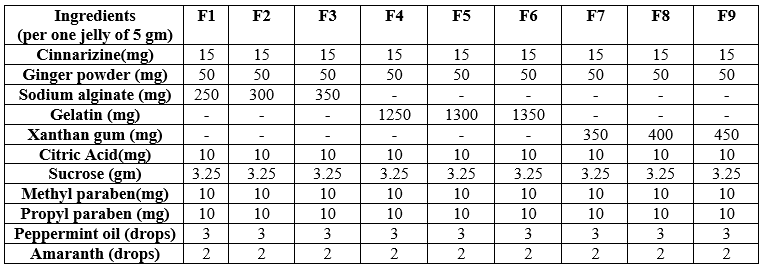
Sugar base jellies are prepared by heating and congealing method. All the ingredients were weighed accurately. All the formulations were prepared using freshly boiled and cooled distilled water. The sugar base was prepared by dissolving 66.7gm of sugar in a beaker, then added water up to 100 ml heated, stirred at 80? for about 30 min. The required quantities of polymers were dispersed to the solution maintained at 90? throughout the process. The dispersion was stirred using a magnetic stirrer (Remi industries, Mumbai) for 20 min to facilitate the hydration of the gelling agent. When the gelling agent was completely dissolved, stabilizer and citric acid were added and again stirred to enhance the softness of jelly maintaining pH respectively and then boiled for a few minutes. After boiling the above solution, preservatives were added, mixed uniformly and finally drug was added. Then the whole mixture was transferred into moulds and then allowed it to cool and settle undisturbed by proper covering the moulds to avoid exposure to outer environment.
Table 1: Cinnarizine Oral Jelly formulations
EVALUATION OF CINNARIZINE ORAL JELLIES
The prepared oral jellies were evaluated for the following parameters:
Compatibility studies
Fourier transform infrared spectroscopy study
FT-IR analysis was carried out for pure Cinnarizine and formulations using KBr pellets methods using FT-IR spectrophotometer (FTIR-8400S, Shimadzu, Japan). The pure Cinnarizine was mixed with KBr in the ratio of 1:3 and pressed in a hydraulic press at 5-6 ton load. The prepared pellets were scanned from 3500 to 1000 cm-1 using FT-IR spectrophotometer. The FT-IR spectra of the formulations F1, F4 and F8 were obtained and compared with the spectra of pure Cinnarizine.
Physical Observations
The prepared jellies were observed visually for clarity, odour, texture and presence of any particles. The texture was evaluated in terms of stickiness and grittiness by mild rubbing the gel between two fingers.
Weight Variation
Ten jelly formulations were taken to determine weight variation. The jellies were taken out of the moulds in a beaker and weighed individually. Average weight of jellies and percent deviation was determined.
Determination of pH
The pH of prepared jellies was measured using a digital pH meter (Elico.LH20pH (type003), Hyderabad, India) at room temperature (25?±5?). For this purpose, 0.5g of jelly was dispersed in 50 ml of distilled water to make a 1% solution and the pH was noted. This study was performed in triplicate.
Spreadability
The spreadability of the oral jellies was determined by taking two glass slides on which 1000 gm weight was kept and placing jelly in between the two slides and pressed for 5 minutes to a uniform thickness and the spreading area of jelly was calculated by using equation (A=?r2) it is represented as area of circle. This procedure was performed in triplicate and data is expressed as mean±standard deviation.
Syneresis
Syneresis is the contraction of the jelly upon storage and separation of water from the gel. All the jellies were observed for signs of syneresis at room temperature (25?±5?) and 8?±1?.
Drug content:
Five oral jellies were selected randomly and were dissolved individually in (50ml) 0.1N HCl, which was further diluted to measure the absorbance at 254 nm using UV-Visible spectrophotometer (Shimadzu UV-1601, Japan ) against a suitable blank. The content of each jelly was calculated. This was performed three times and the average value was calculated and expressed as mean±SD.
In-vitro dissolution study
In-vitro dissolution was performed using USP dissolution Apparatus ? (Model TDT-081 Electro lab, Mumbai, India). The dissolution study was performed in 0.1N HCl (900 ml, 37±0.5?) as a dissolution medium with speed of rotation of 50 rpm. 5ml samples were withdrawn at 5, 10, 15, 20, 25, 30, 35, 40 and 45 min using a prefilter. The sample was replaced by an equal volume of 0.1N HCl to maintain constant volume throughout. The Cinnarizine content was determined using a calibration curve at 254 nm after dilution using suitable blank by UV-Visible spectrophotometer (Shimadzu UV-1601, Japan). The study was performed in triplicate and results are expressed as mean±SD.
Model independent analysis (23)
Dissolution efficiency
Dissolution efficiency is used to translate the profile difference into a single value. Dissolution efficiency was calculated by using following equation
DE%=(?_0^t??y dt?)/y_100 t×100
Where, y is the drug percent dissolved at time t
Mean dissolution time
Mean dissolution time represents the mean time for drug molecules to completely dissolve. It is used to characterize the drug release rate from a dosage form and indicates the drug release retarding efficiency of the polymer. MDT was calculated by using the following equation
MDT=(?_(i=1)^(i=n)??t_mid×?M?)/(?_(i=1)^(i=n)??M)
Where ‘i’ is the dissolution sample number, ‘n’ is the number of dissolution sample time, ‘t_mid’ is the time at the midpoint between ‘i’ and ‘i-1’, and ‘?M’ is the amount of drug dissolved between ‘i’ and ‘i-1’.
Stability studies
For the stability studies, selected jelly formulation was packed in aluminium foil, transferred to high density polyethylene container, tightly closed and stored at room temperature (25?±5?) and 40?±2?/ 75±5% relative humidity for 90 days as per International conference on harmonization guidelines. The samples were characterized for change in physical parameters such as appearance, pH, drug content, and in-vitro studies at the end of 90 days. A freshly made sample was used as a reference standard for subjective evaluations.
RESULTS
Calibration curve of Cinnarizine:
Calibration curve of the pure drug Cinnarizine was prepared in the concentration range of 2 ?g/ml to18 ?g/ml and absorbance was determined at the wavelength of 254 nm. It followed Beer’s and Lamberts law in the above concentration range. The calibration curve showed good linearity and regression coefficient was 0.995(r2 value).
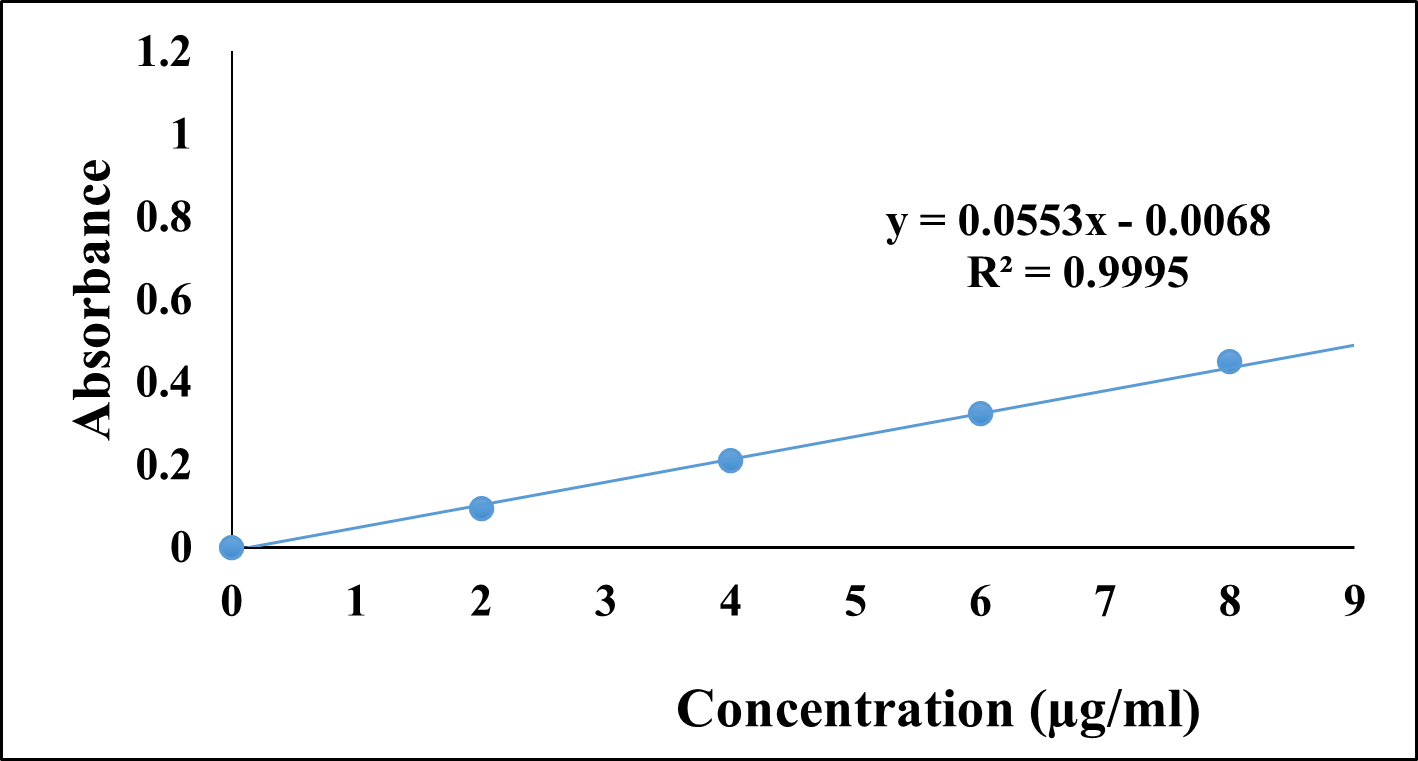
Figure 1: Calibration plot of Cinnarizine
FTIR study
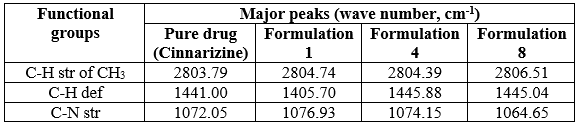
Pure drug Cinnarizine and formulations were subjected for FTIR analysis for compatibility studies and to ascertain whether there was any chemical interaction between the drug and the excipients used. For the pure drug, the peaks were present for C-H stretching at 2803.79 cm-1, C-H deformation at 1441 cm-1 C-H deformation and C-N stretching.at 1072.05 cm-1. The same peaks were observed in the spectra of formulations F1, F4 and F8. This indicated that there was no interaction between drug and excipients.
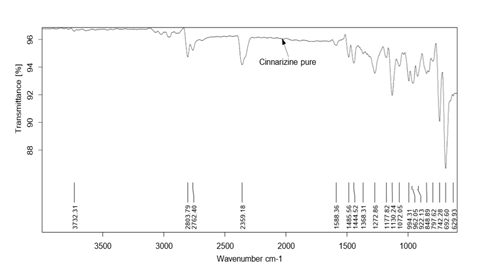
Figure 2: FTIR spectra of pure Cinnarizine
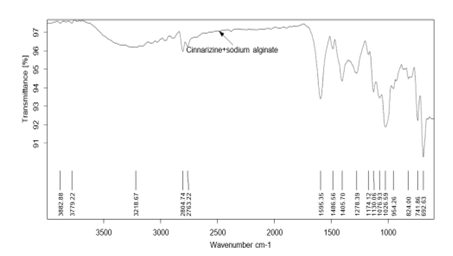
Figure 3: FTIR spectra of Formulation 1
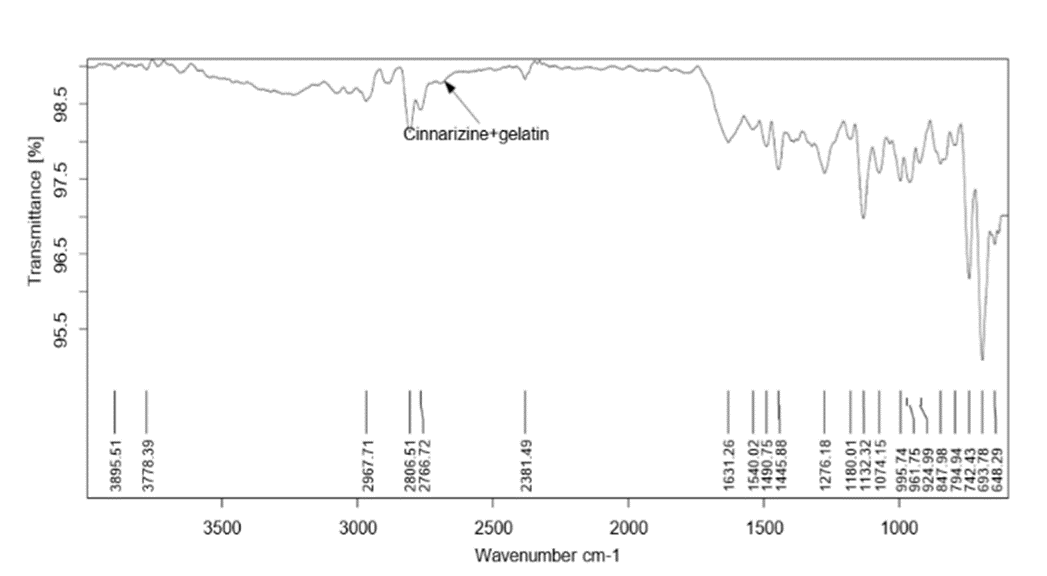
Figure 4: FTIR spectra of Formulation 4
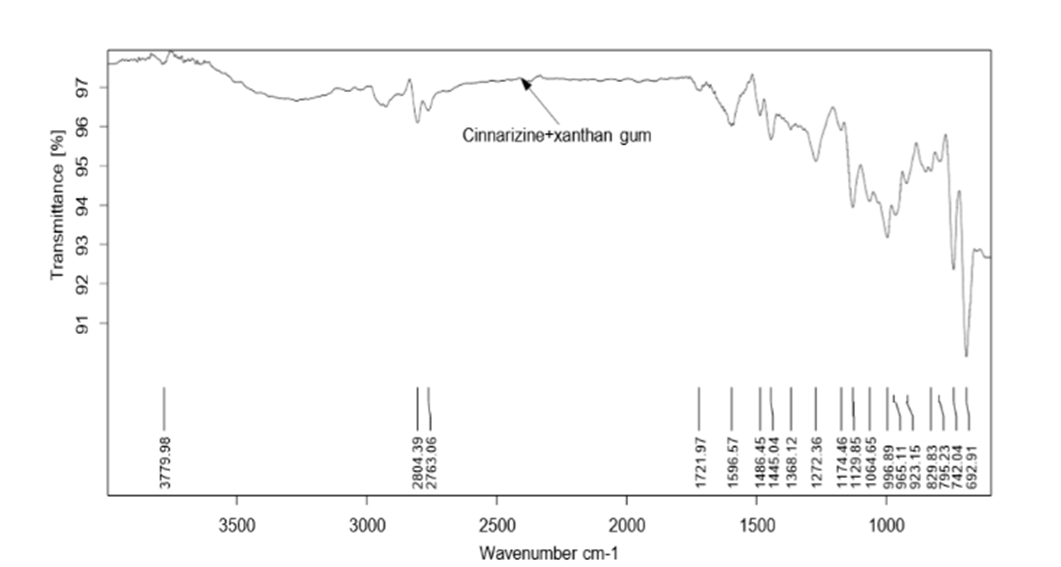
Figure 5: FTIR spectra of Formulation 8
Table 2: FTIR peaks of pure Cinnarizine and Formulations F1, F4 and F8
PRE-FORMULATION STUDIES
Determination of pH and Solubility studies
Table 3: pH and solubility studies of Cinnarizine

EVALUATION OF ORAL JELLY
Physical Appearance

Figure no 6: Formulation F4
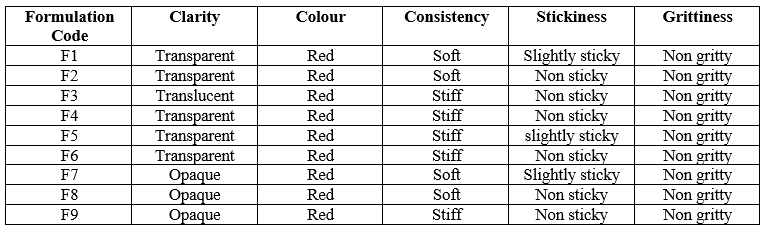
Table 4: Physical observation of medicated jellies (F1-F9)
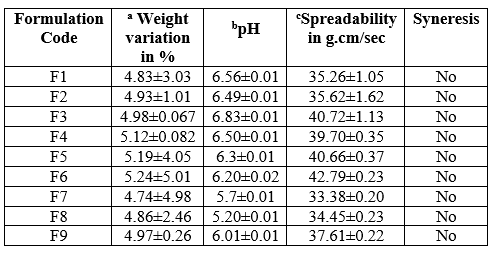
Table 5: Physical properties of Cinnarizine jelly formulations
Values are in Mean ± SD a (n=10) b, c (n=3)
pH of the formulations
The pH of the formulations influences the taste and stability of oral jellies. The pH of the prepared formulations was found in the range of 5.20±0.01 - 6.83±0.01 which was slightly acidic may be due to the presence of citric acid. Sucrose may precipitate in the presence of citric acid on standing. Therefore, Citric acid was used in minimum quantity to maintain the pH.
Spreadability
The spreadability of all the formulations was determined and it ranged between 33.38±0.20 - 42.79±0.23 g.cm/sec. It was inversely proportional to concentration of polymer.
Syneresis
Jellies experience syneresis or de-swelling due to the release of liquid, resulting in shrinkage of gels and reduced quality was observed after 24h of jelly preparation. Syneresis may be pronounced in the gels, where lower concentration of gelling agent was employed. Syneresis was not observed in any of the prepared formulations.
Drug content:
The percentage drug content was found to be in the range of 95.66±2.4- 97.86±1.2 % indicating uniform drug present within all oral jellies formulations.
Table 6: Drug content of Cinnarizine Oral jellies
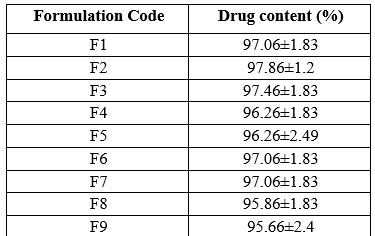
Values are in mean ± S.D (n=3)
In-vitro drug release studies
The in-vitro dissolution study was performed using the USP apparatus ? (paddle type). The in-vitro drug release study of prepared jellies was performed in 0.1N HCl for 45 minutes. Formulations F1, F2, F3 prepared with only sodium alginate showed the drug release of 97.23±0.98, 73.06±0.27, 26.76±0.22 % in 40 min. Formulations F4, F5, F6 prepared with only gelatin showed drug release of 92.59±0.23, 97.74±0.45, 93.78±0.17% in 15 min. The formulations F7, F8, F9 prepared with only xanthan gum showed drug release of 89.86±0.33, 88.35±0.33 and 66.33±0.35 % in 45 min. From the results it was observed that the formulations containing gelatin showed faster and higher % of drug release than other polymers. It was also observed that there was a decrease in drug release with increase in concentration of polymer for all the formulations.
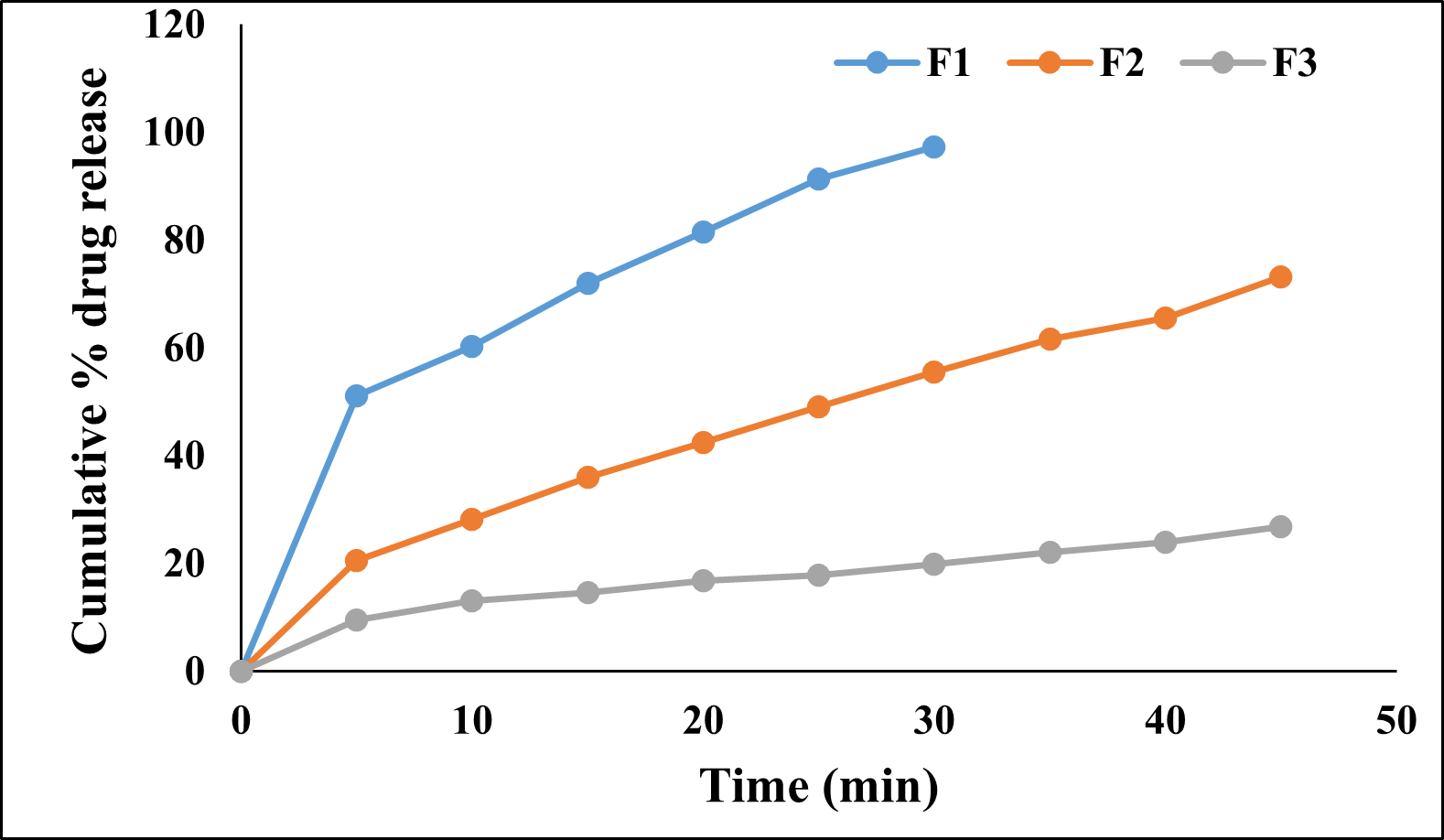
Figure 6 : Cumulative % drug release from formulations F1, F2 and F3
Figure 7: Cumulative % drug release from formulations F4, F5 and F6
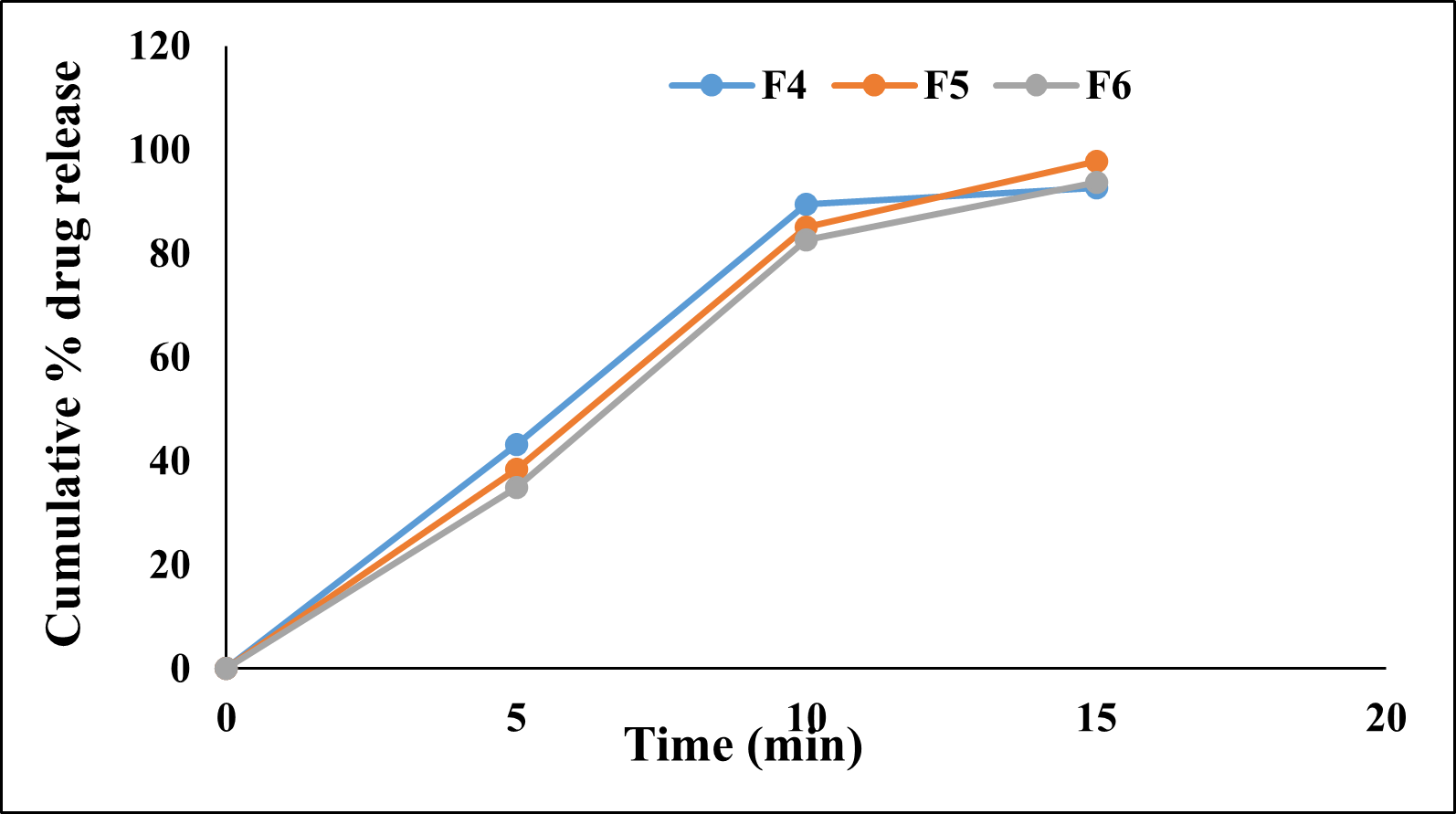

Figure 8: Cumulative % drug release from Formulations F7, F8 and F9
Model Independent Analysis
Table 7: Model independent analysis of dissolution data
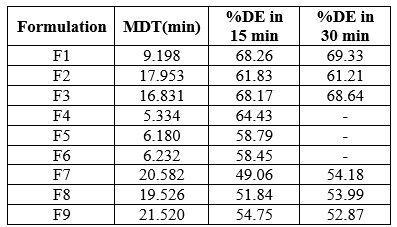
Model independent analysis
All the formulations showed the mean dissolution time between 5.334- 21.520 min. The ? in 15 min ranged between 51.84-68.26% .The ? in 30 min ranged between 52.87-69.33%. Amongst them formulation F4 prepared with gelatin was selected as best formulation because it showed the highest ? of about 64.43 % in 15 min and lowest MDT value of 5.334 min. Hence it will be considered for further studies.
Stability study
The formulation F4 was subjected to accelerated stability studies for 90 days at 25?/75% RH, in-vitro dissolution study was performed and showed negligible change in dissolution profile. The oral jellies subjected for stability studies were found to be smooth and flexible and no change in the physical appearance.
The in-vitro dissolution profile of the formulation F4 after stability study were found to be not significantly different (p>0.05).
Table 8: Cumulative % drug release of formulation F4 after stability study

Values are in mean ± S.D (n=3)
Figure 9: Comparison of drug release from formulation F4 before stability study and after 90 days.
CONCLUSION
In the present study, the sugar based oral jellies loaded with Cinnarizine were prepared by heat and congeal method. The results of FTIR studies showed that there was no interaction between drug and excipients. The prepared formulations showed acceptable Physico-chemical properties. All the oral jelly formulation showed good dissolution in 45 min. Formulation F4 showed max (92.59±0.23%) drug release in 15 min. From the results, it can be concluded that Cinnarizine oral jellies can be successfully formulated using sodium alginate, gelatin and xanthan gum.
REFERENCES
- Ansell H C, Popvich N G, Allen L V. “Pharmaceutical dosage forms and drug delivery system”. 1995; 1st ed: PP 78.
- Mehta R M. Vallabh Prakashan, Pharmaceutics-? 2nd ed; 2003; 168-172.
- Cooper and Gun, Dispensing for pharmaceutics, CBS publishers & distributors, Daraya Ganj New Delhi, 12th ed. 2000; 214-216.
- Sruthi Sunil S, Sharma U K, Arathy S A. Pharmaceutical jellies: A novel way of drug delivery. J Pharm Sci Res. 2020; 12(7):904-909.
- Cinnarizine, www.redpoll.pharmacy.ualberta.ca/drugbank/index.html, 2006.
- British Pharmacopoeia, Cinnarizine, The Stationery Office on behalf of the Medicines and Healthcare products Regulatory Agency (MHRA). 2001;398- 400.
- James E F Reynolds: Martindale, The extra Pharmacopoeia, 31st ed, Royal Pharmaceutical Society, London, 1996; 406.
- Cinnarizine: drug information, www.emc.medicines.org.uk, 2006.
- Lete I and Allue J. The Effectiveness of Ginger in the Prevention of Nausea and Vomiting during Pregnancy and Chemotherapy. Integr Med Insights 2016; 11(1):11–17.
- Britannica, the Editors of Encyclopaedia. "Ginger". Encyclopaedia Britannica, 1 Sep. 2023, https://www.britannica.com/plant/ginger. Accessed 1 Sep 2023.
- Sarojini S, Anusha K, Maneesha C, Mufaquam M A, Mounika B, Reddy Y K, Bhavana M, Deepika B, Dutt K R. Formulation development and evaluation of olmesartan medoxamil ororetentive jellies. European J Biomed Pharm Sci. 2018; 5(4):899-906.
- Akshay J, Shinde Vinay B. Formulation of clotrimazole ororetentive jelly. J Drug Deliv Ther. 2016; 6(2):21-25.
- Gananrajan G, Kaur G, Kothiyal P. Formulation and evaluation of montelukast sodium oral jelly for pediatrics. J Emerg Techno Innov Res. 2018; 5(7):448- 460.
- Taranum R, Mittapally S. Formulation and evaluation of domperidone oral jelly. Int. J Pharm Biolog Sci. 2018; 8(4):870-878.
- Dubey M, Zankhana Sheth. Design and development of oral medicated jelly of palonosetron hydrochloride. Ind J Res. 2015; 4(6):253- 255.
- Prakash K, Satyanarayana V M, Nagiat H T, Fathi A H, Shanta A K, Prameela A R. Formulation development and evaluation of novel oral jellies of carbamazepine using pectin, guar gum, and gellan gum. Asian J Pharm. 2014; 8(1):241-9.
- Kadam V S, Kendre J, Shendarkar G R, Kadam S S. Formulation and evaluation of medicated oral jelly of trazadone hydrochloride. Int J Pharma Sci Res. 2020; 11(12):6251-59.
- Yadav C, Tangri S, Yadav R. A review: Recent advancement in formulation of oral medicated jelly. World J pharm Pharma Sci. 2018; 7(7):417-426.
- Honale V S, Muneshwar S D, Sawale A V. Formulation and evaluation of oral soft jelly containing salbutamol sulphate for the treatment of asthma. J Drug Deliv Ther. 2023; 13(6):118-124.
- Kulkarni S, Londhe V. Oral jelly of Metformin hydrochloride- formulation development using design of experiment and characterization. J Drug Deliv Sci Technol. 2021; 63(1):1773-2247.
- Gomma E, Margrit M Ayoub. Vardenafil oral jellies as a potential approach for management of pediatric irritable bowel syndrome. Saudi Pharma J. 2021; 1(1):1-8.
- Sabri L A. Preparation and evaluation of oral soft chewable jelly containing flurbiprofen. J Adv Pharm Technol Res. 2022; 13(4):306-311.
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001; 13:123-33.


 Ashwini Rajendra*
Ashwini Rajendra*


















 10.5281/zenodo.11114938
10.5281/zenodo.11114938